Abstract
Opioid withdrawal causes a dysphoric state that can lead to complications in pain patients and can propagate use in drug abusers and addicts. Opioid withdrawal changes the activity of neurons in the nucleus accumbens, an area rich in both opioid-binding mu opioid receptors and glutamate-binding NMDA receptors. Because the accumbens is an area important for reward and aversion, plastic changes in this area during withdrawal could alter future behaviors in animals. We discovered an increase in phosphorylation of serine 897 in the NR1 subunit of the NMDA receptor (pNR1) during acute morphine withdrawal. This serine can be phosphorylated by protein kinase A (PKA) and dephosphorylated by calcineurin. We next demonstrated that this increased pNR1 change is associated with an increase in NR1 surface expression. NR1 surface expression and pNR1 levels during acute withdrawal were both reduced by the NMDA receptor antagonist MK-801 (dizocilpine hydrogen maleate) and the PKA inhibitor H-89(N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrochloride hydrate). We also found that pNR1 levels remained high after an extended morphine withdrawal period of 2 months, correlated with reward-seeking behavior for palatable food, and were associated with a decrease in accumbal calcineurin levels. These data suggest that NR1 phosphorylation changes during the acute withdrawal phase can be long lasting and may reflect a permanent change in NMDA receptors in the accumbens. These altered NMDA receptors in the accumbens could play a role in long-lasting behaviors associated with reward and opioid use.
Introduction
Opioid withdrawal is an aversive condition that occurs after a dependent individual no longer takes the drug. This negative state is caused in part by plastic counteradaptations produced by morphine during the dependence/tolerance phase but may have very long-lasting effects on the motivation for rewards (Zhang et al., 2007; Koob, 2009; Anderson et al., 2012a,b; Rouibi and Contarino, 2012), including the incubation of cue-induced drug-seeking behavior (Pickens et al., 2011). N-methyl-d-aspartate receptors (NMDA receptors) are capable of inducing long-term effects (Nikonenko et al., 2002) and are highly involved in many disease states like chronic pain and addiction (Lau and Zukin, 2007). NMDA receptors have the ability to detect neuronal activity and are in turn altered by neuronal activity (Mu et al., 2003). Morphine withdrawal causes changes in activity, and activity changes can modulate NMDA receptors. These activity-dependent changes could be the mechanism for the long-term plastic effects on motivation associated with opioid withdrawal (Zhang et al., 2007; Koob, 2009; Anderson et al., 2012a,b; Rouibi and Contarino, 2012).
One well-characterized aspect of opioid withdrawal is the hyperexcitability of mu opioid receptor containing cells during withdrawal (Chartoff et al., 2003; Fan et al., 2009; Edwards et al., 2009; Yang and Pu, 2009). This effect involves 3′-5′-cyclic adenosine monophosphate (cAMP) superactivation (Chartoff et al., 2003; Fan et al., 2009; Edwards et al., 2009; Yang and Pu, 2009). The changes in neuronal activity, cAMP, and cAMP-dependent protein kinase A (PKA) activation produced by withdrawal could modulate NMDA receptors (Fan et al., 2009; Anderson et al., 2012a,b) because its NR1 subunit can be phosphorylated on serine 897 (pNR1) by PKA (Tingley et al., 1997). Changes in the expression of pNR1 were previously reported in animal models of pain (Caudle et al., 2003; Zhou et al., 2006) as well as ethanol (Ferrani-Kile et al., 2003) and cocaine abuse (Scheggi et al., 2007). These findings indicate that phosphorylation of NR1 could be a common mechanism of plastic disease states. Other studies have linked opioid exposure to NR1 phosphorylation previously (Lin et al., 2010; Rodriguez-Munoz et al., 2012), but little work has been performed during or after withdrawal. PKA activation increases NMDA receptor currents (Westphal et al., 1999), increases the sensitivity of the NMDA receptor to glutamate (Dudman et al., 2003), and increases cell surface delivery of the NR1 subunit of the NMDA receptor (Scott et al., 2003; Lau and Zukin, 2007). Therefore, changes in the surface expression of the NMDA receptor based on cAMP’s activation of PKA could be a mechanism for morphine-induced plastic changes observed during withdrawal.
NMDA receptor phosphorylation can also be altered by calcineurin. Calcineurin (also known as protein phosphatase 2B or calcium/calmodulin-dependent serine/threonine protein phosphatase) dephosphorylates Ser897 on NR1 (Choe et al., 2005) and is viewed as a negative modulator of synaptic plasticity (Biala et al., 2005). Calcineurin can also alter opioid-specific behaviors, because its inhibition decreases naloxone-induced withdrawal symptoms in mice (Dougherty et al., 1987; Dougherty and Dafny, 1988; Homayoun et al., 2003) and can block conditioned place preference for morphine in mice (Suzuki et al., 1993; Motiei Langroudi et al., 2005). Calcineurin has also been linked to glutamate receptors after opioid exposure previously, because it can dephosphorylate AMPA receptors in chronic morphine-exposed primary hippocampal neurons that have undergone no withdrawal period (Kam et al., 2010). Because calcineurin appears to be able to alter NMDA receptors in models of withdrawal and reward, it too may be a component in long-term opioid-induced neural plasticity.
In the following experiments, we demonstrate that NR1 phosphorylation increases after 3 days of withdrawal. This initial observation led us to explore three questions related to this finding. First, do changes in NR1 phosphorylation alter NMDA receptor function during acute withdrawal? Second, what are the mechanisms and intracellular pathways responsible for these withdrawal-induced changes? Third, is this effect transient or could it reflect a long-term change in NMDA receptor function in the accumbens? The following experiments were performed to answer these questions.
Methods
Examining the Effects of Acute Morphine Withdrawal on NR1 Phosphorylation in the NAC
Animal Care.
For all in vivo experiments, male Sprague-Dawley rats (250–300 g, Charles River, Raleigh, NC) were housed in pairs in 22°C temperature- and 31% humidity-controlled rooms with a 12-hour light/dark cycle (6 AM–6 PM lights on). Studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals. Rats had free access to food and water except when fasted for behavioral testing. These facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all procedures were approved by the University of Florida’s Institutional Animal Care and Use Committee.
Morphine Injections and Acute Withdrawal Effects on NR1 Phosphorylation.
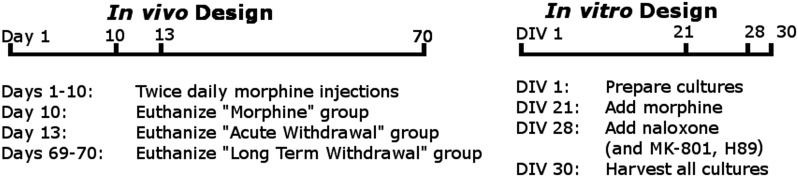
Rats were injected with morphine or an equivalent volume of saline every 12 hours for 10 days in an escalating dose paradigm (days 1–2, 5 mg/kg; days 3–4, 10 mg/kg; days 5–6, 20 mg/kg; days 7–8, 40 mg/kg; days 9–10, 60 mg/kg). Ten morphine-treated and three saline-injected rats were euthanized 30 min after the last injection, whereas the rest were euthanized 3 days later during the acute withdrawal phase (saline N = 7, morphine N = 10, withdrawn N = 9). These experiments are illustrated in Fig. 1. Morphine sulfate (15 mg/ml, Baxter, Deerfield, IL) was obtained from Webster Veterinary (Devens, MA).
Fig. 1.
Experimental timelines. Ten days of escalating morphine injections were followed by either tissue harvesting or a withdrawal period of either a 3-day acute withdrawal or a 2-month extended withdrawal before obtaining samples. Primary neuronal cell cultures were prepared from E17 embryos on day in vitro 1 (DIV 1). On DIV 21, the media from noncontrol plates were replaced with morphine-containing media as needed. On DIV 28, naloxone, MK-801, or H-89 was added to cultures as needed. All cultures were harvested on DIV30.
Rat Brain Tissue Collection.
At the end of each experiment, rats were euthanized by CO2 inhalation followed by rapid decapitation. Brains were removed and placed in an ice-cold acrylic rat brain slicer matrix (Zivic Instruments, Pittsburg, PA). The bilateral NAC (shell and core) was removed from a slice cut from 0 to 2 mm from bregma using a 2 mm Harris Uni-Core puncher with the Paxinos and Watson rat brain atlas (Paxinos and Watson, 1998) as a guide. Tissues were placed in 1.5 ml tubes and immediately frozen in liquid N2. Tissues were later sonicated with a Sonics Vibra-Cell Sonicator (Danbury, CT) at 60 A for 10 seconds in tissue disruption buffer (0.3% SDS, 65 mM dithiothreitol, 1 mM EDTA, 20 mM Tris, pH 8.0) that had 1% protease inhibitor cocktail kit (Thermo Scientific, Waltham, MA) and 1% phosphatase inhibitor cocktail 2 (Sigma, St. Louis, MO) added to it. Samples were centrifuged at 20,000 g for 10 min at 4°C, and supernatant was kept at −80°C.
Western Blotting.
Protein concentration was determined by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, IL), and then a mixture of 20 µg of protein, double distilled H2O (ddH2O), 5% 2–mercaptoethanol, and 50% 2× sodium dodecyl sulfate buffer (Invitrogen, Carlsbad, CA) was heated in a boiling water bath for 5 min, loaded into a 4–20% Tris-glycine gels (Invitrogen) and run at 80 V for 15 min then 150 V for 45 min. Gels were placed in transfer buffer (10% methanol, 48 mM Tris, 39 mM glycine, pH 9.2) for 30 min and then transferred onto a Millipore (Bedford, MA) Immobilon-P polyvinylidene fluoride membrane using a Biorad semi-dry transfer device (Hercules, CA). Membranes were blocked in 5% dry non-fat milk TTBS buffer (20 mM Tris HCl, 0.9% NaCl, 0.05% Tween-20, pH 7.4) for 1 hour. The following primary antibodies were added in blocking buffer and then placed on membranes on a rotator at 4°C overnight: glyceraldehyde 3-phosphate dehydrogenase (1:15000, mouse, Pierce Thermo), calcineurin (1:1000, mouse, BD Transduction Laboratories, San Jose, CA), NR1 (1:2500, rabbit, Epitomics, Burlingame, CA), pNR1 S897 (1:1000, rabbit, Millipore). The next day, blots were washed in TTBS 3 × 10 min and secondary antibody was added (anti-rabbit or anti-mouse IgG, horseradish peroxidase-linked, 1:4000, Cell Signaling, Danvers, MA) for 1 hour. Blots were washed 3 × 5 min each and then detected using ECL Plus or ECL Prime (Amersham, Pittsburg, PA) and Biomax MR film (Kodak, Rochester, NY) or the Carestream Image Station 4000MM (Carestream Health, Rochester, NY). Band density was measured with ImageJ software (National Institutes of Health, Bethesda, MD) for the film or Carestream Molecular Imaging software. Blots were analyzed by taking a percent of the protein level compared with GAPDH. The ratio of pNR1 Ser897 over NR1 total is represented in the Results pNR1/NR1.
Examining the Effect of Acute Morphine Withdrawal on NR1 Phosphorylation and NMDA Receptor Surface Expression
Primary Neuronal Cultures.
Primary neuronal cell cultures were prepared with protocols based on Lutz et al., (2007). Cell culture vials [96 well plates, 12 well plates (Costar) with added 12 mm round glass coverslips or 60 × 15 mm plates (Corning, Corning, NY)] were coated with 0.001% Poly-l-ornithine (Sigma) and 2 hours later were rinsed 2× with ddH2O and then coated with 5 µg/ml Laminin (Invitrogen) and kept in a 37°C incubator in 5% CO2 overnight. The next day, an E17 Sprague-Dawley dam was euthanized with CO2 and decapitation. Both male and female embryos were removed and placed into ice-cold sterile dissecting solution (6.85 mM NaCl, 0.27 mM KCl, 8.5 µM Na2HPO4, 11 µM KH2PO4, 0.27 mM Hepes, 33.3 mM D (+)-glucose, 43.8 mM sucrose, pH 7.4). Frontal areas containing the cortex, striatum, amygdala, and dorsal hippocampus were separated, cut into pieces with razor blades, then placed in 37°C dissociation solution (5 ml TrypLE and 500 µl 1 M Hepes, Thermo Fisher) for 10 min. Cells were dissociated with a fire polished glass pipette, incubated for 5 min, and then redissociated and reincubated for 5 min. After a third dissociation, the solution was spun at 150 g for 5 mins at 4°C. The supernatant was discarded and cells were resuspended in 5 ml of 37°C media [Neurobasal, 1 mM Na pyruvate, 2 mM l-glutamine (Cellgro), Pen-Strep, B27 (GE Healthcare, Logan, UT), with 5% fetal bovine serum (Corning)]. Cells were counted with a hemocytometer (Hausser Scientific, Horsham, PA) and plated with a density of 1 × 106 cells/ml. Starting the next day, the media were replaced with media without fetal bovine serum for the duration of the experiment. On day in vitro 21 (DIV 21), the media from noncontrol plates were replaced with 0.1 µM morphine containing media. On DIV28, 50 µM naloxone (naloxone hydrochloride dehydrate, Sigma) was added. All 60-mm plates were harvested on DIV30. Plates were washed with phosphate-buffered saline (PBS; 137 mM NaCl, 10 mM NaH2PO4, 2.7 mM KCl, pH 7.4), and then 200 μl of ice-cold RIPA buffer [10% 10× RIPA buffer (Cell Signaling, 1% protease inhibitor cocktail (Thermo), 1% phosphatase inhibitor cocktail (Sigma), 1 µM PMSF dissolved in EtOH (Sigma) in ddH2O] was added and cells were loosened with a sterile scraper, pipetted into a 1.5-ml tube, and sonicated with a Sonics Vibra-Cell Sonicator at 20 A for 10 seconds. Samples were centrifuged at 20,000 g and then resonicated and recentrifuged, and supernatant was retained. Protein quantification and Western blot analysis were then performed as described earlier.
Double-Labeled Immunocytochemistry.
Primary rat neuronal cell culture coverslips were removed and fixed in 4°C 10% buffered formalin phosphate (Fisher, Waltham, MA) for 10 min at RT (room temperature), rinsed 3 × 10 min in rinse buffer [PBS with 0.1% Triton-X (Fisher)], and blocked for 60 min with blocking buffer [5% normal donkey serum (Sigma), 5% bovine serum albumin (Sigma) in PBS with 0.1% TritonX]. Primary antibodies to the MOR (1:100, guinea pig, Abcam, Cambridge, MA) and NR1 (1:100, rabbit, Epitomics) were added to new blocking buffer and left overnight at 4°C. Slips were washed with rinse buffer 3 × 10 min, and then fluorescent secondary antibodies [1:1000, Alexa Fluor 594 goat anti-mouse and Alexa Fluor 488 goat anti-rabbit (Invitrogen)] in new blocking buffer were added. After 1 hour of dark incubation at RT and 3 × 10 min washes in rinse buffer, coverslips were flipped onto a slide with Mowiol mounting media [0.1% Mowiol 4-88 (Millipore), 25% glycerol, 0.1 M Tris, in ddH2O, pH 8.5]. Pictures were taken with a Photometrics cascade-cooled EMCCD camera using the Open Source software package MicroManager connected to a spinning disk confocal system with a Leica (Buffalo, NY) DMIRB microscope with a 63× oil immersion objective according to the protocols of Brown et al. (2012). Pictures were analyzed and combined with ImageJ software.
Quantitative Internalization Assay.
Neuronal cultures were plated on DIV1 onto two 96-well plates. Morphine or saline was added on DIV21 as described above. Naloxone was added on DIV28. On DIV30, the buffer was removed and cells were washed once in PBS before being fixed with 4% paraformaldehyde for 30 min at RT. Paraformaldehyde alone was demonstrated to not cause substantial permeabilization of cell membranes in this assay, which allows it to be used as a surface expression assay (Daigle et al., 2008). After fixation, cells were washed 5 × 30 min in PBS (with no added detergent to avoid membrane permeabilization) and blocked for 90 min in LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) at room temperature with gentle rocking. After blocking, cells were incubated overnight at 4°C with an anti-NR1 antibody that detects only the extracellular portion of the NR1 subunit (1:250, BD Pharminogen, San Jose, CA). The cells were washed 5 × 30 min the following day in Tris-buffered saline containing 0.05% Tween-20 (TBST; 137 mM NaCl, 10 mM Tris, 0.05% Tween-20, pH 7.4). A fluorescent antibody (1:1000, Alexa Fluor 594 goat anti-rabbit, Invitrogen) in blocking buffer was added for 90 min, and then plates were washed 5× in TBST and dried. The plates were then rewashed over several days with TBST and dried again to reduce background staining. Plates were read on a Synergy HT plate reader (Biotek, Winooski, VT) with an excitation setting of 590 and an emission setting of 617. Note, this experiment was performed on two separate plates and their results were combined into a single analysis.
Examining the Mechanism of pNR1 Levels and Increased NR1 Surface Expression Using Pharmacological Inhibition
NMDA Antagonism and PKA Inhibition during Morphine Withdrawal.
The same neuronal cultures were grown as above, but in addition to naloxone being added on DIV28, 1 µM MK-801 (dizocilpine hydrogen maleate, Sigma), 10 µM MK-801, or 10 µM H-89 (N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrochloride hydrate, Sigma] were also added to cultures. The cells were harvested at DIV30. These experiments are illustrated in Fig. 1.
The surface expression assay mentioned above was performed but with the addition of 1 µM MK-801, 10 µM MK-801, or 10 µM H-89 added with naloxone on DIV28 before fixation on DIV30.
Examining the Effects of an Extended Withdrawal Period on NR1 Phosphorylation
Behavioral Testing during an Extended Withdrawal Period.
The methods of Anderson et al. (2012a) were used in this study. Briefly, motivational behavior and pain sensitivity during an extended withdrawal period were examined on an operant orofacial pain assay. This assay forces a rat to press its face into two metal tubes to gain access to a reward bottle filled with a 2:1 dilution of sweetened condensed milk. By setting the temperature to aversive temperatures (46 or 50°C), this assay can be used to measure pain (Neubert et al., 2005, 2006, 2007, 2008; Rossi et al., 2006; Rossi and Neubert, 2008, 2009; Rossi et al., 2009; Nolan et al., 2011a,b, 2012), but at nonaversive temperatures (37°C), alterations in motivation and reward can be examined (Anderson et al., 2012a,b). Forty rats were fasted 17 ± 1 hour (from 5 PM the previous night to 9–11 AM the next day) before each behavioral session and then trained for six sessions (three times a week). Two baselines at a nonaversive 37°C were collected and then averaged. Two baselines at an aversive 46°C were also collected and then averaged. Rats were then separated into a morphine (N = 32) and saline (N = 8) group so that there were no significant differences between their time per contact values at both 37°C (a measure of reward-seeking for palatable foods) and 46°C (an operant pain measure). The same morphine or saline doses as described in the acute withdrawal study were administered twice daily for 10 days. During this time, five operant testing sessions took place. After morphine, injections were ceased and rats were tested on average 3 days a week for 2 months. Rats were generally tested at 37°C twice a week and 46°C once a week. At the end of the extended withdrawal period, a behavioral session at 50°C was also performed. Tissue harvesting and Western blotting were performed as above. Half were euthanized on day 59, half on day 60 (N = 4 for saline and N = 16 for morphine on each day). These experiments are illustrated in Fig. 1.
Statistics
Unpaired t tests or one-way analyses of variance (ANOVAs) were used to compare expression levels between animal groups, culture samples, and the surface expression assay values. Pearson’s correlation was used to assess motivational behavior with pNR1/NR1 expression after a Grubbs test was performed. All tests were performed using GraphPad Prism 4 or 5 software (La Jolla, CA). For all tests, P < 0.05 was considered significant.
Results
Acute Morphine Withdrawal Increases NR1 Phosphorylation in the NAC
We first examined changes that occur in the phosphorylation of the NMDA receptor serine 897 (pNR1) during the acute withdrawal phase. We observed a 50% increase in pNR1/NR1 in the NAC 3 days after withdrawal [Fig. 2A, one-way ANOVA, F(2,23) = 3.457, P = 0.0487]. No differences were observed for the morphine only group, demonstrating this is a morphine withdrawal only effect. These changes in pNR1/NR1 during acute withdrawal are not caused by changes in NR1 total protein(Anderson et al., 2012b). These changes are also subregion specific, because we tested samples from the amygdala, hippocampus, and periaqueductal gray and found no changes in pNR1 levels (data not shown).
Fig. 2.
Morphine withdrawal alters NMDA receptors in vivo and in vitro. (A) pNR1 was similar between the saline (N = 7)- and morphine (N = 10)-treated groups but increased about 50% during acute withdrawal (N = 9) [one-way ANOVA, F(2,23) = 3.457, P = 0.0487]. *Significant difference with a one-way ANOVA. (B) pNR1 was similar between the saline- and morphine-treated primary neuronal cultures but increased about 40% after naloxone-induced acute withdrawal [one-way ANOVA, F(2,16) = 3.892, P = 0.0420]. *Significant difference with a one-way ANOVA. (C) NR1 surface expression also increased during naloxone-induced withdrawal but not with morphine alone [one-way ANOVA, F(2,77) = 7.322, P = 0.0012]. *P < 0.05 and **P < 0.01, Bonferroni’s post hoc test. (Right side) Dual-labeled immunohistochemistry with antibodies to the NR1 subunit of the NMDAR and the MOR and fluorescent secondary antibodies colocalize in neurons harvested from E17 embryos.
Morphine Withdrawal Causes an Increase in pNR1 and NMDA Receptor Surface Expression
To determine if our observed effects on pNR1/NR1 levels altered NMDA receptor function, we turned to a primary neuronal cell culture model of morphine withdrawal. These cultures have neurons that express both NMDA receptors and MORs (Fig. 2, right side), so this model was appropriate for studying the changes we detected in adult rat brain tissue. Neurons were exposed to 7 days of morphine followed by 2 days of either morphine alone or with naloxone to induce withdrawal. We first determined if this model mimicked our in vivo findings. As illustrated in Fig. 2B, naloxone addition increased pNR1/NR1 levels 40% compared with saline-treated and morphine-treated samples in this culture model [one-way ANOVA, F(2,16) = 3.892, P = 0.0420]. NR1 levels did not change after naloxone-induced withdrawal in culture [one-way ANOVA, F(2,16) = 0.6338, P = 0.5434, saline mean: 100, saline S.E.M.: 1.705, morphine mean: 110.9, morphine S.E.M.: 3.340, withdrawn mean: 111.4, withdrawn S.E.M.: 15.18]. Because this change was similar to the one we observed in the in vivo study, we moved forward with this model of morphine withdrawal.
Previous studies demonstrated that Ser897 on NR1 can lead to increased surface expression of NMDA receptors (Scott et al., 2003), so we hypothesized that acute withdrawal may do the same. Acute morphine withdrawal in our culture assay led to increased NR1 cell surface expression [Fig. 2C, one-way ANOVA, F(2,77) = 7.322, P = 0.0012], suggesting increased pNR1/NR1 is having a functionally relevant effect on NMDA receptors during withdrawal.
As a control experiment, naloxone alone was added to cultures at DIV28 and harvested at DIV30. With no previous exposure to morphine, naloxone alone had no effect of pNR1/NR1 ratio [t test, t(18)=1.224, P = 0.2366] or NR1 surface expression [t test, t(46) = 0.9054, P = 0.3700].
The Increased pNR1 Levels and Increased NR1 Surface Expression Observed in Acute Withdrawal Are Dependent on the Activity of the NMDA Receptor and PKA
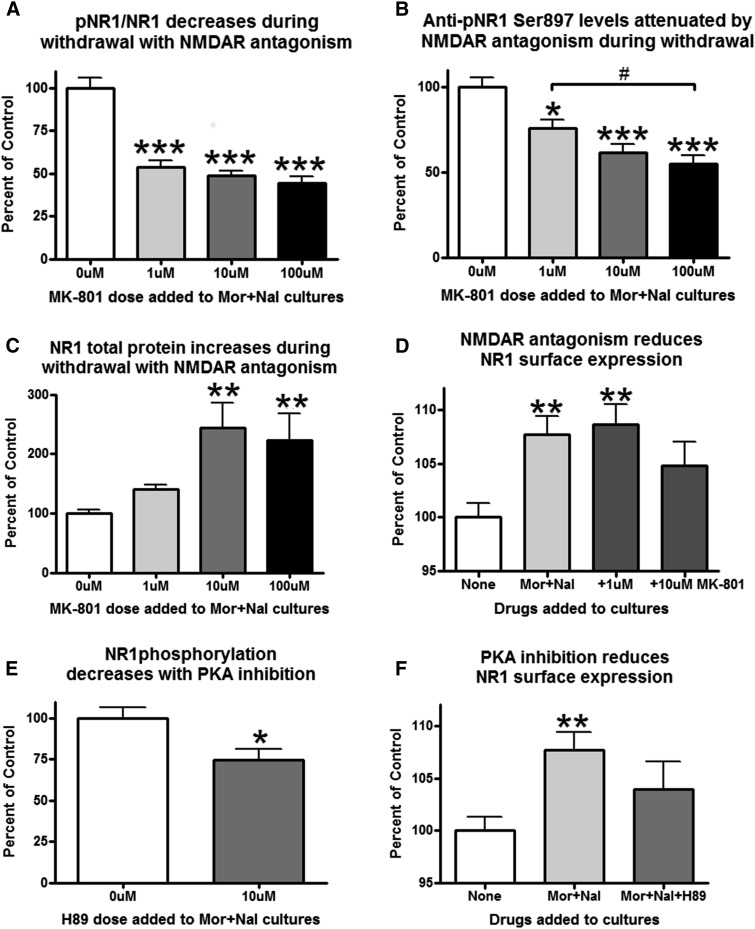
We next determined the possible mechanisms for increased pNR1/NR1 during acute withdrawal through the addition of inhibitors of either PKA or NMDA receptors into the cell culture media. The withdrawal-induced increase in pNR1/NR1 was reduced by the NMDA receptor antagonist MK-801 (Fig. 3A, one-way ANOVA, F(3,36) = 35.78, P < 0.0001). Figure 3, B and C, illustrate that the changes in pNR1/NR1 do not come from decreases in total NR1 protein but rather a combination of pNR1 reductions [one-way ANOVA, F(3,36) = 14.61, P < 0.0001] and NR1 total increases [one-way ANOVA, F(3,36) = 43.02, P < 0.0001]. We also examined the effects of these drugs on NR1 surface expression. When comparing the control group to the morphine/naloxone-treated group, a significant increase in NR1 surface expression was observed. However, the addition of 10 μM, but not 1 μM, MK-801 was able to reduce NR1 surface expression to nonsignificant levels when placed in the bath with morphine/naloxone [Fig. 3D, one-way ANOVA, F(3,92) = 5.660, P = 0.0013]. These data combined show that the 1 μM dose of MK-801 reduces pNR1 in a smaller pool of NR1 total subunits, whereas the 10 and 100 μM doses of MK-801 reduce pNR1 levels even further in an even larger pool of NR1 total proteins. This suggests that the effects on NR1 surface expression are dose dependent and therefore most evident when a larger pool of NR1 subunits is available, like with the 10 μM MK-801 group. The withdrawal-induced increase in pNR1 was also decreased by the PKA inhibitor H-89 (Fig. 3E, t test, t(8) = 2.393, P = 0.0436). NR1 total levels did not change after H89 addition [t test, t(8) = 1.355, P = 0.2125, 0 μM mean: 100, S.E.M.: 13.55, 10 μM mean: 79.06, S.E.M.: 7.420]. PKA inhibition also reduced the effects of morphine/naloxone on NR1 surface expression [Fig. 3F, one-way ANOVA, F(2,77) = 5.808, P = 0.0045]. These experiments demonstrate that both PKA activation and NMDA receptor activation are necessary components in the increased pNR1 levels/NR1 surface expression observed during acute withdrawal.
Fig. 3.
The effects of acute withdrawal on pNR1 and NR1 surface expression are dependent on NMDA receptor activation and protein kinase A activity. (A) NMDA receptor antagonism reduces the pNR1/NR1 ratio in withdrawal [one-way ANOVA, F(3,36) = 35.78, P < 0.0001]. (B) Anti-pNR1 Ser897 densitometry levels decrease dose dependently with MK-801 [one-way ANOVA, F(3,36) = 14.61, P < 0.0001]. (C) NR1 total protein increases dose dependently during withdrawal [one-way ANOVA, F(3,36) = 43.02, P < 0.0001]. (D) NMDA receptor antagonism also decreases NR1 surface expression [one-way ANOVA, F(3,92) = 5.660, P = 0.0013]. (E) PKA inhibition reduces the withdrawal-induced increase in NR1 phosphorylation [t test, t(8) = 2.393, P = 0.0436] and also reduces the withdrawal-induced increase in NR1 surface expression [one-way ANOVA, F(2,77) = 5.808, P = 0.0045]. For all graphs, #P < 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test. Mor, morphine; Nal, naloxone.
NR1 Phosphorylation Remains High in Extended Withdrawal and Correlates with Reward-Seeking Behavior
Finally, we tested the longevity of this effect and its behavioral relevance by examining the effects of an extended withdrawal period on pNR1/NR1 levels and a food reward-seeking behavior. Rats were trained to perform on a rat facial pain assay that can measure reward seeking for palatable foods (Anderson et al., 2012a,b) and changes in pain sensitivity (Neubert et al., 2005, 2006, 2007, 2008; Rossi et al., 2006; Rossi and Neubert, 2008, 2009; Rossi et al., 2009; Nolan et al., 2011a,b, 2012). After training, rats were given 10 days of twice daily escalating morphine injections followed by 2 months of withdrawal. We examined pNR1/NR1 levels in the accumbens after the 2-month withdrawal period and discovered that the increase in pNR1/NR1 had persisted. As illustrated in Fig. 4A, the ratio of pNR1/NR1 is significantly higher in the withdrawn rats versus the saline-only group [t test, t(38) = 3.531, P = 0.0011]. Similarly to the acute study, changes in pNR1/NR1 levels cannot be explained by changes in NR1 total levels, because they did not change during withdrawal [t test, t(38) = 0.8723, P = 0.3885, mean saline: 100, S.E.M. saline: 6.213, mean withdrawn: 91.56, S.E.M. withdrawn: 4.558). Because cAMP levels have returned to normal by this time point and would not likely explain the high pNR1/NR1 levels in the extended withdrawal phase, we looked at another potential mechanism to explain these persistent changes. Ser897 can be dephosphorylated by calcineurin, and we observed that its levels were decreased in the extended withdrawal group [Fig. 4B, t(38) = 2.216, P = 0.0327]. To determine if there were any behavioral correlates with this long-term, persistent change in NMDA receptors in the accumbens, we compared these pNR1/NR1 levels with the final 37°C behavioral session in which we measured motivation behavior for palatable food reward. We found there was a correlation between pNR1/NR1 levels and increased motivation for food reward [Fig. 4C, Pearson’s correlation: r(30) = 0.3688, R2 = 0.1360, P = 0.0449]. We also compared the pNR1/NR1 ratios to the final behavioral sessions at aversive temperatures. No correlations were observed at 46°C [Fig. 4D, Pearson’s correlation: r(32) = −0.2698, R2 = 0.07280, P = 0.1353] or at 50°C [Fig. 4E, Pearson’s correlation: r(32) = 0.1641, R2 = 0.02692, P = 0.3695]. These data demonstrate that changes induced in the acute withdrawal phase persist into the extended withdrawal phase. These changes are associated with behavioral changes in the motivation for rewards, and the mechanism of action may be decreased calcineurin protein expression levels in the accumbens.
Fig. 4.
The increase in pNR1 is still present after 2 months of withdrawal and is correlated with reward-seeking behavior and decreased levels of calcineurin. (A) Increased levels of pNR1 are observed in withdrawn rats (N = 32) after a 2-month withdrawal period compared with saline controls (N = 8) [t test, t(38) = 3.531, P = 0.0011]. (B) Calcineurin levels were significantly decreased in rats after a 2-month withdrawal period [t test, t(38) = 2.216, P = 0.0327]. (C) The level of pNR1/NR1 significantly correlated with the motivation for a palatable food reward on an operant facial assay at a nonaversive 37°C temperature [Pearson’s correlation: r(38) = 0.3688, R2 = 0.1360, P = 0.0449]. (D and E) No correlations were observed at aversive temperatures of 46°C [Pearson’s correlation: r(32) = −0.2698, R2 = 0.07280, P = 0.1353] or at 50°C [Pearson’s correlation: r(32) = 0.1641, R2 = 0.02692, P = 0.3695]. For all graphs, *P < 0.05 and **P < 0.01, Bonferroni’s post hoc test.
Discussion
Opioid withdrawal is a negative affective state caused by removal of the drug after dependence has occurred. This withdrawal state can lead to reuse of the drug to relieve negative symptoms associated with it and poses a problem for both pain patients and opioid addicts. Opioids like morphine alter neuronal function in part by binding to opioid receptors. One brain area rich in opioid receptors is the nucleus accumbens. This is an area associated with motivational behaviors involving seeking rewards and avoiding aversive stimuli and is active during withdrawal. The accumbens is also rich in glutamatergic NMDA receptors that are responsible in part for plastic changes in the brain. The NMDA receptors are modulated by phosphorylation. The opioid withdrawal state causes large changes in neuronal activity in brain areas like the accumbens where opioid receptors are located. Therefore, we hypothesized that morphine withdrawal could lead to altered activity-dependent plastic changes in NMDA receptors. One marker of upregulated NMDA receptors is phosphorylation of Ser897 on the NR1 subunit, so we hypothesized that pNR1 levels in the accumbens may increase in opioid withdrawal due to the increased activity present during this time.
Acute Morphine Withdrawal Increases NR1 Phosphorylation
We examined NR1 phosphorylation in the accumbens in rats that had undergone saline injections, morphine injections with no withdrawal, or morphine injections with 3 days of withdrawal. We found that pNR1 increased significantly during acute withdrawal in vivo in the accumbens after withdrawal. No differences in pNR1 were observed for morphine-treated samples that did not undergo withdrawal. This result may be clinically significant, because phosphorylating Ser897 on NR1 is a way of upregulating NMDA receptors (Scott et al., 2003) and is associated with other diseases of altered plasticity like chronic pain (Caudle et al., 2003; Zhou et al., 2006) and drug abuse (Ferrani-Kile et al., 2003; Scheggi et al., 2007). Our findings are similar to those obtained by another group that injected heroin for 10 days but observed an increase in accumbal pNR1 after a naloxone-precipitated withdrawal instead of a natural withdrawal as in our study (Jiang et al., 2012). However, we are the first to demonstrate that NR1 phosphorylation increases in the accumbens after morphine withdrawal.
This initial observation led us to ask three major questions in this study: 1) Does this increase in NR1 phosphorylation have a measurable effect on NMDA receptor function during withdrawal? 2) What is the mechanism for increased accumbal pNR1 levels in withdrawal? 3) Is the increase in pNR1 transient or long lasting, suggesting a plastic change in the accumbens caused by morphine withdrawal?
Morphine Withdrawal Increases NR1 Surface Expression
We first sought to determine if the pNR1 changes we observed led to any differences in NMDA receptor upregulation. The increase in pNR1 occurred on Ser897, and previous reports demonstrated that this can lead to an increase in the surface expression levels of NMDA receptors (Scott et al., 2003). In the opposite direction, knocking in an alanine mutation to the Ser897 site reduces NMDA receptor synaptic incorporation and glutamatergic (both NMDA and AMPA) transmission (Li et al., 2009). We turned to our primary neuronal cell culture model and demonstrated that in addition to an increase in pNR1, NR1 surface expression also increased. The increase in NR1 surface expression we observed suggests that pNR1 is upregulating NMDA receptors during withdrawal. NR1 subunits do not reach the cell surface without accompanying NR2 subunits (Cik et al., 1993; Grimwood et al., 1995; Varney et al., 1996), therefore this change reflects an increased presence of complete NMDA receptors on the surface. Increased NMDA receptor expression could alter the plastic properties of neurons by increasing the probability of activation by glutamate and allowing more calcium into the cell (Cull-Candy et al., 2001). As a result, plasticity in the accumbens may be altered in the future due to this change. The ability to reduce pNR1 increases in the accumbens may be beneficial to relieving withdrawal symptoms or reducing unwanted effects of withdrawal like craving.
Because the same results were observed both in vivo and in vitro, this suggests that this increase is due to intracellular changes and not circuit-level changes. We observed colocalization of MOR and NR1 receptors in our cultures as well, which demonstrates that these effects can be intracellular. However, morphine and/or naloxone could be causing increased NMDA receptor phosphorylation by inducing other cells in the cultures to alter glutamate release, which mimic the in vivo effects in the accumbens. Further experiments with the addition of tetrodotoxin or another neurotransmitter blocker like cobalt could be performed to confirm the intracellular nature of our effects.
Morphine Withdrawal Increases NR1 Phosphorylation and NR1 Surface Expression through a Mechanism Dependent on NMDA Receptor Activation and PKA Activity
We next wanted to determine what mechanisms are responsible for the changes in pNR1 and NR1 surface expression during morphine withdrawal. We were able to demonstrate that NMDA receptor antagonism and PKA inhibition reduced both pNR1 levels and NMDA receptor surface expression. These results further suggest that pNR1 levels are responsible for the increase in NR1 surface expression, because their levels changed together with pharmacological manipulation. If pNR1 levels in the accumbens are an important factor in withdrawal and NMDA receptor antagonism reduces pNR1 levels during withdrawal, then NMDA receptor antagonism may reduce certain aspects of withdrawal. Behaviorally, withdrawal symptoms can be assessed after naloxone-precipitated withdrawal and Ji et al. (2004) demonstrated that intra-accumbal NMDA antagonism reduces these morphine withdrawal symptoms in animals. These data combined with our results suggest that blocking pNR1 levels during withdrawal could be the mechanism responsible for reduced withdrawal symptoms.
Our finding that PKA inhibition reduces pNR1 is not surprising due to numerous reports demonstrating that the adenylate cyclase/cAMP/PKA pathway is hyperactivated during withdrawal. This is a well-supported effect found both in culture(Chartoff et al., 2003; Fan et al., 2009) and in brain regions like the accumbens, amygdala, hippocampus, striatum, and prefrontal cortex(Edwards et al., 2009). Our finding of PKA activity being responsible for NMDA receptor changes during withdrawal fits well into this model of cellular change after opioid exposure, because PKA activation of NR1 increases the surface expression of the NMDA receptor (Scott et al., 2003).
Increased NR1 Phosphorylation Is Still Increased after Two Months of Morphine Withdrawal
Our last experiment assessed whether the effect of pNR1 during acute withdrawal was transient or long lasting. We discovered that this effect was still present after a 2-month withdrawal period and was therefore very long lasting and possibly even permanent. Increased pNR1 could play a role in other long-term effects of chronic morphine exposure and subsequent withdrawal such as increased excitatory postsynaptic current inactivation rates (Martin et al., 1999), alterations in dendritic spines (Robinson and Kolb, 1999), craving, or relapse (Chartoff and Connery, 2014).
This long-lasting increase in pNR1 was correlated with an increased motivation to obtain a sweet food reward and decreased levels of calcineurin. This suggests that decreased phosphatase activity was responsible for the increased pNR1 at this late withdrawal time point. Calcineurin was previously demonstrated to exert its effects on the NR1 subunit in medium spiny neurons by dephosphorylating S897 (Choe et al., 2005). Therefore, calcineurin’s increase of pNR1 would likely increase the surface expression of NMDA receptors (Scott et al., 2003). Calcineurin can directly alter synaptic transmission as it is anchored in synaptic complexes along with PKA, NMDA receptors, AMPA receptors, and PSD-95 (Ehlers, 2003). Because of this close association, inhibiting calcineurin can prolong NMDA receptor channel openings (Liu et al., 1991; Lieberman and Mody, 1994; Li et al., 2002), allowing increased calcium influx and the strengthening of synapses. This could allow for an increased response to glutamate release in the accumbens. An increase in the response to glutamate by NMDA receptors in the accumbens after a 10-day withdrawal was recently reported (Wu et al., 2012). Although these authors did not examine phosphorylation in this study, this increase in pNR1 is a possible mechanism for this effect. Our effect on feeding behavior was only correlative, but nevertheless altered pNR1 levels and NR1 surface expression could be a mechanism for the long-term correlation with reward-seeking behavior observed in the accumbens during withdrawal. Furthermore, the long lasting changes in pNR1 and calcineurin levels are also only correlative in nature but could be related to long-term changes in behaviors associated with opioid withdrawal.
In conclusion, this study demonstrates that increases in pNR1 levels in the accumbens occurred during both an acute (3 day) and extended (2 month) withdrawal from morphine. The drastic changes in activity and cAMP superactivation during acute withdrawal may have long-lasting effects that cause cellular changes in the extended withdrawal phase. Some of these effects include the expression changes in calcineurin and pNR1 in the accumbens and may underlie alterations in motivational reward seeking behavior. Because pNR1 is responsible in part for NMDA receptor surface expression levels and surface expression alters the strength of synapses, reducing these events in the accumbens may reduce the negative plastic effects of the acute withdrawal stage. Finding ways to block or reduce these effects acutely could provide treatment options for patients undergoing this aversive extended withdrawal and could prevent long-term alterations in plasticity in this population.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- ddH2O
double deuterated H2O
- DIV
day in vitro
- NAC
nucleus accumbens
- NMDA
N-methyl-d-aspartate
- NR1
subunit 1 of the NMDA receptor
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- pNR1
phosphorylated NR1 on Ser897
- RT
room temperature
- Ser897
serine 897 of the NR1 subunit
Authorship Contributions
Participated in research design: Anderson, Neubert, and Caudle.
Conducted experiments: Anderson, Reeves, and Kapernaros.
Performed data analysis: Anderson.
Wrote or contributed to the writing of the manuscript: Anderson and Caudle.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA030044].
References
- Anderson EM, Del Valle-Pinero AY, Suckow SK, Nolan TA, Neubert JK, Caudle RM. (2012b) Morphine and MK-801 administration leads to alternative N-methyl-D-aspartate receptor 1 splicing and associated changes in reward seeking behavior and nociception on an operant orofacial assay. Neuroscience 214:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EM, Neubert JK, Caudle RM. (2012a) Long-term changes in reward-seeking following morphine withdrawal are associated with altered N-methyl-D-aspartate receptor 1 splice variants in the amygdala. Neuroscience 223:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Betancur C, Mansuy IM, Giros B. (2005) The reinforcing effects of chronic D-amphetamine and morphine are impaired in a line of memory-deficient mice overexpressing calcineurin. Eur J Neurosci 21:3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HM, Knowlton AE, Grieshaber SS. (2012) Chlamydial infection induces host cytokinesis failure at abscission. Cell Microbiol 14:1554–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle RM, Perez FM, King C, Yu CG, Yezierski RP. (2003) N-methyl-D-aspartate receptor subunit expression and phosphorylation following excitotoxic spinal cord injury in rats. Neurosci Lett 349:37–40. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Connery HS. (2014) It’s MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol 5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Konradi C, Carlezon WA., Jr (2003) Dopamine-dependent increases in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J Neurochem 87:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Shin EH, Wang JQ. (2005) Inhibition of protein phosphatase 2B upregulates serine phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in striatal neurons in vivo. Neurosci Lett 384:38–43. [DOI] [PubMed] [Google Scholar]
- Cik M, Chazot PL, Stephenson FA. (1993) Optimal expression of cloned NMDAR1/NMDAR2A heteromeric glutamate receptors: a biochemical characterization. Biochem J 296:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. (2008) Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Aronowski J, Drath D, Dafny N. (1987) Evidence of neuro-immunologic interactions: cyclosporine modifies opiate withdrawal by effects on the brain and immune components. J Neuroimmunol 13:331–342. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Dafny N. (1988) Cyclosporine affects central nervous system opioid activity via direct and indirect means. Brain Behav Immun 2:242–253. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macías W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. (2003) Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem 87:922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. (2009) Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse 63:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. (2003) Eppendorf 2003 prize-winning essay. Ubiquitin and the deconstruction of synapses. Science 302:800–801. [DOI] [PubMed] [Google Scholar]
- Fan P, Jiang Z, Diamond I, Yao L. (2009) Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol 76:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrani-Kile K, Randall PK, Leslie SW. (2003) Acute ethanol affects phosphorylation state of the NMDA receptor complex: implication of tyrosine phosphatases and protein kinase A. Brain Res Mol Brain Res 115:78–86. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Le Bourdellès B, Whiting PJ. (1995) Recombinant human NMDA homomeric NR1 receptors expressed in mammalian cells form a high-affinity glycine antagonist binding site. J Neurochem 64:525–530. [DOI] [PubMed] [Google Scholar]
- Hilgenberg LG, Smith MA. (2007) Preparation of dissociated mouse cortical neuron cultures. J Vis Exp 10:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Khavandgar S, Mehr SE, Namiranian K, Dehpour AR. (2003) The effects of FK506 on the development and expression of morphine tolerance and dependence in mice. Behav Pharmacol 14:121–127. [DOI] [PubMed] [Google Scholar]
- Ji D, Sui ZY, Ma YY, Luo F, Cui CL, Han JS. (2004) NMDA receptor in nucleus accumbens is implicated in morphine withdrawal in rats. Neurochem Res 29:2113–2120. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Wang J, Wei XL, Liang QY, Cheng TT. (2012) Exogenous sodium hydrosulfide can attenuate naloxone-precipitated withdrawal syndromes and affect cAMP signaling pathway in heroin-dependent rat’s nucleus accumbens. Eur Rev Med Pharmacol Sci 16:1974–1982. [PubMed] [Google Scholar]
- Kam AY, Liao D, Loh HH, Law PY. (2010) Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci 30:15304–15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2009) Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 (Suppl 1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426. [DOI] [PubMed] [Google Scholar]
- Li B, Devidze N, Barengolts D, Prostak N, Sphicas E, Apicella AJ, Malinow R, Emamian ES. (2009) NMDA receptor phosphorylation at a site affected in schizophrenia controls synaptic and behavioral plasticity. J Neurosci 29:11965–11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, Kato K, Tomizawa K, Matsushita M, Moriwaki A, Matsui H, Mikoshiba K. (2002) Calcineurin plays different roles in group II metabotropic glutamate receptor- and NMDA receptor-dependent long-term depression. J Neurosci 22:5034–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. (1994) Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature 369:235–239. [DOI] [PubMed] [Google Scholar]
- Lin SL, Tsai RY, Shen CH, Lin FH, Wang JJ, Hsin ST, Wong CS. (2010) Co-administration of ultra-low dose naloxone attenuates morphine tolerance in rats via attenuation of NMDA receptor neurotransmission and suppression of neuroinflammation in the spinal cords. Pharmacol Biochem Behav 96:236–245. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. (1991) Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807–815. [DOI] [PubMed] [Google Scholar]
- Martin G, Ahmed SH, Blank T, Spiess J, Koob GF, Siggins GR. (1999) Chronic morphine treatment alters NMDA receptor-mediated synaptic transmission in the nucleus accumbens. J Neurosci 19:9081–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiei Langroudi R, Khoshnoodi MA, Abadi NY, Tahsili Fahadan P, Ghahremani MH, Dehpour AR. (2005) Effect of cyclosporin A on morphine-induced place conditioning in mice: involvement of nitric oxide. Eur J Pharmacol 507:107–115. [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. (2003) Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40:581–594. [DOI] [PubMed] [Google Scholar]
- Neubert JK, King C, Malphurs W, Wong F, Weaver JP, Jenkins AC, Rossi HL, Caudle RM. (2008) Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Mol Pain 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Rossi HL, Malphurs W, Vierck C J, Jr, Caudle RM. (2006) Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behav Brain Res 170:308–315. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Rossi HL, Pogar J, Jenkins AC, Caudle RM. (2007) Effects of mu- and kappa-2 opioid receptor agonists on pain and rearing behaviors. Behav Brain Funct 3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. (2005) Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain 116:386–395. [DOI] [PubMed] [Google Scholar]
- Nikonenko I, Jourdain P, Alberi S, Toni N, Muller D. (2002) Activity-induced changes of spine morphology. Hippocampus 12:585–591. [DOI] [PubMed] [Google Scholar]
- Nolan TA, Caudle RM, Neubert JK. (2011a) Effect of caloric and non-caloric sweet reward solutions on thermal facial operant conditioning. Behav Brain Res 216:723–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TA, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. (2011b) Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behav Brain Res 217:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TA, Price DD, Caudle RM, Murphy NP, Neubert JK. (2012) Placebo-induced analgesia in an operant pain model in rats. Pain 153:2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA. [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. (1999) Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33:160–162. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Berrocoso E, Garzón J. (2012) The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 37:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Neubert JK. (2008) Effects of environmental enrichment on thermal sensitivity in an operant orofacial pain assay. Behav Brain Res 187:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Neubert JK. (2009) Effects of hot and cold stimulus combinations on the thermal preference of rats. Behav Brain Res 203:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Vierck CJ, Jr, Caudle RM, Neubert JK. (2006) Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Mol Pain 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Vierck CJ, Jr, Caudle RM, Yezierski RP, Neubert JK. (2009) Dose-dependent effects of icilin on thermal preference in the hindpaw and face of rats. J Pain 10:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouibi K, Contarino A. (2012) Increased motivation to eat in opiate-withdrawn mice. Psychopharmacology (Berl) 221:675–684. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Raone A, De Montis MG, Tagliamonte A, Gambarana C. (2007) Behavioral expression of cocaine sensitization in rats is accompanied by a distinct pattern of modifications in the PKA/DARPP-32 signaling pathway. J Neurochem 103:1168–1183. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. (2003) Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology 45:755–767 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshiike M, Funada M, Mizoguchi H, Kamei J, Misawa M. (1993) Effect of cyclosporine A on the morphine-induced place preference. Neurosci Lett 160:159–162. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. (1997) Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 272:5157–5166. [DOI] [PubMed] [Google Scholar]
- Varney MA, Jachec C, Deal C, Hess SD, Daggett LP, Skvoretz R, Urcan M, Morrison JH, Moran T, Johnson EC, et al. (1996) Stable expression and characterization of recombinant human heteromeric N-methyl-D-aspartate receptor subtypes NMDAR1A/2A and NMDAR1A/2B in mammalian cells. J Pharmacol Exp Ther 279:367–378. [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. (1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285:93–96. [DOI] [PubMed] [Google Scholar]
- Wu X, Shi M, Wei C, Yang M, Liu Y, Liu Z, Zhang X, Ren W. (2012) Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. J Neurosci Res 90:1270–1283. [DOI] [PubMed] [Google Scholar]
- Yang HY, Pu XP. (2009) Chronic morphine administration induces over-expression of aldolase C with reduction of CREB phosphorylation in the mouse hippocampus. Eur J Pharmacol 609:51–57. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhou X, Wang X, Xiang X, Chen H, Hao W. (2007) Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addict Biol 12:152–157. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Caudle RM, Price DD, Del Valle-Pinero AY, Verne GN. (2006) Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]