Abstract
Background: Resveratrol may play a protective role against the frailty syndrome (FS) because of its antioxidant and anti-inflammatory properties.
Objective: We prospectively evaluated the association between habitual dietary resveratrol exposure and the development of FS after 3-, 6-, and 9-y follow-up periods in a community-dwelling older population.
Design: We conducted a longitudinal analysis with the use of data from 769 participants aged ≥65 y from the Invecchiare in Chianti (Aging in Chianti) study. Total dietary resveratrol (TDR) intake was estimated at baseline with the use of a validated food-frequency questionnaire, which was developed to assess participants’ usual food intakes over the previous year, and an ad hoc resveratrol database. Total urinary resveratrol (TUR) was analyzed with the use of liquid chromatography–tandem mass spectrometry with a previous solid-phase extraction at baseline. The combination of both measures [total dietary resveratrol plus total urinary resveratrol (TDR+TUR)] was computed with the use of the Howe’s method. FS was assessed at baseline and at 3-, 6-, and 9-y of follow-up and was defined as the presence of ≥3 of the following 5 criteria: shrinking, exhaustion, sedentariness, slowness, and weakness.
Results: TDR+TUR concentrations were inversely associated with FS risk over 3-y of follow-up (OR for comparison of extreme tertiles: 0.11; 95% CI: 0.03, 0.45; P-trend = 0.002) but not after 6- and 9-y of follow-up in multinomial logistic regression models adjusted for baseline frailty status and potential confounders. These results did not differ when analyses were further adjusted for inflammatory markers.
Conclusion: Higher habitual dietary resveratrol exposure was associated with lower risk of older community dwellers developing FS during the first 3 y of follow-up but not after longer follow-up periods.
Keywords: aging, biomarker, diet, epidemiology, frailty, InCHIANTI, resveratrol
INTRODUCTION
Frailty is a geriatric syndrome that is characterized by a decrease in both the physiologic reserve and resistance to stressors that embodies elevated risks of illness, disability, hospitalization, institutionalization, and mortality (1–3). The clinical phenotype of frailty is characterized by an unintentional weight loss, global muscle weakness, exhaustion or poor endurance, slowed performance, and low physical activity (4). Studies have suggested that oxidative stress and chronic inflammation are key factors in the causal link between aging and the development of frailty syndrome (FS)8 (5, 6).
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) and its glycoside piceid (3,5,4′-trihydroxystilbene-3-O-β-d-glucopyranoside) are bioactive compounds that are mainly present in grapes and red wine and in very low concentrations in peanuts, pistachios, berries, tomatoes, chocolate, apples, and beer (7–11). Several studies have shown the potential effects of resveratrol against aging-related diseases through the activation of sirtuins, thereby mimicking the benefits of energy restriction (12), as well as its antioxidant and anti-inflammatory activities (13). To the best of our knowledge, there has been no epidemiologic evidence linking resveratrol with FS although 2 recent randomized clinical trials have shown protective effects of resveratrol on markers of inflammation and oxidative stress such as reactive oxygen species, the total antioxidant capacity, C-reactive protein, and TNF-α in healthy subjects (14, 15).
Although cognitive impairment is not included in the phenotypic definition of FS, its prevalence in frail subjects varies between 20% and 50% in the literature (6). For that reason, the use of self-reported dietary questionnaires in cognitively impaired participants may be a limitation; thus, the use of nutritional biomarkers is essential to confirm and reinforce the results obtained with the use of questionnaires. Recently, a mass-spectrometric methodology was improved to fully characterize the resveratrol metabolome in humans through the measurement of total urinary resveratrol (TUR) (16). In epidemiologic studies, TUR has been validated as a biomarker of habitual dietary resveratrol and wine intake (17, 18). In studies of diet-disease associations, the combination of both measures has provided higher statistical power of the true exposure than has each method used separately (19).
In the current prospective study, we examined the association between risk of FS and each of its 5 criteria at the 3-, 6- and 9-y follow-ups and the habitual dietary resveratrol exposure measured at baseline with the use of a validated food-frequency questionnaire (FFQ), an objective dietary biomarker, and a combination of both measures in older adults from the InCHIANTI [Invecchiare in Chianti (Aging in Chianti)] study.
METHODS
Study population
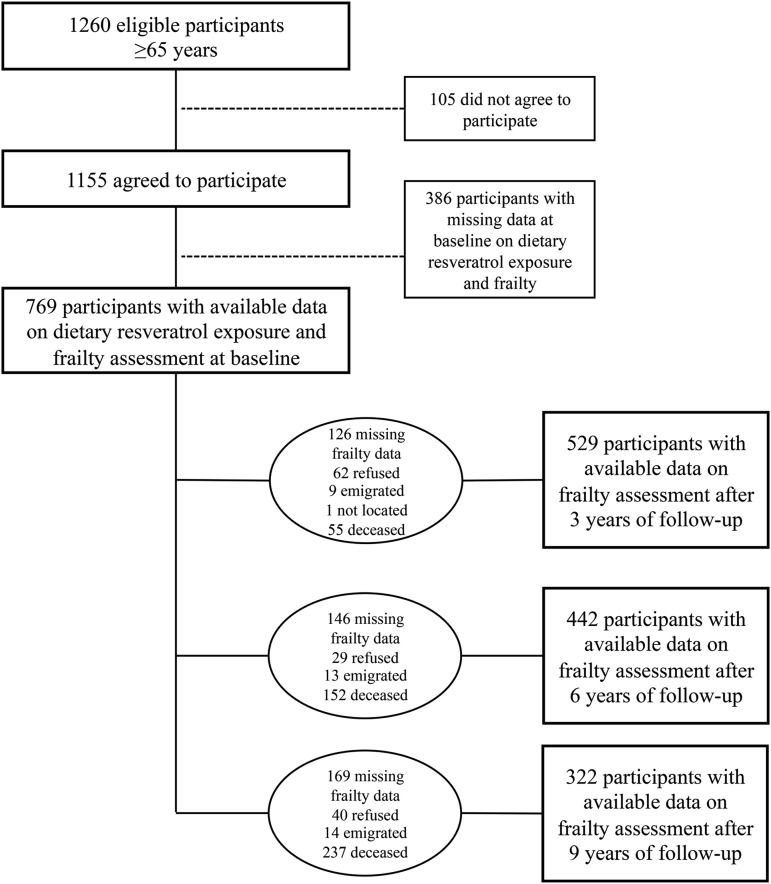
The InCHIANTI study is a cohort study of risk factors for late-life disability conducted in Bagno a Ripoli and Greve in Chianti, which are 2 municipalities adjacent to the city of Florence (Italy). A detailed description of the study rationale, design, and methods has been provided elsewhere (20). Briefly, 1299 participants aged ≥65 y were randomly selected from the population registries with the use of a multistage stratified sampling method. Because 39 subjects had died or emigrated, 1260 subjects were eligible; 1155 participants agreed to take part in the study. The participation rate was 91.7%. The current study was based on data collected at baseline (1998–2000) and after the 3-y (2001–2003), 6-y (2004–2006), and 9-y (2007–2009) follow-up periods. Finally, 769 participants had both measures of resveratrol exposure (biomarker and FFQ data) and frailty data available at baseline of whom 529 subjects (68.8%), 442 subjects (57.5%), and 322 subjects (41.9%) completed the frailty assessment at 3-, 6-, and 9-y follow-up visits, respectively. The main reasons for the unavailability of data at the 3-y follow-up were participation refusal (8.1%) and death (7.2%), whereas at 6- and 9-y follow-ups, the main reason was death, which accounted for 19.8% and 30.8% of the total initial cohort, respectively (Figure 1). The National Institute of Research and Care of Aging Ethical Committee approved the study protocol, and all participants signed an informed consent.
FIGURE 1.
Flowchart of participants at each stage of the study.
Assessment of resveratrol exposure
At baseline, 24-h urine samples were collected from participants. Urine samples were divided into aliquots, coded, and stored at −80°C until analysis. TUR was extracted with the use of solid-phase extraction and analyzed by liquid chromatography–tandem mass spectrometry as previously described (16, 21). Briefly, 1 mL urine with the internal standard was loaded into a preconditioned Waters Oasis HLB 96-well plate for solid-phase extraction (Milford). TUR was eluted with acidified methanol solution and ethyl acetate after washing the plate. After evaporation to dryness, the samples were reconstituted with 100 μL of the mobile phase and analyzed in the liquid chromatography–tandem mass spectrometry system. TUR was calculated as the sum of both total individual metabolites from phase II enzymes (17) and total dihydroresveratrol metabolites, which are produced by intestinal microbiota (16, 22), and was expressed as the 24-h volume as nmol/24 h.
Total dietary resveratrol (TDR) intake was assessed at baseline with the use of the Italian version of the FFQ developed and validated in the European Prospective Study into Cancer and Nutrition (23) and an ad hoc food-composition database on resveratrol (7, 8). TDR intake was computed as the sum of trans- and cis-resveratrol and trans- and cis-piceid (7) and was reported as mg/d.
Assessment of FS
FS was defined according to the 5-component criteria proposed by Fried et al. (4) as follows: shrinking or unintended weight loss, self-reported exhaustion, muscle weakness or poor grip strength, slowness or slow walking speed, and low physical activity or sedentariness. Each criterion was operationalized with the use of previously published methods (24). Shrinking was defined as self-reported weight loss >4.5 kg within the past year for reasons other than dieting. Exhaustion was defined as a response of occasionally (3–4 d in the past week) or often or always (5–7 d in the past week) to the statement “I felt that everything was an effort.” Muscle weakness at baseline was defined as grip strength in the lowest quintile stratified by sex and BMI (in kg/m2) quartiles, and muscle weakness at follow-up visits was determined with the use of the baseline cutoffs. Grip strength was measured with a handheld dynamometer (Nicholas Muscle Tester; Sammon Preston Inc.) by a standard method. Slowness at baseline was defined as the time to walk 4.57 m or 15 ft (the mean of 2 repetitions) in the slowest quintile stratified by sex and standing height. Slow walking speed at follow-up visits was determined with the use of the baseline cutoffs. Sedentariness was defined as either complete inactivity or spending <1 h/wk performing low-intensity activities. Frailty, prefrailty, and robustness were defined as the presence of ≥3, one or 2, and zero of the 5-component criteria, respectively (25).
Baseline covariate assessment
Trained geriatricians conducted a comprehensive assessment of health, functional status, and anthropometric measures by standardized methods. BMI was calculated as weight divided by the square of height. A smoking habit, which was based on self-report, was classified as current smokers and “nonsmokers” (including former and never smokers). The level of education was recorded as years of education. Wine (g/d) consumption was assessed with the use of the validated FFQ (23). Total energy (kcal/d) and alcohol (g/d) intakes were calculated with the use of an Italian food-composition database (26). Global cognitive performance was assessed with the use of the Mini-Mental State Examination (MMSE). Participants with an MMSE score <24 were considered cognitively impaired (27). Depressive symptoms were evaluated with the use of the Center for Epidemiologic Studies Depression Scale (CES-D). A CES-D score ≥16 was defined as a depressed mood (28). Functional status was assessed with the use of the Activities of Daily Living (ADL) scale (29) and the Instrumental Activities of Daily Living (IADL) scale (30). Comorbidities were ascertained by combining information from self-reports, medical records, and clinical examinations (31). Specific comorbidities considered in this analysis were angina pectoris, myocardial infarction, hypertension, congestive heart failure, stroke, peripheral arterial disease, diabetes, osteoarthritis, cancer, renal disease, and chronic obstructive pulmonary disease. Comorbidities were defined as the presence of at least one chronic disease.
Inflammatory markers were measured in serum samples. IL-6 and IL-1 receptor antagonist were measured with high-sensitivity enzyme-linked immunoabsorbent assays (ELISAs) with commercial kits (BIOSOURCE International Inc.). TNF-α was measured with the use of multiplex technology (Human Serum Adipokine Panel B LINCOplex kit; Linco Research Inc.). High-sensitivity C-reactive protein was measured with the use of an ELISA colorimetric competitive immunoassay that used purified protein and polyclonal anti–C-reactive protein antibodies (20, 32).
Statistical analysis
Habitual dietary resveratrol exposure was assessed with the use of TDR, TUR, and the combination of both measures [i.e., total dietary resveratrol plus total urinary resveratrol (TDR+TUR)] in a score according to Howe’s method (19). Briefly, participants were ranked from lowest to highest values for TDR intake and TUR concentrations, and the 2 ranks were summed.
Descriptive analyses of baseline characteristics across habitual dietary resveratrol exposure tertiles were assessed by with age- and sex-adjusted generalized linear models. Spearman rank correlation analyses were performed to examine the relation between dietary resveratrol exposure and alcohol and wine consumption.
Multinomial logistic regression models were used to estimate ORs and 95% CIs between FS (using robust as the reference category) and habitual dietary resveratrol exposure tertiles. A parsimonious approach was used, and therefore, only confounding variables associated (P < 0.10) with FS and habitual dietary resveratrol exposure in univariate analyses were considered as covariates for adjustment in the subsequent multivariate models. Three separate models were presented as follows: an unadjusted model (model 1); model 2, which was adjusted for the status of frailty (or its components) at baseline, age, sex, study municipality, education, BMI, total energy intake (except for TUR), smoking status, comorbidities, depression mood, and cognitive impairment; and model 3, which was further adjusted for inflammatory markers (IL-6, TNF-α, high-sensitivity C-reactive protein, and IL-1 receptor antagonist ). The last model was used to evaluate the mediation effect of inflammation in the associations between dietary resveratrol exposure and FS. Moreover, we estimated the percentage of excess risk mediated by inflammatory markers for FS with the use of inverse ORs of the dietary resveratrol exposure as a continuous variable (33).
Logistic regression models were used to evaluate the association between each of the 5 criteria of FS and TDR, TUR, and TDR+TUR. These risk estimates were similar; thus, only data from TDR+TUR models are presented. TDR and TDR+TUR were continuously analyzed as log-transformed variables and TUR as a Box-Cox transformed variable (α = 0.00001, λ = 0.25), because they were not normally distributed. TDR, TUR, and TDR+TUR were also analyzed as tertiles. Tests for linear trend were performed by considering the median of each tertile as an ordinal variable for TDR and TUR. For the TDR+TUR score, the linear trend was performed by treating the tertiles as a continuous variable in the model. Interactions between TDR+TUR and sex, age, BMI, and cognitive impairment in relation to FS were tested by including product terms in fully adjusted multinomial logistic regression models. In the sensitivity analysis, we excluded heavy drinkers (>56 g alcohol/d) (34) in multinomial logistic regression models. There was no evidence of collinearity. Repeated-measures general linear models were used to assess changes in log-transformed TDR intakes over time. All statistical tests were 2-tailed, and the significance level was P < 0.05. Statistical tests were conducted with SPSS software (version 18.0; SPSS Inc.).
RESULTS
The main characteristics of the study population according to TDR+TUR tertiles at baseline adjusted for age and sex are reported in Table 1. The mean ± SD age of the cohort at baseline was 72.7 ± 5.8 y; 55.4% of the population were women. Participants in the highest TDR+TUR tertile were more likely to be younger and men than were subjects in the lowest tertile. Participants in the top TDR+TUR tertile were more likely to be located in the wine-growing Chianti municipality and to have a higher total energy intake than were those in the bottom tertile. The proportion of participants with IADL disability tended to decrease progressively with increasing TDR+TUR tertiles. There were no significant differences across the dietary resveratrol exposure by BMI, current smokers, comorbidities, cognitive impairment, and ADL disabilities, whereas there were significant differences across TDR and TUR tertiles by sex, total energy intake, depressed mood, and IADL disabilities (Supplemental Tables 1 and 2).
TABLE 1.
Characteristics of the study population according to TDR+TUR tertiles at baseline1
| TDR+TUR tertile |
|||||
| Total | Lowest | Intermediate | Highest | P | |
| Participants, n (%) | 529 | 176 (33.3) | 177 (33.4) | 176 (33.3) | — |
| Age, y | 72.7 ± 5.82 | 72.6 ± 6.0 | 73.5 ± 6.2 | 71.9 ± 4.9 | 0.07 |
| Sex, F, n (%) | 293 (55.4) | 138 (47.1) | 115 (39.2) | 40 (13.7) | <0.001 |
| Study municipality, n (%) | 0.04 | ||||
| Greve in Chianti (rural) | 245 (46.3) | 78 (44.3) | 75 (42.4) | 92 (52.3) | |
| Bagno a Ripoli (urban) | 284 (53.7) | 98 (55.7) | 102 (57.6) | 84 (47.7) | |
| BMI, kg/m2 | 27.7 ± 4.0 | 28.0 ± 4.3 | 27.5 ± 3.7 | 27.7 ± 3.9 | 0.51 |
| Education, y | 5.7 ± 3.3 | 5.0 ± 2.4 | 5.8 ± 3.6 | 6.2 ± 3.7 | 0.10 |
| Current smokers, n (%) | 312 (59.0) | 128 (72.7) | 107 (60.5) | 77 (43.8) | 0.28 |
| Total energy intake, kcal/d | 1957 ± 573 | 1742 ± 508 | 1885 ± 548 | 2245 ± 543 | <0.001 |
| Alcohol intake, g/d | 15.2 ± 21.1 | 0.6 ± 2.3 | 10.0 ± 13.7 | 35.0 ± 22.6 | <0.001 |
| Wine consumption, g/d | 53.6 (0.0–250.0)3 | 0.0 (0.0–0.0) | 53.6 (17.9–125.0) | 250.0 (134.0–375.0) | <0.001 |
| TDR, mg/d | 0.5 (0.0–1.7) | 0.0 (0.0–0.0) | 0.5 (0.1–1.0) | 2.1 (1.1–3.2) | <0.001 |
| TUR, nmol/24h | 4596.4 (1596.0–14,263.6) | 876.6 (207.0–1877.4) | 4742.5 (2855.7–8360.1) | 24,211.0 (12,771.4–49,766.6) | <0.001 |
| Inflammatory markers | |||||
| IL-6, pg/mL | 2.8 (2.0–3.8) | 2.6 (1.8–3.6) | 2.8 (2.0–3.9) | 2.9 (2.1–3.9) | 0.98 |
| TNF-α, pg/mL | 4.4 (3.0–5.7) | 4.5 (3.0–5.7) | 4.3 (3.0–5.7) | 4.3 (3.2–5.6) | 0.56 |
| hsCRP, μg/mL | 2.7 (1.3–5.1) | 2.4 (1.5–5.4) | 2.8 (1.3–5.9) | 2.7 (1.2–4.6) | 0.38 |
| IL-1ra, pg/mL | 128.9 (95.0–177.0) | 129.6 (93.9–170.9) | 126.1 (94.6–180.8) | 132.9 (97.3–188.4) | 0.92 |
| Comorbidities, n (%) | 473 (90.4) | 158 (90.8) | 163 (93.1) | 152 (87.4) | 0.57 |
| Cognitive impairment, MMSE score ≤24, n (%) | 112 (21.2) | 47 (26.7) | 41 (23.2) | 24 (13.6) | 0.40 |
| Depressed mood, CES-D score ≥16, n (%) | 148 (28.0) | 65 (36.9) | 52 (29.4) | 31 (17.6) | 0.14 |
| Frailty, n (%) | 23 (4.4) | 8 (4.6) | 10 (5.7) | 5 (2.9) | 0.14 |
| Prefrailty, n (%) | 196 (37.4) | 74 (42.3) | 70 (40.0) | 52 (29.9) | 0.76 |
| ADL disabilities, n (%) | 8 (1.5) | 3 (1.7) | 4 (2.3) | 1 (0.6) | 0.60 |
| IADL disabilities, n (%) | 63 (11.9) | 31 (17.6) | 26 (14.7) | 6 (3.4) | 0.06 |
Descriptive analyses were compared between habitual dietary resveratrol exposure tertiles with the use of age- and sex-generalized linear models. ADL, Activities of Daily Living; CES-D, Center for Epidemiologic Studies Depression Scale; hsCRP, high-sensitivity C-reactive protein; IADL, Instrumental Activities of Daily Living; IL-1ra, IL-1 receptor antagonist; MMSE, Mini-Mental State Examination; TDR, total dietary resveratrol; TDR+TUR, total dietary resveratrol plus total urinary resveratrol; TUR, total urinary resveratrol.
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Frail participants at the 3-y follow-up were older and had higher BMI, lower habitual dietary resveratrol exposure (TDR), and a higher frequency of depressed mood and ADL and IADL disabilities than nonfrail participants did (data not shown). The most-common frailty criterion for frail participants at the 3-y follow-up period was slowness, and at the 6- and 9-y follow-up visits, it was sedentariness. TDR+TUR was significantly correlated with TDR (ρ = 0.925, P < 0.0001), TUR (ρ = 0.924, P < 0.0001), and wine (ρ = 0.908, P < 0.0001) and alcohol (ρ = 0.907, P < 0.0001) consumption at the 3-y follow-up visit. TDR and TUR were correlated with each other (ρ = 0.713, P < 0.0001).
At the 3-y follow-up, participants who were included in the study, compared with subjects who were excluded from the study because of incomplete data (n = 626), were younger (72.7 ± 5.7 vs. 77.8 ± 8.2 y, respectively; P < 0.001), had higher education (5.6 ± 3.3 vs. 5.0 ± 3.3 y, respectively; P = 0.001), had lower rates of ADL and IADL disabilities (1.5% vs. 17.3%, respectively, and 11.9% vs. 41.9%, respectively; P < 0.001), and had lower prevalences of depressed mood (28.0% vs. 38.5%, respectively; P < 0.001) and cognitive impairment (21.2% vs. 43.0%, respectively; P < 0.001). Subjects excluded at the 6- and 9-y follow-ups (n = 713 and n = 833, respectively) were older (77.7 ± 8.0 and 77.3 ± 7.8 y, respectively; P < 0.001). ADL and IADL disability rates were significantly much lower in subjects included at the 6- and 9-y follow-ups (15.7% vs. 0.9% and 13.7% vs. 0.6%, respectively, and 39.6% vs. 9.7% and 37.0% vs. 5.3%, respectively). Prevalences of depressed mood and cognitive impairment were significantly lower in subjects included at the 6- and 9-y follow-ups (25.6% vs. 38.8% and 23.6% vs. 37.5%, respectively, and 18.6% vs. 41.9% and 11.8% vs. 41.2%, respectively).
The associations between habitual dietary resveratrol exposure measures at baseline and FS over the 3 y of follow-up are shown in Table 2. In unadjusted multinomial logistic regression models (model 1), participants in the highest tertile of all measures of habitual dietary resveratrol exposure (TDR, TUR, and TDR+TUR) had lower risk of developing FS than did subjects in the lowest tertile. The strength of these associations remained after adjustments for FS at baseline and potential confounders (model 2) for TDR (OR: 0.17; 95% CI: 0.05, 0.63; P trend = 0.02), TUR (OR: 0.32; 95% CI: 0.09, 1.11; P trend = 0.03), and TDR+TUR (OR: 0.11; 95% CI: 0.03, 0.45; P trend = 0.002). After adjustments for all inflammatory markers (model 3), inverse associations between resveratrol exposure and FS risk were almost identical and remained significant (Table 2). The percentages of excess risk mediated by inflammatory markers for TDR, TUR and TDR+TUR after 3 y of follow-up were only 0%, 4.5%, and 2.9%, respectively. These results showed that the effect of dietary resveratrol exposure on FS risk was not mediated by inflammatory markers. After the 6- and 9-y follow-ups, inverse associations between measures of dietary resveratrol exposure and FS risk were also observed although the results were not statistically significant in the fully adjusted models (Supplemental Tables 3 and 4, respectively).
TABLE 2.
Association of dietary resveratrol exposure measures at baseline with the probability of developing frailty during a follow-up of 3 y (first follow-up)1
| TDR (FFQ) |
TUR (biomarker) |
TDR+TUR (FFQ plus biomarker)2 |
||||||||||||
| Prefrailty |
Frailty |
Prefrailty |
Frailty |
Prefrailty |
Frailty |
|||||||||
| Cutoff, mg/d | n | OR (95% CI) | n | OR (95% CI) | Cutoff, nmol/d | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | |
| Model 1 | ||||||||||||||
| Lowest tertile | <0.1 | 66 | 1 (referent) | 26 | 1 (referent) | <2273.0 | 74 | 1 (referent) | 18 | 1 (referent) | 70 | 1 (referent) | 23 | 1 (referent) |
| Intermediate tertile | 0.1–1.1 | 74 | 1.04 (0.66, 1.62) | 12 | 0.43 (0.20, 0.90) | 2273.0–10,342.7 | 63 | 0.76 (0.49, 1.19) | 20 | 0.99 (0.49, 2.00) | 70 | 0.92 (0.59, 1.44) | 17 | 0.68 (0.34, 1.37) |
| Highest tertile | >1.1 | 64 | 0.78 (0.50, 1.21) | 7 | 0.22 (0.09, 0.52) | >10,342.7 | 67 | 0.75 (0.48, 1.16) | 7 | 0.32 (0.13, 0.80) | 64 | 0.71 (0.46, 1.11) | 5 | 0.17 (0.06, 0.46) |
| P-trend | 0.19 | 0.001 | 0.33 | 0.009 | 0.12 | <0.001 | ||||||||
| Continuous | 204 | 0.96 (0.88, 1.04) | 45 | 0.75 (0.65, 0.86) | 204 | 0.99 (0.98, 1.00) | 45 | 0.98 (0.96, 0.99) | 204 | 0.84 (0.65, 1.08) | 45 | 0.50 (0.34, 0.73) | ||
| Model 2 | ||||||||||||||
| Lowest tertile | <0.1 | 66 | 1 (referent) | 26 | 1 (referent) | <2273.0 | 74 | 1 (referent) | 18 | 1 (referent) | 70 | 1 (referent) | 23 | 1 (referent) |
| Intermediate tertile | 0.1–1.1 | 74 | 1.02 (0.62, 1.66) | 12 | 0.33 (0.11, 0.98) | 2273.0–10,342.7 | 63 | 0.90 (0.55, 1.46) | 20 | 1.34 (0.49, 3.65) | 70 | 0.86 (0.52, 1.40) | 17 | 0.48 (0.17, 1.35) |
| Highest tertile | >1.1 | 64 | 0.94 (0.53, 1.66) | 7 | 0.17 (0.05, 0.63) | >10,342.7 | 67 | 1.05 (0.61, 1.80) | 7 | 0.32 (0.09, 1.11) | 64 | 0.96 (0.55, 1.69) | 5 | 0.11 (0.03, 0.45) |
| P-trend | 0.78 | 0.02 | 0.71 | 0.03 | 0.85 | 0.002 | ||||||||
| Continuous | 204 | 1.01(0.91, 1.12) | 45 | 0.67 (0.54, 0.84) | 204 | 0.99 (0.98, 1.01) | 45 | 0.98 (0.95, 1.00) | 204 | 1.01 (0.74, 1.38) | 45 | 0.47 (0.26, 0.85) | ||
| Model 3 | ||||||||||||||
| Lowest tertile | <0.1 | 66 | 1 (referent) | 26 | 1 (referent) | <2273.0 | 74 | 1 (referent) | 18 | 1 (referent) | 70 | 1 (referent) | 23 | 1 (referent) |
| Intermediate tertile | 0.1–1.1 | 74 | 1.01 (0.61, 1.66) | 12 | 0.37 (0.12, 1.11) | 2273.0–10,342.7 | 63 | 0.90 (0.55, 1.49) | 20 | 1.85 (0.64, 5.35) | 70 | 0.84 (0.51, 1.39) | 17 | 0.55 (0.19, 1.57) |
| Highest tertile | >1.1 | 64 | 0.84 (0.47, 1.50) | 7 | 0.17 (0.04, 0.64) | >10,342.7 | 67 | 1.02 (0.59, 1.77) | 7 | 0.34 (0.10, 1.24) | 64 | 0.89 (0.50, 1.58) | 5 | 0.11 (0.02, 0.46) |
| P-trend | 0.50 | 0.02 | 0.81 | 0.03 | 0.65 | 0.003 | ||||||||
| Continuous | 204 | 1.00 (0.90, 1.12) | 45 | 0.67 (0.53, 0.84) | 204 | 1.00 (0.98, 1.01) | 45 | 0.98 (0.95, 1.01) | 204 | 0.97 (0.71, 1.34) | 45 | 0.47 (0.26, 0.85) | ||
Multinomial logistic regression models were used, and the following 3 separate models are presented: an unadjusted model (model 1); model 2, which was adjusted for the status of frailty at baseline, age, sex, study municipality, education, BMI, total energy intake (except for TUR), smoking status, comorbidities, depression mood, and cognitive impairment; and model 3, which was further adjusted for inflammatory markers (IL-6, TNF-α, high-sensitivity C-reactive protein, and IL-1 receptor antagonist). P-trend values were obtained by assigning the median of each tertile as scores or entered as an ordinal variable as appropriate. FFQ, food-frequency questionnaire; TDR, total dietary resveratrol; TDR+TUR, total dietary resveratrol plus total urinary resveratrol; TUR, total urinary resveratrol.
Computed with the use of the Howe’s method on the basis of ranks.
No significant interactions were detected for sex, age, BMI, and cognitive impairment in relation to the association between TDR+TUR and FS after any of the follow-up periods in the fully adjusted models. Sensitivity analyses were performed by repeating the models after the exclusion of 31, 30, and 24 participants at the 3-, 6-, and 9-y follow-ups, respectively, because they consumed >56 g alcohol/d. The associations between TDR, TUR, and TDR+TUR and FS at the 3-, 6- and 9-y follow-ups were almost identical to results that were based on the whole population (data not shown).
The associations of TDR+TUR with individual FS criteria during the 3 y of follow-up are shown in Table 3. After adjustment for FS at baseline and potential covariates, participants in the highest tertile of TDR+TUR had lower risk of feeling exhaustion than did those in the lowest tertile (OR: 0.20; 95% CI: 0.09, 0.43; P-trend < 0.001). No associations were observed for other FS criteria. A low level of physical activity showed a significant inverse association with TDR exposure between participants in raw models at the 3-, 6-, and 9-y follow-up periods but not in the adjusted models (Table 3, Supplemental Tables 5 and 6).
TABLE 3.
Association of TDR+TUR tertiles at baseline with the probability of frailty components during a follow-up of 3 y (first follow-up)1
| Unintentional weight loss |
Feeling of exhaustion |
Low-level physical activity |
Low walking speed |
Poor muscle strength |
||||||
| n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | |
| Model 1 | ||||||||||
| Lowest tertile | 16 | 1 (referent) | 58 | 1 (referent) | 43 | 1 (referent) | 46 | 1 (referent) | 11 | 1 (referent) |
| Intermediate tertile | 15 | 0.93 (0.44, 1.94) | 32 | 0.45 (0.27, 0.74) | 37 | 0.82 (0.50, 1.35) | 46 | 0.99 (0.62, 1.60) | 15 | 1.39 (0.62, 3.12) |
| Highest tertile | 11 | 0.67 (0.30, 1.48) | 12 | 0.15 (0.07, 0.29) | 20 | 0.40 (0.22, 0.71) | 35 | 0.70 (0.43, 1.16) | 13 | 1.20 (0.52, 2.75) |
| P-trend | 0.33 | <0.001 | 0.002 | 0.17 | 0.68 | |||||
| Continuous | 42 | 0.87 (0.58, 1.32) | 102 | 0.47 (0.35, 0.62) | 100 | 0.63 (0.48, 0.84) | 127 | 0.83 (0.64, 1.08) | 39 | 1.19 (0.74, 1.91) |
| Model 2 | ||||||||||
| Lowest tertile | 16 | 1 (referent) | 58 | 1 (referent) | 43 | 1 (referent) | 46 | 1 (referent) | 11 | 1 (referent) |
| Intermediate tertile | 15 | 0.77 (0.34, 1.72) | 32 | 0.40 (0.23, 0.70) | 37 | 0.81 (0.44, 1.46) | 46 | 0.85 (0.48, 1.49) | 15 | 1.27 (0.49, 3.27) |
| Highest tertile | 11 | 0.64 (0.24, 1.70) | 12 | 0.20 (0.09, 0.43) | 20 | 0.68 (0.32, 1.46) | 35 | 0.74 (0.38, 1.46) | 13 | 0.57 (0.19, 1.68) |
| P-trend | 0.36 | <0.001 | 0.30 | 0.38 | 0.30 | |||||
| Continuous | 42 | 0.88 (0.54, 1.43) | 102 | 0.56 (0.40, 0.78) | 100 | 0.81 (0.56, 1.18) | 127 | 0.89 (0.63, 1.25) | 39 | 0.90 (0.51, 1.58) |
| Model 3 | ||||||||||
| Lowest tertile | 16 | 1 (referent) | 58 | 1 (referent) | 43 | 1 (referent) | 46 | 1 (referent) | 11 | 1 (referent) |
| Intermediate tertile | 15 | 0.65 (0.27, 1.54) | 32 | 0.41 (0.23, 0.73) | 37 | 0.76 (0.41, 1.39) | 46 | 1.01 (0.54, 1.88) | 15 | 1.39 (0.51, 3.84) |
| Highest tertile | 11 | 0.55 (0.19, 1.60) | 12 | 0.17 (0.07, 0.39) | 20 | 0.66 (0.30, 1.45) | 35 | 0.79 (0.37, 1.67) | 13 | 0.42 (0.13, 1.40) |
| P-trend | 0.24 | <0.001 | 0.27 | 0.57 | 0.15 | |||||
| Continuous | 42 | 0.84 (0.50, 1.42) | 102 | 0.55 (0.39, 0.79) | 100 | 0.80 (0.54, 1.16) | 127 | 0.97 (0.67, 1.43) | 39 | 0.83 (0.45, 1.51) |
Logistic regression models were used, and the following 3 separate models are presented: an unadjusted model (model 1); model 2, which was adjusted for its components at baseline, age, sex, study municipality, education, BMI, total energy intake, smoking status, comorbidities, depression mood, and cognitive impairment; and model 3, which was further adjusted for inflammatory markers (IL-6, TNF-α, high-sensitivity C-reactive protein, and IL-1 receptor antagonist). P-trend values were entered as an ordinal variable. TDR+TUR, total dietary resveratrol plus total urinary resveratrol.
Because TDR intake could have changed over time, we assessed TDR over the 9-y follow-up visits in the 247 participants with both dietary and frailty data in all 3 follow-ups. Median (IQR) TDR intakes at 3-, 6-, and 9-y follow-up visits were 0.44 (0.04–1.68), 0.46 (0.04–1.12), and 0.60 (0.03–1.15) mg/d, respectively. The observed change in TDR intake over the 9-y follow-up visits was not statistically significant (P = 0.18). The intraclass correlation of TDR intake over the 9-y follow-up period was 0.65. Spearman rank correlations between TDR intake at the 3-, 6-, and 9-y follow-up visits and urinary resveratrol concentrations at baseline were 0.62 (P < 0.001), 0.63 (P < 0.001), and 0.61 (P < 0.001), respectively (data not shown).
DISCUSSION
Our study showed that high habitual dietary resveratrol exposure was associated with lower risk of developing FS in older adults who were followed over a 3-y period. No significant associations were observed over the 6- and 9-y follow-up periods although the results were in the same direction.
First, the loss of significance at the 6- and 9-y follow-ups could have been be partially due to the reduction in the sample size (42.5% and 58.1% losses at 6- and 9-y follow-ups, respectively, which were mainly due to death) and, consequently, a decrease in statistical power. Furthermore, changes in other dietary and environmental factors and changes of the health status of participants during follow-up may have been the causes of the attenuated association with long-term FS risk.
Frailty is a multifactorial and complex syndrome. The potential underlying mechanisms of FS are many and include oxidative stress, inflammation, decreased immune function, and endocrine-system and musculoskeletal alterations (6). Energy restriction has been shown to increase longevity and decrease FS (6). These effects were suggested to be mediated by sirtuins, particularly sirtuin 1 (SIRT1), which is an important molecular target for regulating cellular energy metabolism and mitochondrial homeostasis (35). There is a vast interest in resveratrol regarding its role in the prevention of common clinical conditions of aging through the activation of SIRT1 in the liver, skeletal muscle, heart, neocortex, and adipose tissue (36–38). Resveratrol could attenuate the oxidative stress-mediated modification of SIRT1 by increasing the SIRT1 deacetylase activity and amount, which leads to the inhibition of inflammatory and apoptotic signaling and, thereby, improving skeletal muscle after disuse because of aging (39). Randomized clinical trials have shown that the potential effects of resveratrol may be involved in the interaction with markers of inflammatory status and oxidative stress (14, 15). However, in our study, as previously described (34), the correlation of resveratrol exposure and inflammatory markers was not statistically significant. Furthermore, our data suggested that the association between habitual resveratrol exposure and FS was not mediated by inflammation because the ORs did not change after adjustment for inflammatory markers, and the percentage of excess risk mediated by inflammation was <5%.
Frailty is related to an array of diseases such as obesity, hypertension, cardiovascular disease, diabetes, osteoporosis, cancer, cognitive impairment, and depression (6). Although there is limited epidemiologic evidence of the protective effects of resveratrol on these chronic diseases; a cross-sectional study showed that dietary resveratrol via wine consumption, which was evaluated as TUR, was associated with beneficial effects on fasting blood glucose, triglycerides, and heart rate in subjects at high cardiovascular disease risk (40). However, several clinical trials have assessed the health benefits of with the use of high resveratrol doses in these chronic diseases and their associated risk factors (13). The daily ingestion of 250 mg resveratrol for 3 mo improved systolic and diastolic blood pressures, glycated hemoglobin, and total cholesterol in type 2 diabetic subjects receiving oral hypoglycemic treatment (41), whereas the daily ingestion of 10 mg resveratrol for 4 wk improved insulin resistance, decreased blood glucose concentrations, and delayed the appearance of glucose peaks after a test meal (42). A recent pilot trial also described an improvement in insulin sensitivity and postmeal plasma glucose in overweight and obese and moderately insulin-resistant older adults with intakes of 1, 1.5, or 2 g resveratrol for 1 mo (43). In healthy obese men, supplementation with 150 mg resveratrol/d for 1 mo improved the metabolic profile that mimics energy restriction, reduced sleeping and resting metabolic rates, blood pressure, insulin concentrations, and the hepatic lipid content, and improved the skeletal muscle intrinsic mitochondrial function (38). Resveratrol has also been postulated as a neuroprotective and antidepressant molecule (44, 45). As shown in an animal study, trans-resveratrol at doses of 40 and 80 mg/kg increased serotonin and noradrenaline concentrations in brain regions (45). The effects of resveratrol on cognition were postulated in a recent double-blind, placebo-controlled, interventional study that showed that 26 wk of supplementation with resveratrol (200 mg/d) improved memory performance in healthy older adults (46). We showed that habitual dietary intake of resveratrol was correlated with depressed mood (Spearman correlation coefficient between TDR+TUR and CES-D: −0.25; P < 0.001) and cognitive function (Spearman correlation coefficient between TDR+TUR and MMSE: 0.17; P < 0.001) at baseline.
Major strengths of our study were its longitudinal design, long follow-up, and relatively large sample size of old subjects who are the target population for studying FS. Moreover, the cohort was Italian, which is a population with a high heterogeneity in wine consumption, the main food source of resveratrol (7). Another important strength was the assessment of dietary resveratrol exposure with a combinative approach with the use of TUR concentrations in 24-h urine samples as a nutritional biomarker (17, 40) and FFQ-reported TDR intakes (7, 23). This approach provided a more-accurate estimation than provided by only one measure and may have attenuated the misclassification of habitual exposure (19). An additional advantage was the standardized assessment of FS by trained geriatricians and, consequently, the identification of frail and prefrail cases with the use of a validated methodology (25).
However, this study had some limitations. First, a moderate wine-drinking habit has been associated with optimal social, cognitive, and personality factors (47). Moreover, participants excluded from the study because of data unavailability were older and less healthy (with more disabilities and cognition impairment). Although we adjusted our models for several important indicators of psychological functioning and social status, the presence of residual confounding could not be excluded. Second, TUR were analyzed only a single time at baseline; however, TDR intake in the diets of our whole population tended to be conservative as was also observed in a previous InCHIANTI study (34). Third, our results could also have been affected by measurement error in TDR intake although the FFQ was previously validated (23). Finally, shrinking was self-reported, and therefore, it may have been influenced by the memory of older participants. To minimize recall bias, the data were collected by trained personnel in a home interview with the use of a standardized protocol.
In conclusion, our study showed that a higher habitual exposure to resveratrol was associated with lower risk of developing frailty in a cohort of older individuals who were studied during a 3-y period but not during 6 and 9 y of follow-up. Our data did not suggest that inflammation mediated the protective effects of resveratrol against FS. Future clinical trials on the relations between dietary resveratrol exposure and FS are needed to clarify this potential association and the underlying mechanisms with the avoidance of the limitations of observational studies.
Acknowledgments
The authors’ responsibilities were as follows—MR: wrote the manuscript; MR and RZ-R: conducted the statistical analysis; MR, RZ-R, CA-L, and AC: conducted the research; MR, MU-S, and CA-L: performed the samples analyses; RZ-R, MU-S, SB, LF, CA-L, and AC: provided critical revision of the manuscript; SB, LF, CA-L, and AC: designed the research; CA-L and AC: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ADL, Activities of Daily Living; CES-D, Center for Epidemiologic Studies Depression Scale; FFQ, food-frequency questionnaire; FS, frailty syndrome; IADL, Instrumental Activities of Daily Living; InCHIANTI, Invecchiare in Chianti; MMSE, Mini-Mental State Examination; SIRT1, sirtuin 1; TDR, total dietary resveratrol; TDR+TUR, total dietary resveratrol plus total urinary resveratrol; TUR, total urinary resveratrol.
REFERENCES
- 1.Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res 2009;35:61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med 2005;118:1225–31. [DOI] [PubMed] [Google Scholar]
- 3.Hastings SN, Purser JL, Johnson KS, Sloane RJ, Whitson HE. Frailty predicts some but not all adverse outcomes in older adults discharged from the emergency department. J Am Geriatr Soc 2008;56:1651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 5.Mulero J, Zafrilla P, Martinez-Cacha A. Oxidative stress, frailty and cognitive decline. J Nutr Health Aging 2011;15:756–60. [DOI] [PubMed] [Google Scholar]
- 6.Heuberger RA. The frailty syndrome: a comprehensive review. J Nutr Gerontol Geriatr 2011;30:315–68. [DOI] [PubMed] [Google Scholar]
- 7.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventós RM, Berenguer T, Jakszyn P, Martínez C, Sánchez MJ, Navarro C, Chirlaque MD, Tormo MJ, et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br J Nutr 2008;100:188–96. [DOI] [PubMed] [Google Scholar]
- 8.Chiva-Blanch G, Urpi-Sarda M, Rotchés-Ribalta M, Zamora-Ros R, Llorach R, Lamuela-Raventós RM, Estruch R, Andrés-Lacueva C. Determination of resveratrol and piceid in beer matrices by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A 2011;1218:698–705. [DOI] [PubMed] [Google Scholar]
- 9.Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem 2008;56:8374–8. [DOI] [PubMed] [Google Scholar]
- 10.Ragab AS, Van Fleet J, Jankowski B, Park JH, Bobzin SC. Detection and quantitation of resveratrol in tomato fruit (Lycopersicon esculentum Mill.). J Agric Food Chem 2006;54:7175–9. [DOI] [PubMed] [Google Scholar]
- 11.Farneti B, Masuero D, Costa F, Magnago P, Malnoy M, Costa G1, Vrhovsek U, Mattivi F. Is there room for improving the nutraceutical composition of apple? J Agric Food Chem 2015;63:2750–9. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 2014;35:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 2013;19:6064–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab 2010;95:E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P, Durazzo M, Cavallo-Perin P, Cassader M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem 2013;20:1323–31. [DOI] [PubMed] [Google Scholar]
- 16.Rotches-Ribalta M, Andres-Lacueva C, Estruch R, Escribano E, Urpi-Sarda M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol Res 2012;66:375–82. [DOI] [PubMed] [Google Scholar]
- 17.Zamora-Ros R, Urpí-Sardà M, Lamuela-Raventós RM, Estruch R, Martínez-González MA, Bulló M, Arós F, Cherubini A, Andres-Lacueva C. Resveratrol metabolites in urine as a biomarker of wine intake in free-living subjects: the PREDIMED Study. Free Radic Biol Med 2009;46:1562–6. [DOI] [PubMed] [Google Scholar]
- 18.Zamora-Ros R, Urpí-Sardà M, Lamuela-Raventós RM, Estruch R, Vázquez-Agell M, Serrano-Martínez M, Jaeger W, Andres-Lacueva C. Diagnostic performance of urinary resveratrol metabolites as a biomarker of moderate wine consumption. Clin Chem 2006;52:1373–80. [DOI] [PubMed] [Google Scholar]
- 19.Freedman LS, Kipnis V, Schatzkin A, Tasevska N, Potischman N. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies? Epidemiol Perspect Innov 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–25. [DOI] [PubMed] [Google Scholar]
- 21.Urpi-Sarda M, Zamora-Ros R, Lamuela-Raventos R, Cherubini A, Jauregui O, de la Torre R, Covas MI, Estruch R, Jaeger W, Andres-Lacueva C. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clin Chem 2007;53:292–9. [DOI] [PubMed] [Google Scholar]
- 22.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 2012;95:1323–34. [DOI] [PubMed] [Google Scholar]
- 23.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 1997;26(Suppl 1):S152–60. [DOI] [PubMed] [Google Scholar]
- 24.Shardell M, D’Adamo C, Alley DE, Miller RR, Hicks GE, Milaneschi Y, Semba RD, Cherubini A, Bandinelli S, Ferrucci L. Serum 25-hydroxyvitamin D, transitions between frailty states, and mortality in older adults: the Invecchiare in Chianti Study. J Am Geriatr Soc 2012;60:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 2006;61:262–6. [DOI] [PubMed] [Google Scholar]
- 26.Salvini S. A food composition database for epidemiological studies in Italy. Cancer Lett 1997;114:299–300. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 29.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–9. [DOI] [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- 31.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME editors. The women’s health and aging study. In: Health and social characteristics of older women with disability. NIH publication no. 95-4009. Bethesda (MD): National Institute on Aging; 1995.; [Google Scholar]
- 32.Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, Manwani B, Reiner A, Jenny N, Parekh N, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci 2014;69:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014;383:970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semba RD, Ferrucci L, Bartali B, Urpí-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, Andres-Lacueva C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern Med 2014;174:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster BR, Lu Z, Sack MN, Scott I. The role of sirtuins in modulating redox stressors. Free Radic Biol Med 2012;52:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 2008;8:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 2008;3:e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 2010;501:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventós RM, Martínez-González MÁ, Salas-Salvadó J, Arós F, Fitó M, Lapetra J, Estruch R, Andres-Lacueva C, et al. High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high-risk patients. Pharmacol Res 2012;65:615–20. [DOI] [PubMed] [Google Scholar]
- 41.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 2012;32:537–41. [DOI] [PubMed] [Google Scholar]
- 42.Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 2011;106:383–9. [DOI] [PubMed] [Google Scholar]
- 43.Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 2012;67:1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci 2009;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Wang Z, You W, Zhang X, Li S, Barish PA, Vernon MM, Du X, Li G, Pan J, et al. Antidepressant-like effect of trans-resveratrol: involvement of serotonin and noradrenaline system. Eur Neuropsychopharmacol 2010;20:405–13. [DOI] [PubMed] [Google Scholar]
- 46.Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci 2014;34:7862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortensen EL, Jensen HH, Sanders SA, Reinisch JM. Better psychological functioning and higher social status may largely explain the apparent health benefits of wine: a study of wine and beer drinking in young Danish adults. Arch Intern Med 2001;161:1844–8. [DOI] [PubMed] [Google Scholar]