Abstract
Background: Excessive gestational weight gain (GWG) is associated with postpartum weight retention (PPWR) and abdominal adiposity, but long-term effects are understudied in low-income and minority populations at high risk of obesity and associated sequelae.
Objective: We examined associations between GWG and long-term PPWR and adiposity in a prospective cohort of African American and Dominican mothers in the Bronx and Northern Manhattan.
Design: Women (n = 302) were enrolled during pregnancy and were followed for 7 y postpartum. Linear regression was used to relate excessive GWG [greater than 2009 Institute of Medicine (IOM) guidelines] to outcomes [percentage body fat and long-term PPWR (change in weight from prepregnancy to 7 y postpartum)], adjusting for covariates and included an interaction term between prepregnancy body mass index (BMI; in kg/m2) and GWG.
Results: Mean ± SD prepregnancy BMI and total GWG were 25.6 ± 5.8 (42% of women had BMI ≥25) and 16.6 ± 7.8 kg (64% of women had total GWG greater than IOM guidelines), respectively. Associations between GWG and long-term PPWR and the percentage body fat varied by prepregnancy BMI (P-interaction ≤ 0.06); excessive GWG was associated with a higher percentage body fat and greater long-term PPWR in mothers with lower prepregnancy BMI. To illustrate the interaction, a predicted covariate-adjusted model, which was used to derive estimates for the percentage body fat and PPWR associated with excessive GWG, was estimated for 2 prepregnancy BMI examples. For a woman with prepregnancy BMI of 22, excessive GWG was associated with 3.0% higher body fat (P < 0.001) and a 5.6-kg higher PPWR (P < 0.001); however, for a woman with a prepregnancy BMI of 30, excessive GWG was associated with 0.58% higher body fat (P = 0.55) and 2.06 kg PPWR (P = 0.24).
Conclusions: Long-term adiposity and PPWR in low-income African American and Dominican mothers were predicted by interacting effects of prepregnancy BMI and excessive GWG. The provision of support for mothers to begin pregnancy at a healthy weight and to gain weight appropriately during pregnancy may have important lasting implications for weight-related health in this population. This study was registered at clinicaltrials.gov as NCT00043498.
Keywords: body composition, gestational weight gain, maternal health, pregnancy, body fat, maternal, prepregnancy, African American, Dominican
INTRODUCTION
Overweight and obesity disproportionally affect minority populations. In women of childbearing age, 59% of all women are overweight or obese [BMI (in kg/m2) ≥25]; however, the prevalence of overweight or obesity is strikingly higher in non-Hispanic black (80%) and Hispanic (70%) women (1). The high prevalence of overweight and obesity in these subgroups may contribute to disparities observed for a number of maternal and child health outcomes such as preterm birth, childhood obesity, and postpartum weight retention (PPWR)10 (2). Gestational weight gain (GWG) may play a role in these health disparities (2) and, importantly, is potentially modifiable.
In 2009, the Institute of Medicine (IOM) issued revised recommendations for GWG that were specific to a prepregnancy BMI group (3). Despite the recommendations, most women in the United States gain above these guidelines; in recent estimates from 28 states, 47% of women had excessive GWG (greater than IOM guidelines), 32% of women gained within the guidelines, and 21% of women had inadequate GWG (less than IOM guidelines) (4). Prepregnancy BMI influences adherence; compared with normal-weight women, women with prepregnancy overweight or obesity are more likely to have excessive GWG, whereas inadequate GWG is more common in underweight women and in women with class II (BMI: 35.0–39.9) and class III (BMI ≥40) obesity (4). Although the prevalence of overweight and obesity is higher in non-Hispanic black and Hispanic women, these women are not more likely to gain excessively than other racial-ethnic groups (4).
There has been consistent evidence that high GWG is positively associated with PPWR (5, 6). From a meta-analysis of 11 studies, women with high GWG retained 3.21 kg at 1–15 y postpartum. Similar associations were observed in a meta-analysis of 9 studies that focused on longer-term PPWR (≥3 y), whereby women with high GWG retained ∼3.06 and ∼4.7 kg body weight after 3 and ≥15 y postpartum, respectively (7).
Although it has been presumed that much of PPWR reflects adipose tissue, the composition of retained weight is understudied. GWG includes maternal, fetal, and supporting components (8); the maternal component of GWG includes increments of fat mass. Women with high GWG gain greater fat mass during pregnancy (9, 10); but whether these women retain this fat mass beyond the immediate postpartum period (and, thus, have a greater percentage body fat) is unclear. To our knowledge, no previous studies have examined the association between GWG and long-term PPWR and overall body fat in a low-income, urban, and minority population, which is a population at high risk of obesity. Our objective was to evaluate the association between GWG and maternal body composition and size at 7 y postpartum in African American and Dominican women. We hypothesized that excessive GWG and total GWG (kg; continuous) would be positively associated with long-term body fat, PPWR, and BMI, and, in addition, that effects of GWG would vary by prepregnancy BMI.
METHODS
Participants were a subset of mothers (n = 302) enrolled in the Columbia Center for Children’s Environmental Health prospective birth cohort located in Northern Manhattan and the South Bronx, which has been previously described (clinicaltrials.gov; NCT00043498) (11, 12). From 1998 to 2006, women who self-identified as African American or Dominican (n = 727) were enrolled during pregnancy. To be eligible for the study, mothers needed to be 18–35 y old, reside in the study area for at least 1 y, and attend a prenatal care visit before 20 wk of gestation. Women were excluded if they self-reported having diabetes, hypertension, HIV, or used illicit drugs or cigarettes during pregnancy. The prenatal visit occurred during the third trimester and included measurements and questionnaires. During this visit, a self-report of prepregnancy weight, education, receipt of public assistance, ability to afford food during pregnancy, and previous pregnancy history was obtained.
Mothers and their children have been followed prospectively since delivery. This report focuses on data collected from 1998 to 2013. After delivery, medical records were abstracted to obtain the prenatal medical history, birth outcomes, and maternal weight at the last prenatal visit. A maternal report of breastfeeding was obtained at 3, 6, 9, 12, and 24 mo postpartum. Height was obtained ≤3 times in participants; mothers self-reported height at the prenatal interview, the medical record height was abstracted after delivery, and height was assessed with a stadiometer at 7 y postpartum (before January 2010: Cardinal Scale Manufacturing Co.; after January 2010: Holtain Ltd.). The protocol for addressing disparate maternal height data has been previously described in detail (13). At 7 y postpartum, maternal body weight (kg) and bioelectrical impedance estimates of the percentage body fat were obtained with the use of the Tanita Digital Body Mass Indicator Scale BC-418 (Tanita Corp.) according to standard procedures. Mothers were assessed during the study visit, which was held at varying times of day; thus, measurements were obtained in the nonfasting state. Bioelectrical impedence measurements were not conducted if the participant was pregnant. A maternal report of interim pregnancies was obtained at 7 y postpartum. Interim pregnancies reported at 5 y were used for one women with missing data at 7 y. Study procedures at enrollment and follow-up were approved by the Columbia University Institutional Review Board. Written informed consent was obtained from all enrolled women.
Analyses were conducted with Stata 12.0 software (StataCorp LP). An α = 0.05 was used for statistical tests, and an α = 0.1 was used for statistical tests of interactions. To evaluate for selection bias, baseline characteristics were compared between included and excluded mothers with the use of parametric tests for continuously, normally distributed variables and nonparametric tests as appropriate.
Total GWG was calculated by subtracting the last prenatal visit weight from the prepregnancy weight. The GWG percentage of adequacy was determined by dividing the observed total GWG by the expected GWG as follows:
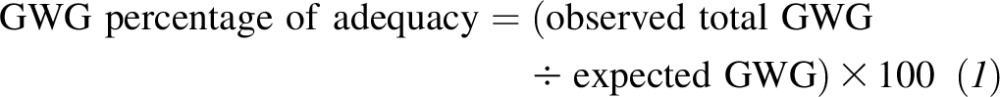 |
Expected GWG was determined with the following equation (IOM 2009 guidelines) (3):
 |
First-trimester gains for the expected GWG calculation were 2 kg for underweight and normal-weight women, 1 kg for overweight women, and 0.5 kg for obese women. Ratios that exceeded the 2009 IOM recommendations were coded as an excessive GWG as previously described (13). This calculation for the determination of excessive GWG incorporates gestational age and reflects that women with longer gestations may have a greater total weight gain (13).
Multivariable linear and logistic regression models were used to assess the association between GWG and outcomes. GWG was modeled as both the total gain (kg; continuous variable) and the adequacy of GWG (dichotomous variable: excessive or not excessive (referent) (IOM 2009 guidelines) (3). The primary dependent variables were 1) long-term PPWR, which was calculated as the weight change from prepregnancy to 7 y postpartum; 2) body fat assessed with bioelectrical impedance analysis; 3) BMI at 7 y postpartum and in mothers who were not obese prepregnancy (n = 235); and 4) risk of obesity at 7 y postpartum (BMI >30). We evaluated if the effects of GWG varied by prepregnancy BMI by including an interaction term between these variables. Because interaction effect estimation can be susceptible to the choice of cutoffs used when one interaction partner is modeled as a dichotomous or categorical variable, prepregnancy BMI was modeled as a continuous variable. This choice allowed for the observation of any interaction effects across the full range of the prepregnancy BMI data. Although this approach provides more-valid estimates of interaction effects, the interpretation of the linear combinations of β coefficients is more challenging. Therefore, to inform the interpretation of the interaction effects, model-derived, covariate-adjusted estimates of the association between excessive GWG and the outcomes were made for specific prepregnancy BMI values. Specifically, the effect of excessive GWG on outcomes was estimated for a prepregnancy BMI of 22 and 30. Other covariates in the multivariate model included maternal education (>12 y; yes or no), maternal race-ethnicity (categorical), receipt of public assistance (yes or no), ability to afford food in pregnancy (yes or no), parity (continuous), prenatal demoralization (i.e., psychological distress) previously described (14, 15) (score >1.55 was coded as high; yes or /no), live births since the index pregnancy (range: 0–3; continuous), and months postpartum at measurement (mo; continuous). Gestational age at the last measured prenatal weight was also included in the models (wk; continuous). To be consistent with the literature, we added the breastfeeding duration (coded as weeks of any breastfeeding) to an additional set of models because this variable may be on the causal pathway between GWG and long-term weight retention and adiposity.
As in our previous report on GWG in this cohort (13), many women were missing weights within close proximity to delivery. Of women with prepregnancy weight, covariates, and data at 7 y (n = 302), the last prenatal weight was obtained at a mean ± SD of 4.2 ± 4.9 d before delivery (range: 0–26 d). However, for 50 of these women, weights were obtained from 8 to 26 d before delivery. A sensitivity analysis was conducted to assess whether the exclusion of these 50 women influenced associations.
In addition, we conducted inverse probability weighting to assess effects of attrition and incomplete follow-up on observed associations with the use of methods previously described in this cohort (11, 13, 16); this assessment allowed for the estimation of biases that were due to sample attrition and missing data, which could have potentially affected associations if biases were differential by GWG or other important covariates. A logistic regression model estimated the probability of a successful follow-up with baseline cohort data; the model included the following variables: prepregnancy BMI, parity, total GWG, race-ethnicity, maternal age, education, linguistic isolation, neighborhood poverty rate, child sex, receipt if public assistance, a report of dissatisfaction with conditions, and gestational age. The inverse of this predicted probability of follow-up was used to weight the sample in a reanalysis of the data with the use of survey commands in Stata 12.0 software.
RESULTS
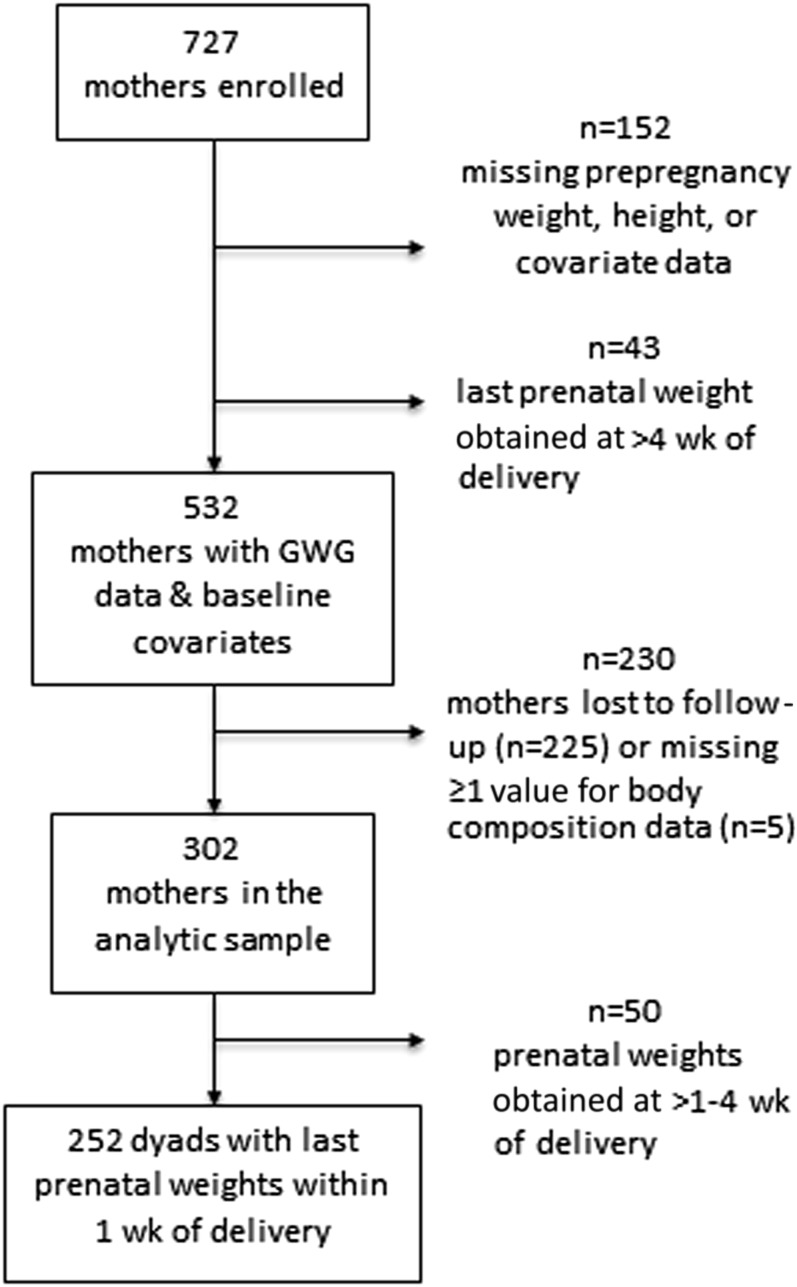
Data on pregnancy weight gain and key covariates at 7 y postpartum was available for 302 women (Figure 1). Mothers were followed a mean ± SD of 7.1 ± 0.20 y postpartum. Table 1 shows sample characteristics at baseline and follow-up. Most characteristics were similar between women who were included and excluded from the analytic sample; however, mothers in the analytic sample were older and lived in neighborhoods that were more linguistically isolated than were mothers excluded from the analysis because of a loss to follow-up or missing data.
FIGURE 1.
Participant flow diagram. GWG, gestational weight gain.
TABLE 1.
Sample characteristics
| Enrolled cohort with pregnancy weight-gain data (n = 532) | Excluded because of loss to follow-up or missing covariate data (n = 230) | Analytic sample (n = 302) | P1 | |
| Prepregnancy BMI, kg/m2 | 25.6 ± 5.82 | 25.6 ± 5.9 | 25.6 ± 5.8 | 0.94 |
| Prepregnancy BMI, n (%) | 0.07 | |||
| Underweight | 27 (5.1) | 13 (5.7) | 14 (4.6) | |
| Normal | 268 (50.4) | 107 (46.5) | 161 (53.3) | |
| Overweight | 127 (23.9) | 67 (29.1) | 60 (19.9) | |
| Obese3 | 110 (20.7) | 42 (18.7) | 672 (22.2) | |
| Total GWG,4 kg | 16.7 ± 7.7 | 16.7 ± 7.5 | 16.6 ± 7.8 | 0.93 |
| Excessive total GWG,5 n (%) | 346 (65.0) | 152 (66.1) | 194 (64.2) | 0.64 |
| Ethnicity | 0.17 | |||
| African American, n (%) | 191 (35.9) | 75 (32.6) | 116 (38.4) | |
| Dominican, n (%) | 341 (64.1) | 155 (67.4) | 186 (61.6) | |
| Age, y | 25.0 ± 4.9 | 24.4 ± 4.7 | 25.6 ± 5.0 | 0.003 |
| Received public assistance in pregnancy, n (%) | 216 (40.6) | 93 (40.4) | 123 (40.7) | 0.95 |
| Prenatal demoralization score | 1.15 ± 0.63 | 1.17 ± 0.65 | 1.13 ± 0.62 | 0.40 |
| High prenatal demoralization (score >1.55), n (%) | 137 (25.8) | 60 (26.1) | 77 (25.5) | 0.88 |
| Education >12 y, n (%) | 155 (29.1) | 65 (28.3) | 90 (29.8) | 0.70 |
| Parity at index pregnancy, n | 1.9 ± 1.8 | 1.9 ± 1.9 | 2.0 ± 1.8 | 0.88 |
| Unable to afford food during pregnancy, n (%) | 92 (17.3) | 40 (17.4) | 52 (17.2) | 0.96 |
| Dissatisfied with conditions, n (%) | 69 (13.0) | 26 (11.3) | 43 (14.2) | 0.32 |
| Neighborhood poverty, % | 35.8 ± 4.9 | 35.8 ± 5.2 | 35.8 ± 4.6 | 0.53 |
| Linguistic isolation, % | 36.7 ± 4.7 | 37.2 ± 4.5 | 36.3 ± 4.8 | 0.03 |
| Breastfeeding duration, weeks | — | — | 11.8 ± 14.6 | — |
| Live births after index pregnancy, n | — | — | 0.61 ± 0.71 | — |
| Weight change from prepregnancy to 7 y postpartum, kg | — | — | 9.2 ± 11.2 | — |
| Percentage body fat at 7 y postpartum, % | — | — | 36.6 ± 8.3 | — |
| BMI at 7 y postpartum, kg/m2 | — | — | 29.0 ± 6.8 | — |
| Obese at 7 y postpartum, n (%) | — | — | 114 (38.0) | — |
For comparisons between excluded and included subjects in the analytic sample.
Mean ± SD (all such values).
Of obese women, 16 subjects (23.9%) had class II obesity (BMI: 35.0–39.9), and 6 subjects (9.0%) had class III obesity (BMI: ≥40.0).
GWG, gestational weight gain.
Greater than the Institute of Medicine 2009 guidelines.
Before pregnancy, 5% of women in the study population were underweight, 53% of women were normal weight, and 42% of women were overweight or obese. A majority of mothers (64%) gained more weight in pregnancy than recommended in the 2009 IOM guidelines (3). In univariate analyses, the proportion of women who had excessive GWG varied by prepregnancy BMI (P = 0.001); compared with overweight (25%), obese (26%), or underweight women (4%), a larger proportion of normal-weight women (46%) had excessive GWG. The proportion of women with excessive GWG did not vary by maternal race-ethnicity, prenatal distress, education, parity, food insecurity, or receipt of public assistance (all P > 0.05).
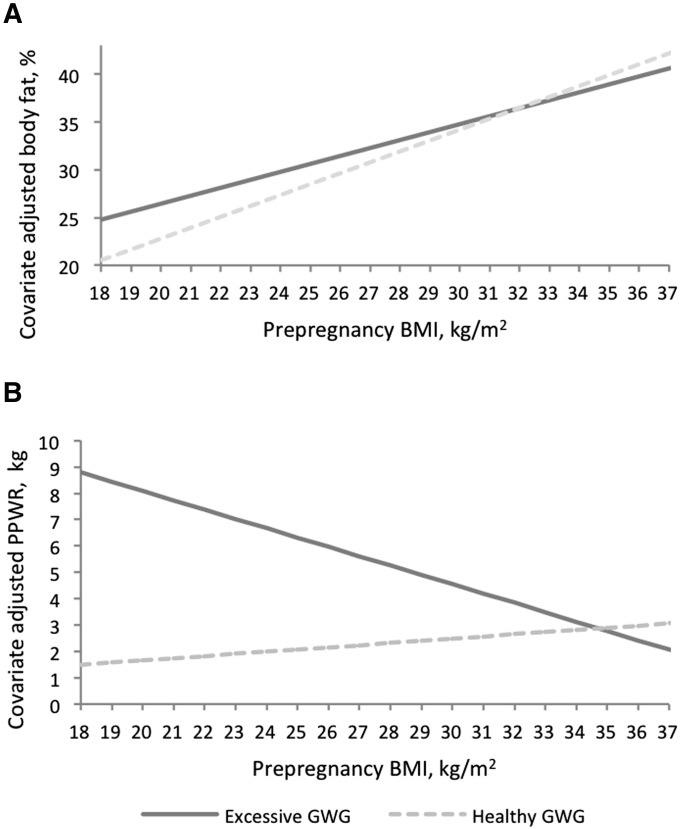
At 7 y postpartum, mean weight retention was ∼9.2 ± 11.2 kg from prepregnancy to 7 y (range: −34.6 to 50.6 kg). A total of 38% of participants were obese, whereas almost one-half of the obese women (55 of 114 women; 48.2%) had class II obesity [31 of 114 (27.2%); BMI: 35.0–39.9] or class III obesity [24 of 114 (21.1%); BMI ≥40.0]. Table 2 shows the covariate-adjusted β coefficients for prepregnancy BMI, excessive GWG, and the interaction term that predicted the percentage body fat and PPWR. Predicted effects of excessive GWG for women at specific prepregnancy BMI are also shown. As an illustration of the interaction effect, for a women with a prepregnancy BMI of 22, excessive weight gain was associated with a 3.04 (P < 0.001) difference in the percentage body fat and a 5.56-kg (P < 0.001) difference in PPWR 7 y postpartum, whereas for a women with a prepregnancy BMI of 30, excessive weight gain was associated with a 0.58 (P = 0.55) difference in the percentage body fat and a 2.06-kg (P = 0.24) difference in PPWR 7 y postpartum. Because the interaction effect appeared as a crossing over of the prepregnancy BMI effect by weight-gain status (Figure 2), the prepregnancy BMI above which excessive weight gain was no longer significantly associated with the outcomes was calculated on the basis of covariate-adjusted β coefficients (Table 2); for the percentage body fat, this value was a prepregnancy BMI ≥27, and for PPWR, this value was a prepregnancy BMI ≥28. The addition of breastfeeding duration to the models had minimal effects on the estimates between GWG and maternal health outcomes (data not shown). Similar associations were observed when analyses were redone with the use of total GWG (kg; continuous); in women with relatively lower BMI, every one-unit increase in GWG was positively associated with higher body fat and greater PPWR at 7 y postpartum (Supplemental Table 1).
TABLE 2.
Model effect estimates and model-derived predicted effects of excessive GWG on long-term weight retention and percentage body fat for specific prepregnancy BMI1
| Body fat, % |
PPWR, kg |
|||
| β (95% CI) | P | β (95% CI) | P | |
| Model effect estimates | ||||
| Prepregnancy BMI | 1.14 (0.94, 1.34) | <0.001 | 0.08 (−0.29, 0.46) | 0.66 |
| Excessive GWG | 9.81 (3.40, 16.2) | 0.003 | 15.19 (3.38, 26.97) | 0.01 |
| Excessive GWG × prepregnancy BMI | −0.31 (−0.56, −0.06) | 0.02 | −0.44 (−0.89, 0.02) | 0.06 |
| Predicted effect estimate for excessive GWG for specific prepregnancy BMI,2 kg/m2 | ||||
| 18 | 4.27 (1.99, 6.55) | <0.001 | 7.31 (3.12, 11.51) | 0.001 |
| 19 | 3.96 (1.87, 6.05) | <0.001 | 6.88 (3.03, 10.72) | 0.001 |
| 20 | 3.65 (1.74, 5.57) | <0.001 | 6.44 (2.91, 9.97) | <0.001 |
| 21 | 3.35 (1.58, 5.11) | <0.001 | 6.00 (2.76, 9.24) | <0.001 |
| 22 | 3.04 (1.41, 4.67) | <0.001 | 5.56 (2.56, 8.56) | <0.001 |
| 23 | 2.73 (1.20, 4.26) | 0.001 | 5.13 (2.31, 7.94) | <0.001 |
| 24 | 2.42 (0.96, 3.88) | 0.001 | 4.69 (2.00, 7.38) | 0.001 |
| 25 | 2.11 (0.68, 3.55) | 0.004 | 4.25 (1.61, 6.89) | 0.002 |
| 26 | 1.81 (0.36, 3.26) | 0.02 | 3.81 (1.14, 6.49) | 0.005 |
| 27 | 1.50 (−0.01, 3.01) | 0.05 | 3.38 (0.60, 6.16) | 0.02 |
| 28 | 1.19 (−0.41, 2.80) | 0.15 | 2.94 (−0.02, 5.89) | 0.05 |
| 29 | 0.88 (−0.85, 2.61) | 0.32 | 2.50 (−0.68, 5.69) | 0.12 |
| 30 | 0.58 (−1.30, 2.46) | 0.55 | 2.06 (−1.40, 5.53) | 0.24 |
| 31 | 0.27 (−1.78, 2.32) | 0.80 | 1.63 (−2.15, 5.40) | 0.40 |
| 32 | −0.04 (−2.27, 2.19) | 0.97 | 1.19 (−2.93, 5.31) | 0.57 |
| 33 | −0.35 (−2.78, 2.08) | 0.78 | 0.75 (−3.72, 5.23) | 0.74 |
| 34 | −0.66 (−3.29, 1.98) | 0.63 | 0.31 (−4.54, 5.17) | 0.90 |
| 35 | −0.96 (−3.81, 1.88) | 0.51 | −0.12 (−5.37, 5.12) | 0.96 |
| 36 | −1.27 (−4.34, 1.79) | 0.42 | −0.56 (−6.20, 5.09) | 0.85 |
| 37 | −1.58 (−4.87, 1.71) | 0.35 | −1.00 (−7.05, 5.06) | 0.75 |
Effect estimates are from a multivariate linear regression model for effects of high GWG on maternal weight retention and body fat at 7 y postpartum by prepregnancy BMI. Effects of excessive GWG on maternal outcomes varied by prepregnancy BMI (P-interaction ≤ 0.06). GWG, gestational weight gain; PPWR, postpartum weight retention.
Effects shown are predicted estimates of excessive GWG for percentage body fat and long-term weight retention by specific maternal prepregnancy BMI values adjusted for covariates. Predicted estimates are shown for approximately the 5th to 95th percentiles of prepregnancy BMI (18–37) in the study population. Results presented for the model were adjusted for maternal education, age, parity, race, receipt of public assistance, report of food insecurity in pregnancy, prenatal demoralization, gestational age at delivery, and interim pregnancies between the index pregnancy and 7 y postpartum.
FIGURE 2.
Effects of GWG on covariate-adjusted predicted percentages of body fat at 7 y postpartum (A) and weight changes from prepregnancy to 7 y postpartum (PPWR) (B) by prepregnancy BMI in African American and Dominican women from the Bronx and Northern Manhattan (n = 302). Effects of excessive GWG on maternal outcomes varied by prepregnancy BMI (in kg/m2) (P-interaction < 0.1). Effects shown are predicted estimates from multivariable linear regression models for the percentage body fat and long-term weight retention by maternal prepregnancy BMI adjusted for covariates. Results presented for the model were adjusted for maternal education, age, parity, race, receipt of public assistance, report of food insecurity in pregnancy, prenatal demoralization, gestational age at delivery, and interim pregnancies between the index pregnancy and 7 y postpartum. Predicted estimates are shown for approximately the 5th to 95th percentiles of prepregnancy BMI (18–37) in the study population. GWG, gestational weight gain; PPWR, postpartum weight retention.
Excessive GWG was positively associated with maternal BMI at 7 y postpartum [β = 2.21 (95% CI: 1.15, 3.26); P < 0.001]; however, associations between GWG and maternal BMI at 7 y postpartum did not vary by prepregnancy BMI (P-interaction > 0.1). With prepregnancy BMI and covariates controlled for, mothers with excessive GWG had BMI values at 7 y postpartum that were 2.21 kg/m2 higher than those of mothers who gained within or below the IOM guidelines. In the subsample of mothers who were not obese before pregnancy (n = 225), mothers with excessive GWG were at almost 400% increased risk of obesity at 7 y postpartum [RR: 3.86 (95% CI: 1.47, 10.14); P = 0.006] in multivariate-adjusted models. Similarly, in these women (n = 225), total GWG (kg; continuous) was positively associated with maternal obesity at 7 y postpartum [RR: 1.1 (95% CI: 1.04, 1.15); P = 0.001]. Specifically, every 1-kg increase in total GWG corresponded to 10% increase in risk of obesity at 7 y postpartum.
Additional analyses were conducted to evaluate whether our findings were sensitive to the timing of the last prenatal weight measurement relative to that at delivery. Last prenatal weight measurements were obtained between 1 and 4 wk before delivery in 50 women (16.6%) included in this analysis. In these women, the last prenatal weight was obtained at 1.8 ± 0.7 wk before delivery (range: 1.1–3.7 wk). In sensitivity analyses, where these women were excluded from the analytic sample, associations between GWG and maternal size outcomes at 7 y postpartum were essentially the same or negligibly different from associations in the larger analytic sample (data not shown).
To evaluate for effects of sample attrition and incomplete follow-up, we conducted analyses with inverse-probability weighting for successful follow-up. Older maternal age was associated with successful follow-up [OR: 1.07 (95% CI: 1.03, 1.11); P = 0.002]; no other covariates were associated with follow-up (P > 0.05). A weighting analysis for successful follow-up had minimal effects on associations between GWG and PPWR, later maternal body fat, and BMI (data not shown).
DISCUSSION
To our knowledge, this is the first study to evaluate the effects of GWG on long-term body fatness in a contemporary, low-income, urban population of African American and Dominican women, who are a population at high risk of obesity and related comorbidities. In our analysis, we showed that excessive GWG was associated with a higher percentage body fat and PPWR at 7 y postpartum in mothers with underweight, normal, and some overweight prepregnancy BMI values (<27 for the percentage body fat and <28 for weight retention). Strikingly, excessive GWG was not associated with body fat or long-term weight retention in mothers with prepregnancy obesity. Although effects of excessive GWG on body fat and PPWR varied by prepregnancy weight status, no evidence of effect modification by prepregnancy BMI was observed for maternal BMI outcomes (obesity or continuous). Excessive GWG was associated with 2-kg/m2 higher maternal BMI at 7 y postpartum. Moreover, in the subset of mothers who were not obese before pregnancy (n = 225), excessive GWG was associated with markedly increased risk of obesity at 7 y postpartum.
Our findings are generally consistent with previous research that showed associations between high GWG and long-term (≥3 y) PPWR (7, 17–20), waist circumference (WC) (18, 20, 21), BMI (20–22), and the transition to a higher BMI group (22). In another cohort of predominately normal-weight women (64%) who were non-Hispanic African Americans (27%) or other races (73%) including white, Asian and Pacific Islander, Native American, and Hispanic, excessive GWG was associated with a 3.5-cm greater WC and 300% increased risk of abdominal adiposity compared with values for adequate GWG (20). In Project Viva, which was a study of predominately normal-weight women (57%) who were white (65%), African American (18%), Hispanic (8%), Asian (4%), and other races (4%), the rate of total GWG (kg/wk) was positively associated with WC at 7 y postpartum in normal-weight and obese women but not in overweight women (18). In normal-weight women, the GWG rate was only associated with the postpartum weight change (18). In a United Kingdom birth cohort (the Avon Longitudinal Study of Parents and Children), a high overall GWG was associated with higher BMI (2.90), greater WC (5.84 cm), and increased likelihoods of overweight or obesity (OR: 3.58) and central adiposity (WC ≥ 80 cm) (OR: 2.67) (21). In a Danish birth cohort [the DNBC (Danish National Birth Cohort)] (n = 23,701), no associations were observed between GWG (continuous) and BMI-adjusted WC at 7 y postpartum (19), but a significant indirect effect of GWG on later WC was mediated through early postpartum weight changes at 6–18 mo.
For body fat and PPWR, effects of excessive GWG varied by prepregnancy BMI. In women with heavier prepregnancy BMI, excessive GWG was not associated with later adiposity and weight retention. However, we showed that the effects of high GWG were stronger in women with lower prepregnancy BMIs (<27 for the percentage body fat and <28 for weight retention). This result corroborates a recent meta-analysis that reported that the average PPWR decreased with increasing prepregnancy BMI (5), an observational study that reported stronger effects in thinner women (23), and other observational studies with central adiposity outcomes (18, 21). In Project Viva, the total GWG rate was associated with PPWR only in normal-weight women (18). Similarly, in the Avon Longitudinal Study of Parents and Children, GWG (kg/wk; from 19 to 28 wk) was associated with greater maternal BMI and central adiposity (WC ≥80 cm) at 16 y postpartum in women with prepregnancy BMI <25, but effects of later GWG (>29 wk) on central adiposity were stronger in women with BMI ≥25 (21). Effects of the timing of GWG did not vary by prepregnancy BMI for later WC (continuous) or overweight and obesity (21). Other reports of long-term effects of GWG have not observed effect modification by prepregnancy BMI (20), which may have been attributable to an insufficient number of overweight and obese women, whereas another study observed stronger associations in heavier women (19). In the DNBC, the effect of a one-unit increase in the total GWG z score on maternal weight at 7 y postpartum was greater for overweight and obese women than for normal-weight women (19). This cohort of Danish women was predominately normal weight (69%) and of high- or middle-income socio-occupational status (91%) (19). On average, PPWR in the DNBC (2.1 ± 6.3 kg) was substantially less than in our study population of low-income, urban, minority women (Columbia Center for Children’s Environmental Health weight retention: 9.2 ± 11.2 kg) (19). These differential effects of prepregnancy BMI on long-term maternal body size may have been due to these study population differences.
Although effects of excessive GWG varied by prepregnancy BMI for the percentage of fat and long-term weight retention, effect modification was not observed for maternal BMI at 7 y postpartum. BMI is an index of weight relative to height and is assumed to be associated with body fatness and associated sequelae (24). However, BMI is not a measure of fatness but a surrogate measure of weight status (i.e., underweight, overweight, and obesity). Because body weight is the sum of fat mass and fat-free mass, BMI cannot partition body weight into these 2 components. Accordingly, BMI cannot discriminate when an individual is normal weight with higher body fat or overweight with lower body fat (e.g., high muscle mass). Because of this potential measurement error, we may have been underpowered to detect effect modification for this outcome.
There were some limitations that should be noted. As with most studies on GWG, prepregnancy weight was estimated by maternal report; previous evidence has suggested that weight reporting may be impacted by maternal prepregnancy weight status and ethnicity (25–27). If weight was underreported, prepregnancy BMI could have been underestimated, and GWG could have been inflated (13). Although the majority of women in this analysis had last prenatal weight measurement ≤1 wk of delivery, some women did not have weight measurements close to delivery. We conducted a sensitivity analysis in which women with weights measured between 1 and 4 wk before delivery were excluded. In this analysis, effects of GWG were not appreciably altered. Unfortunately, data were not available on the timing of pregnancies after the index pregnancy, which could have potentially affected associations if the interim pregnancy was near the 7-y measures. An adjustment for a pregnancy after the index pregnancy did not independently predict maternal size outcomes at 7 y postpartum. During the course of this study, there was an overall increase in obesity nationwide (1), and macroscale drivers of these changes, such as food policies, changes in the retail food environment, portion sizing, and changes in physical activity patterns, likely affected our study participants. In our interpretation of the findings, we assumed that mothers with different GWG experienced similar obesogenic environments; however, this possibility was not explicitly measured as part of the study. Furthermore, we could not determine how GWG was associated with the change in body fat from prepregnancy to 7 y postpartum because body fat was not assessed before pregnancy, although our analyses did adjust for prepregnancy BMI.
In conclusion, our results suggest that GWG greater than the IOM recommendations has long-term implications for maternal weight-related health for African American and Dominican women, particularly for women with lower prepregnancy BMI. These findings and others (5, 18, 21) suggest that normal and modestly overweight weight women may be more physiologically sensitive to effects of high GWG and, therefore, need to be further supported to gain weight appropriately during pregnancy and adhere to the IOM guidelines. Interventions, policies, and clinical management that support healthy GWG may have lasting implications for maternal weight-related health, particularly in women with normal and modestly overweight prepregnancy BMI.
Acknowledgments
We thank Virginia Rauh, Greg Freyer, Howard Andrews, Deliang Tang, Diurka Diaz, Fred Hua, and Darrell Holmes.
The authors’ responsibilities were as follows—EMW: conceptualized the research question, designed and performed the analysis, wrote the manuscript, and had primary responsibility for the final content of the manuscript; EMW, LAH, and AGR: contributed to the data cleaning; EMW, SEO, AH, DG, and AGR: contributed to the interpretation of the data; RMW, FPP, and AGR: contributed to the original cohort design; RMW, LAH, and AGR: contributed to the design of the age 7-y wave of follow-up; LAH: contributed to the database management; JR-C: contributed to the data collection; and all authors: reviewed all versions of the manuscript and contributed to the intellectual content of the manuscript. The Irving General Clinical Research Center, the Educational Foundation of America, the John and Wendy Neu Family Foundation, the New York Community Trust, the Trustees of the Blanchette Hooker Rockefeller Fund, and PepsiCo Global R+D had no role in the design, implementation, analysis, or interpretation of the data. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DNBC, Danish National Birth Cohort; GWG, gestational weight gain; IOM, Institute of Medicine; PPWR, postpartum weight retention; WC, waist circumference.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headen IE, Davis EM, Mujahid MS, Abrams B. Racial-ethnic differences in pregnancy-related weight. Adv Nutr 2012;3:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academy of Sciences; 2009. [Google Scholar]
- 4.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol 2015;125:773–81; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rong K, Yu K, Han X, Szeto IM, Qin X, Wang J, Ning Y, Wang P, Ma D. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr 2015;18:2172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol 2009;201:339.e1–14. [DOI] [PubMed] [Google Scholar]
- 7.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 2011;94:1225–31. [DOI] [PubMed] [Google Scholar]
- 8.Widen EM, Gallagher D. Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 2014;68:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol 2003;189:1423–32. [DOI] [PubMed] [Google Scholar]
- 10.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN Jr. Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol 1997;90:483–8. [DOI] [PubMed] [Google Scholar]
- 11.Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, Reyes M, Quinn J, Camann D, Perera F, et al. . Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol 2012;175:1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, et al. . Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect 2003;111:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widen EM, Whyatt RM, Hoepner LA, Mueller NT, Ramirez-Carvey J, Oberfield SE, Hassoun A, Perera FP, Gallagher D, Rundle AG. Gestational weight gain and obesity, adiposity and body size in African-American and Dominican children in the Bronx and Northern Manhattan. Matern Child Nutr 2015. Mar 5 (Epub ahead of print; DOI: 10.1111/mcn.12174). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes M, Perzanowski MS, Whyatt RM, Kelvin EA, Rundle AG, Diaz DM, Hoepner L, Perera FP, Rauh V, Miller RL. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allergy Asthma Immunol 2011;107:42–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace D, Wallace R, Rauh V. Community stress, demoralization, and body mass index: evidence for social signal transduction. Soc Sci Med 2003;56:2467–78. [DOI] [PubMed] [Google Scholar]
- 16.Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, Hassoun A, Perera F, Rundle A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 2015;39:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol 2009;170:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter JR, Perng W, Kleinman KP, Rifas-Shiman SL, Rich-Edwards JW, Oken E. Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol 2015;212:499.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkegaard H, Stovring H, Rasmussen KM, Abrams B, Sorensen TI, Nohr EA. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. Am J Clin Nutr 2014;99:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure CK, Catov JM, Ness R, Bodnar LM. Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. Am J Clin Nutr 2013;98:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, Lawlor DA. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 2011;93:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez DC. Gestational weight gain as a predictor of longitudinal body mass index transitions among socioeconomically disadvantaged women. J Womens Health (Larchmt) 2012;21:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley-Martin J, Woolcott C. Gestational weight gain and postpartum weight retention in a cohort of Nova Scotian women. Matern Child Health J 2014;18:1927–35. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996;143:228–39. [DOI] [PubMed] [Google Scholar]
- 25.Richmond TK, Thurston I, Sonneville K, Milliren CE, Walls CE, Austin SB. Racial/ethnic differences in accuracy of body mass index reporting in a diverse cohort of young adults. Int J Obes (Lond) 2015;39:546–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol 1992;2:715–21. [DOI] [PubMed] [Google Scholar]
- 27.Wen M, Kowaleski-Jones L. Sex and ethnic differences in validity of self-reported adult height, weight and body mass index. Ethn Dis 2012;22:72–8. [PMC free article] [PubMed] [Google Scholar]