Abstract
(1R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine (lorcaserin) is approved by the United States Food and Drug Administration for treating obesity, and its therapeutic effects are thought to result from agonist activity at serotonin (5-HT)2C receptors. Lorcaserin has affinity for other 5-HT receptor subtypes, although its activity at those subtypes is not fully described. The current study compared the behavioral effects of lorcaserin (0.0032–32.0 mg/kg) to the effects of other 5-HT receptor selective agonists in rats (n = 8). The 5-HT2C receptor selective agonist 1-(3-chlorophenyl)piperazine (mCPP, 0.032–1.0 mg/kg) and lorcaserin induced yawning which was attenuated by the 5-HT2C receptor selective antagonist 6-chloro-5-methyl-N-(6-[(2-methylpyridin-3-yl)oxy]pydidin-3-yl)indoline-1-carboxamide (1.0 mg/kg). The 5-HT2A receptor selective agonist 2,5-dimethoxy-4-methylamphetamine (0.1–3.2 mg/kg) induced head twitching, which was attenuated by the 5-HT2A receptor selective antagonist R-(+)-2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol] (MDL 100907, 0.01 mg/kg), lorcaserin (3.2 mg/kg), and mCPP (3.2 mg/kg). In rats pretreated with MDL 100907 (1.0 mg/kg), lorcaserin also induced head twitching. At larger doses, lorcaserin produced forepaw treading, which was attenuated by the 5-HT1A receptor selective antagonist N-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-N-(2-pyridyl)cyclohexanecarboxamide (0.178 mg/kg). While the behavioral effects of lorcaserin in rats are consistent with it having agonist activity at 5-HT2C receptors, these data suggest that at larger doses it also has agonist activity at 5-HT2A and possibly 5-HT1A receptors. Mounting evidence suggests that 5-HT2C receptor agonists might be effective for treating drug abuse. A more complete description of the activity of lorcaserin at 5-HT receptor subtypes will facilitate a better understanding of the mechanisms that mediate its therapeutic effects.
Introduction
(1R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine [lorcaserin (Belviq®; Arena Pharmaceuticals, San Diego, CA)] (Smith et al., 2008) was recently approved by the U.S. Food and Drug Administration for treating obesity (Colman et al., 2012). The therapeutic effects of lorcaserin are thought to be due to agonist activity at serotonin (5-HT)2C receptors. Drugs that have agonist activity at 5-HT2C receptors decrease food intake in rats (Kennett and Curzon, 1991; Clifton et al., 2000; Vickers et al., 2001a) and have long been considered for treating obesity [for a review, see Bickerdike (2003)]. Agonists acting at 5-HT2C receptors have other behavioral effects in rats. For example, the prototypical 5-HT2C receptor agonist 1-(3-chlorophenyl)piperazine (mCPP) induces yawning and penile erections (Kennett and Curzon, 1988; Protais et al., 1995). In addition to 5-HT2C receptors, lorcaserin also has affinity for other 5-HT receptor subtypes, including 5-HT2A, 5-HT2B, and 5-HT1A (Thomsen et al., 2008). Although lorcaserin has been shown to have efficacy at 5-HT2A receptors in vitro (Thomsen et al., 2008), it is unclear whether lorcaserin has agonist properties in vivo that are mediated by 5-HT2A receptors. In humans, drugs with agonist effects at 5-HT2A receptors are sometimes abused and can produce hallucinations (Kennett and Clifton, 2010). In rodents, drugs with agonist activity at 5-HT2A receptors [e.g., (+/−)-2,5-dimethoxy-4-indoamphetamine hydrochloride (DOI), 2,5-dimethoxy-4-methylamphetamine (DOM), and lysergic acid diethylamide (LSD)] induce a characteristic head twitch response [Corne et al., 1963; Darmani et al., 1992; Fantegrossi et al., 2010; for a review, see Canal and Morgan (2012)]. One study compared the behavioral effects of lorcaserin to DOI, a prototypic 5-HT2A receptor selective agonist (Thomsen et al., 2008). Lorcaserin did not produce behavioral effects like those produced by DOI (e.g., wet dog shakes and back contractions), suggesting that lorcaserin does not have agonist activity at 5-HT2A receptors.
Several decades of preclinical research suggest that 5-HT2C receptor agonists might be effective for treating substance use disorders [for a review, see Howell and Cunningham (2015)]. For example, 5-HT2C receptor agonists can attenuate the positive reinforcing effects of cocaine as well as reinstatement of responding by cocaine and cocaine-associated stimuli (Callahan and Cunningham, 1995; Burbassi and Cervo, 2008; Navarra et al., 2008; Anastasio et al., 2011; Cunningham et al., 2011). Lorcaserin reduces nicotine self-administration in rats (Higgins et al., 2012) and clinical trials were recently initiated to investigate lorcaserin for smoking cessation (http://www.clinicaltrials.gov).
Despite having been approved for treating obesity, the pharmacological profile of lorcaserin has yet to be fully described. This study tested the hypothesis that, in rats, the behavioral effects of lorcaserin are due to its agonist actions at 5-HT2C receptors by examining directly observable behavior induced by lorcaserin and comparing these effects to behavior induced by prototypic agonists that have selectivity for particular 5-HT receptor subtypes. To determine the receptor subtypes mediating the behavioral effects of these drugs, agonists were also examined in combination with 5-HT receptor subtype selective antagonists. Lastly, lorcaserin was also examined in combination with other agonists in instances when a particular behavior was not observed with lorcaserin alone to test whether it had antagonist properties at specific 5-HT receptor subtypes.
Materials and Methods
Subjects.
Eight male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g upon arrival were housed individually in an environmentally controlled room (24 ± 1°C; 50 ± 10% relative humidity) under a 12-hour light/dark cycle (light period from 7:00 a.m. to 7:00 p.m.). Except during testing, rats had free access to food and water in the home cage. Rats were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, University of Texas Health Science Center at San Antonio, and the 2011 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, and National Academy of Sciences).
Directly Observable Behavior.
On the day of testing, rats were transferred from a clear plastic home cage to a test cage—the home and test cages were identical, with the exception that no food, water, or bedding was present in the test cage—and allowed to habituate for 15 minutes. Experiments were conducted at the same time on each test day (2:00 p.m.) by an independent observer blind to the treatment. Behavior was assessed after injection of vehicle, followed by injection of increasing doses of the drug administered i.p. (except where otherwise noted) every 25 minutes according to a cumulative dosing procedure. Behavior was scored either in real time or by video recordings. Beginning 5 minutes after each injection, the total number of head twitches and yawns observed for 20 minutes was recorded. Head twitch was defined as an irregularly occurring horizontal head movement resembling a strong pinna reflex (Corne et al., 1963; Li and France, 2008). Yawning was defined as an opening and closing of the mouth, such that the lower incisors were completely visible (Sevak et al., 2008; Baladi and France, 2009). The presence or absence of forepaw treading, lower lip retraction, and flat body posture were assessed in 5-second periods every minute during the 20-minute observation period following each injection (i.e., maximum possible score = 20). Forepaw treading was scored as present when repeated flexion and extension of the forepaws occurred (at least three times and involving both forepaws) (Colpaert et al., 1989). Lower lip retraction was scored as present if the lower incisors were visible, and flat body posture was scored as present if the entire ventral surface of the rat was in contact with the cage floor (Kleven et al., 1997). For tests of antagonism, an injection of one drug (e.g., an antagonist) was administered immediately after the injection of vehicle.
Data Analyses.
The results are expressed as the average (± 1 S.E.M.) number of yawns or head twitches during a 20-minute observation period and plotted as a function of dose. The results are also expressed as an average (± 1 S.E.M.) forepaw treading score (during a 20-minute observation period) and plotted as a function of dose. For each group, the differences between dose-response curves were analyzed by comparing the following: maximal number for head twitches and yawns and maximal score for forepaw treading, lower lip retraction, and flat body posture. A two-way (dose x test) repeated measures analysis of variance, followed by post hoc Bonferroni tests, was used to determine statistical differences between maximal effects for dose-response curves determined in the presence and absence of an antagonist. Doses producing the maximum number of yawns, maximum number of head twitches, and maximum forepaw treading score were also determined for each rat and dose-response curve. These doses were log transformed for individual subjects and analyzed using one-way repeated measures analysis of variance, followed by Dunnett’s post hoc analyses. For all tests, the P value was less than 0.05.
Drugs.
Lorcaserin hydrochloride (MedChem Express, Monmouth Junction, NJ), 2,5-dimethoxy-4-methylamphetamine (DOM hydrochloride) (NIDA Research Technology Branch, Rockville, MD), 7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol (8-OH-DPAT) (Sigma Aldrich, St. Louis, MO), N-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-N-(2-pyridyl)cyclohexanecarboxamide (WAY 100635) (a gift from Dr. Adrian Newman-Tancredi; Centre de Recherche Pierre Fabre, Castres, France), mCPP (Sigma Aldrich), and ritanserin (Sigma Aldrich) were dissolved in sterile 0.9% saline. 6-Chloro-5-methyl-N-(6-[(2-methylpyridin-3-yl)oxy]pydidin-3-yl)indoline-1-carboxamide (SB 242084 hydrochloride) (ABCAM, Cambridge, MA) was dissolved in a mixture of saline (0.9%) containing hydroxypropyl-β-cyclodextrin (8% by weight) plus citric acid (25 mM). Sodium hydrochloride was then added to achieve a more basic pH. R-(+)-2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol] (MDL 100907) was synthesized by Kenner Rice (Ullrich and Rice, 2000) and dissolved in 20% dimethylsulfoxide (v/v). Doses of SB 242084 are expressed as the base, and doses of other drugs are expressed as the salt. Drugs were administered i.p., except for 8-OH-DPAT and WAY 100635, which were administered s.c. All drugs were typically administered in a volume of 1 ml/kg body weight.
Results
Yawning.
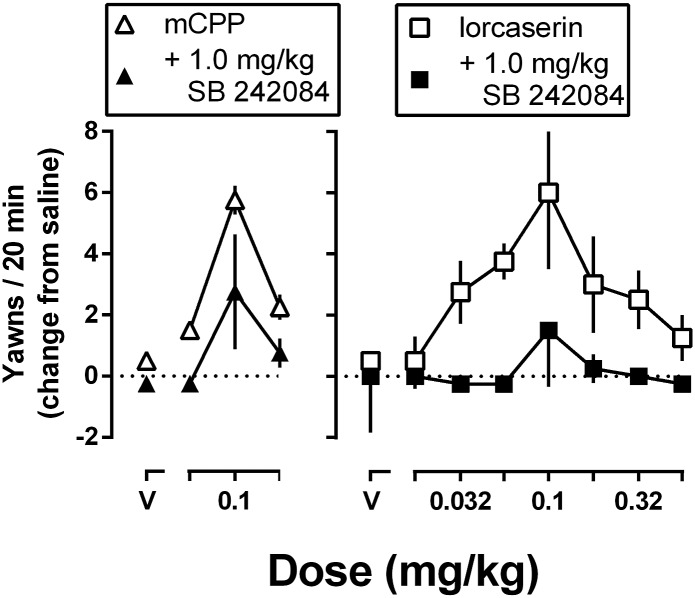
With increasing cumulative doses, mCPP increased and then decreased yawning (Fig. 1, left panel), resulting in an inverted U-shaped dose-response curve. Similarly, increasing cumulative doses of lorcaserin also produced an inverted U-shaped dose-response curve for yawning (Fig. 1, right panel). Pretreatment with the 5-HT2C receptor selective antagonist SB 242084 (1.0 mg/kg) significantly attenuated yawning produced by lorcaserin [F(1,7) = 35.48; P = 0.006] or mCPP [F(1,7)=21.00; P = 0.0025]. Up to a cumulative dose of 3.2 mg/kg, DOM did not increase yawning (data not shown). A smaller dose of SB 242084 (0.1 mg/kg) significantly attenuated lorcaserin-induced yawning (data not shown) in a manner that was not different from antagonism observed with 1.0 mg/kg SB 242084. Pretreatment with the 5-HT2A receptor selective antagonist MDL 100907 (0.01 mg/kg) did not significantly affect lorcaserin-induced yawning (data not shown).
Fig. 1.
Average (± 1 S.E.M.) number of yawns observed in a 20-minute observation period in rats that received cumulative doses of mCPP (left panel) or lorcaserin (right panel) alone (open symbols) or in combination with 1.0 mg/kg SB 242084 (closed symbols). The average number of yawns (ordinate) is expressed as a change from a saline test (eight saline injections spaced 25 minutes apart to match the temporal conditions of the drug tests) and plotted as a function of dose (abscissae; V, vehicle).
Head Twitching.
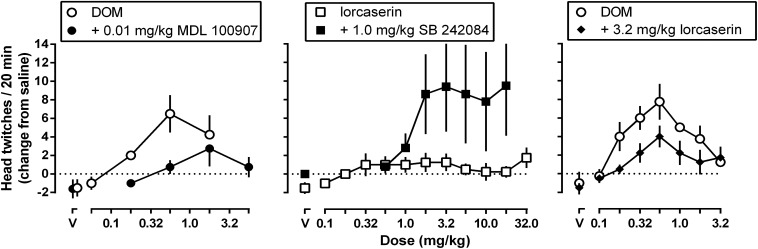
Cumulative doses of DOM increased and then decreased head twitching, resulting in an inverted U-shaped dose-response curve (Fig. 2, left and right panels). In contrast, lorcaserin did not produce head twitching up to a cumulative dose of 32.0 mg/kg (Fig. 2, middle panel, open squares). Pretreatment with 0.01 mg/kg of the 5-HT2A receptor selective antagonist MDL 100907 (Fig. 2, left panel) attenuated DOM-induced head twitching [F(1,7) = 21.11; P = 0.0025], as did pretreatment with either 3.2 mg/kg lorcaserin [Fig. 2, right panel, closed diamonds; F(1,7) = 13.24; P = 0.0083] or 3.2 mg/kg mCPP (data not shown). While not producing head twitching when administered alone, lorcaserin significantly increased head twitching in rats pretreated with 1.0 mg/kg of the 5-HT2C receptor selective antagonist SB 242084 [Fig. 2, middle panel; F(1,7) = 5.765; P = 0.0474].
Fig. 2.
Average (± 1 S.E.M.) number of head twitches observed in a 20-minute observation period in rats that received cumulative doses of DOM (left and right panels, open circles) or lorcaserin (middle panel, open squares). DOM was also studied in combination with 0.01 mg/kg MDL 100907 (left panel, closed circles) and in combination with 3.2 mg/kg lorcaserin (right panel, closed diamonds). Lorcaserin was examined in combination with 1.0 mg/kg SB 242084 (middle panel, closed squares). The average number of head twitches (ordinate) is expressed as a change from a saline test (eight saline injections spaced 25 minutes apart to match the temporal conditions of the drug tests) and plotted as a function of dose (abscissae; V, vehicle).
Other Behavioral Effects.
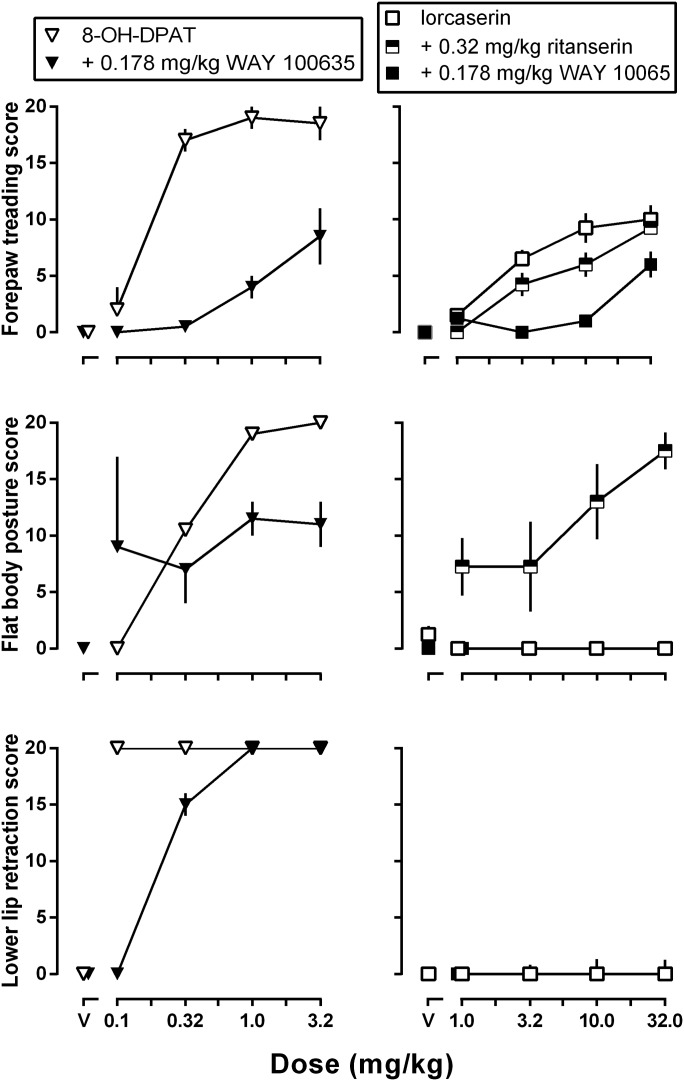
Forepaw treading, flat body posture, and lower lip retraction were all induced by 8-OH-DPAT (Fig. 3, left panels, open inverted triangles). Large doses of lorcaserin produced forepaw treading but not flat body posture or lower lip retraction (Fig. 3, right panels, open squares).The maximal effect for 8-OH-DPAT–induced forepaw treading was significantly larger than the maximal effect for lorcaserin-induced forepaw treading [Fig. 3, upper panels; F(1,7) = 175.0; P < 0.0001]. Pretreatment with 0.178 mg/kg of the 5-HT1A receptor selective antagonist WAY 100635 attenuated forepaw treading produced by 8-OH-DPAT [F(1,7) = 171.5; P < 0.0001] or lorcaserin [F(1,7) = 42.0; P = 0.0003; Fig. 3, upper panels]. WAY 100635 also attenuated 8-OH-DPAT–induced flat body posture [i.e., maximal effect; F(1,7) = 84.29; P < 0.0001] and lower lip retraction [i.e., maximally effective dose; F(1,7) = 91.93; P < 0.0001; Fig. 3, middle and lower left panels, respectively]. Pretreatment with the nonselective 5-HT2 receptor antagonist ritanserin (0.32 mg/kg) did not significantly impact lorcaserin-induced forepaw treading (Fig. 3, upper right). While not producing flat body posture when administered alone, lorcaserin significantly increased flat body posture in rats pretreated with 0.178 mg/kg ritanserin [Fig. 3, middle right panel; F(1,7) = 1980.0; P < 0.0001]. Lower lip retraction was not observed with lorcaserin alone or in combination with ritanserin (Fig. 3, lower right panel).
Fig. 3.
Average (± 1 S.E.M.) forepaw treading, flat body posture, and lower lip retraction in a 20-minute observation period (maximum possible effect = 20) in rats that received cumulative doses of 8-OH-DPAT (left panels, open inverted triangles) or lorcaserin (right panels, open squares) alone or in combination with 0.0178 mg/kg WAY 100635 (left panels, closed inverted triangles; right panels, closed squares) or 0.32 mg/kg ritanserin (right panels, half closed squares). Average scores (ordinate) are plotted as a function of dose (abscissae; V, vehicle).
Discussion
Lorcaserin has highest affinity for 5-HT2C receptors, although it also binds to other 5-HT receptor subtypes and at still higher concentrations to the 5-HT transporter (Thomsen et al., 2008). It is well established that many of the behavioral effects of lorcaserin are mediated by agonist activity at 5-HT2C receptors. For example, lorcaserin decreases food intake in rats and that effect is prevented by the 5-HT2C receptor selective antagonist SB 242084 (Thomsen et al., 2008). In the present study, lorcaserin increased yawning as well as other behavioral effects (e.g., penile erections and grooming, both of which were observed but not recorded) that are produced by drugs with agonist activity at 5-HT2C receptors (Kennett and Curzon, 1988; Protais et al., 1995). When administered alone, lorcaserin did not produce head twitching; however, lorcaserin significantly increased head twitching in rats that were pretreated with the 5-HT2C receptor selective antagonist SB 242084. Drugs with agonist activity at 5-HT2A receptors increase head twitching (Canal and Morgan, 2012). At doses larger than those producing head twitching, lorcaserin induced forepaw treading, an effect produced by drugs with agonist activity at 5-HT1A receptors (Haberzettl et al., 2013). These results extend an earlier study (Thomsen et al., 2008) by confirming in vivo agonist properties of lorcaserin that appear to be mediated by 5-HT2C receptors and by demonstrating other agonist properties of lorcaserin that appear to be mediated by 5-HT2A and 5-HT1A receptors.
The prototypical 5-HT2C receptor agonist mCPP as well as lorcaserin produced yawning that was attenuated by the 5-HT2C receptor selective antagonist SB 242084, suggesting that yawning induced by both drugs is mediated by agonist activity at 5-HT2C receptors. When administered alone up to a cumulative dose of 32.0 mg/kg, lorcaserin did not induce head twitching; these data are consistent with a previous report (Thomsen et al., 2008) in which lorcaserin administered via oral gavage did not share behavioral effects with the prototypic 5-HT2A receptor selective agonist DOI (e.g., wet dog shakes and back contractions).
Lorcaserin did not produce head twitching when administered alone, although it attenuated head twitching produced by the 5-HT2A receptor selective agonist DOM. The dose-response curve for head twitching produced by DOM or other 5-HT2A receptor selective agonists (e.g., DOI) is an inverted U-shape, with the ascending limb of the curve being mediated by 5-HT2A receptors and the descending limb being mediated by 5-HT2C receptors [Vickers et al., 2001b; Fantegrossi et al., 2010; see Canal and Morgan (2012) for a review]. Thus, it was possible that attenuation of DOM-induced head twitching by lorcaserin could have resulted from agonism at 5-HT2C receptors, antagonism at 5-HT2A receptors, or both mechanisms. Similarly, that lorcaserin did not induce head twitching when administered alone could have occurred either because it does not have agonist activity at 5-HT2A receptors or because its agonist activity at 5-HT2C receptors inhibits the expression of (masks) 5-HT2A-receptor mediated head twitching. When administered in combination with the 5-HT2C receptor selective antagonist SB 242084, lorcaserin significantly increased head twitching. This result suggests that lorcaserin has agonist activity at both 5-HT2C and 5-HT2A receptors and that its agonist activity at 5-HT2C receptors can inhibit the expression of a behavior (i.e., head twitching) that is mediated by agonist activity at 5-HT2A receptors. The minimally effective dose of lorcaserin to induce head twitching in rats was 1.78 mg/kg, whereas the therapeutic dose for treating obesity is 10 mg twice daily (i.e., 0.29 mg/kg for a 70-kg human). Lorcaserin is under evaluation for its potential in treating substance use disorders (e.g., tobacco smoking and cocaine use disorder) and it is unclear whether the therapeutic dose of lorcaserin for treating obesity will be effective for treating substance abuse disorders. Any agonist activity of lorcaserin at other 5-HT receptor subtypes (e.g., 5-HT2A) might be particularly important when considering its possible use in treating individuals with a history of substance use disorder.
Indirect-acting 5-HT receptor agonists [e.g., selective 5-HT reuptake inhibitors as well as direct-acting 5-HT receptor agonists that are selective for 5-HT1A receptors (e.g., 8-OH-DPAT)] produce a characteristic pattern of behavioral effects in rats that include forepaw treating, lower lip retraction, and flat body posture [for a review, see Haberzettl et al. (2013)]. In the current study, 8-OH-DPAT produced forepaw treading, lower lip retraction, and flat body posture [see Li and France (2008) for similar results using i.p. cumulative dosing]. In contrast, lorcaserin produced forepaw treading but not lower lip retraction or flat body posture. Other results from this study (see above) suggest that lorcaserin has agonist activity at both 5-HT2C and 5-HT2A receptors and it is possible that activity at those 5-HT receptor subtypes inhibits the expression of (masks) lower lip retraction and flat body posture. Consistent with this possibility, both 5-HT2C receptor agonists (mCPP) as well as 5-HT2A receptor agonists (DOI) attenuate 8-OH-DPAT–induced lower lip retraction (Berendsen et al., 1989). In the present study, pretreatment with the nonselective 5-HT2 receptor antagonist ritanserin did not significantly impact lorcaserin-induced forepaw treading; however, doses that failed to produce any flat body posture when lorcaserin was administered alone caused significant, dose-related increases in flat body posture in rats that were pretreated with ritanserin, suggesting that activity of lorcaserin at 5-HT2 receptors might prevent the expression of a behavior (i.e., flat body posture) that is thought to be mediated by agonist activity at 5-HT1A receptors. Lower lip retraction was not observed with lorcaserin alone or in combination with ritanserin.
In summary, these results provide further evidence that lorcaserin has agonist activity at 5-HT2C receptors, the site of action that is thought to be important for its therapeutic actions (i.e., treating obesity). However, in addition to agonist activity at 5-HT2C receptors, these results also provide evidence that lorcaserin has agonist activity (in vivo) at 5-HT2A and 5-HT1A receptors. Moreover, the rank order potency of lorcaserin for producing yawning (5-HT2C receptor mediated), head twitching (5-HT2A receptor mediated), forepaw treading, and flat body posture (5-HT1A receptor mediated) is the same as its rank order binding affinities for these 5-HT receptor subtypes (Thomsen et al., 2008). It is unclear whether actions at these 5-HT receptor subtypes might contribute to the adverse effects that can occur with lorcaserin. Lorcaserin is a controlled substance (Drug Enforcement Administration Schedule IV), and its adverse effects include hallucinations and euphoria (http://www.belviq.com; http://onlinelibrary.wiley.com/doi/10.1111/obr.12015/epdf). Overweight patients receiving lorcaserin are also encouraged to diet and exercise. Food restriction decreases the sensitivity of rats to some of the behavioral effects of drugs acting on 5-HT receptors (Li and France, 2008; Serafine and France, 2014). It is possible that food restriction and the resulting body weight changes might also impact the therapeutic and/or adverse effects of lorcaserin. Finally, lorcaserin is under evaluation for smoking cessation and for treating cocaine abuse. The results of the current study underscore the importance of full characterization of the behavior profile of lorcaserin to identify any effects that could be problematic for individuals with a history of a substance use disorder (e.g., 5-HT2A agonist activity). A better understanding of the mechanisms of action of lorcaserin at 5-HT receptor subtypes might also facilitate the development of new, improved drugs for treating obesity as well as substance use disorders.
Abbreviations
- DOI
(+/−)-2,5-dimethoxy-4-indoamphetamine hydrochloride
- DOM
2,5-dimethoxy-4-methylamphetamine
- 8-OH-DPAT
7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol
- 5-HT
serotonin
- lorcaserin
(1R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- LSD
lysergic acid diethylamide
- mCPP
1-(3-chlorophenyl)piperazine
- MDL 100907
R-(+)-2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol]
- SB 242084
6-chloro-5-methyl-N-(6-[(2-methylpyridin-3-yl)oxy]pydidin-3-yl)indoline-1-carboxamide
- WAY 100635
N-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-N-(2-pyridyl)cyclohexanecarboxamide
Authorship Contributions
Participated in research design: Serafine, France.
Conducted experiments: Serafine.
Contributed new reagents or analytic tools: Rice.
Performed data analysis: Serafine.
Wrote or contributed to the writing of the manuscript: Serafine, France.
Footnotes
This work was supported, in part, by grants from the National Institutes of Health National Institute on Drug Abuse [Grants K05DA017918 and T32DA031115]. A portion of this work was supported by the National Institutes of Health Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institute of Alcohol Abuse and Alcoholism, or the National Institutes of Health. The authors have no conflict of interest.
References
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, Cunningham KA. (2011) Serotonin (5-hydroxytryptamine) 5-HT(2A) receptor: association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol 22:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. (2009) High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol 610:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HH, Jenck F, Broekkamp CL. (1989) Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav 33:821–827. [DOI] [PubMed] [Google Scholar]
- Bickerdike MJ. (2003) 5-HT2C receptor agonists as potential drugs for the treatment of obesity. Curr Top Med Chem 3:885–897. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. (2008) Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 196:15–27. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. (1995) Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther 274:1414–1424. [PubMed] [Google Scholar]
- Canal CE, Morgan D. (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4:556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Dourish CT. (2000) Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 152:256–267. [DOI] [PubMed] [Google Scholar]
- Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. (2012) The FDA’s assessment of two drugs for chronic weight management. N Engl J Med 367:1577–1579. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Koek W, Lategan A. (1989) 1-5-Hydroxytryptophan-induced flat body posture in the rat: antagonism by ritanserin and potentiation after 5,7-dihydroxytryptamine. Eur J Pharmacol 169:175–178. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. (1963) A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br Pharmacol Chemother 20:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. (2011) Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. (1992) Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (+/- )-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J Pharmacol Exp Ther 262:692–698. [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl R, Bert B, Fink H, Fox MA. (2013) Animal models of the serotonin syndrome: a systematic review. Behav Brain Res 256:328–345. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. (2012) The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37:1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Clifton PG. (2010) New approaches to the pharmacological treatment of obesity: can they break through the efficacy barrier? Pharmacol Biochem Behav 97:63–83. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Curzon G. (1988) Evidence that mCPP may have behavioural effects mediated by central 5-HT1C receptors. Br J Pharmacol 94:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Curzon G. (1991) Potencies of antagonists indicate that 5-HT1C receptors mediate 1-3(chlorophenyl)piperazine-induced hypophagia. Br J Pharmacol 103:2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Assié MB, Koek W. (1997) Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther 282:747–759. [PubMed] [Google Scholar]
- Li JX, France CP. (2008) Food restriction and streptozotocin treatment decrease 5-HT1A and 5-HT2A receptor-mediated behavioral effects in rats. Behav Pharmacol 19:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Comery TA, Graf R, Rosenzweig-Lipson S, Day M. (2008) The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav Brain Res 188:412–415. [DOI] [PubMed] [Google Scholar]
- Protais P, Windsor M, Mocaër E, Comoy E. (1995) Post-synaptic 5-HT1A receptor involvement in yawning and penile erections induced by apomorphine, physostigmine and mCPP in rats. Psychopharmacology (Berl) 120:376–383. [DOI] [PubMed] [Google Scholar]
- Serafine KM, France CP. (2014) Restricted access to standard or high fat chow alters sensitivity of rats to the 5-HT(2A/2C) receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane. Behav Pharmacol 25:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. (2008) Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol 592:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, et al. (2008) Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem 51:305–313. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587. [DOI] [PubMed] [Google Scholar]
- Ullrich T, Rice KC. (2000) A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorg Med Chem 8:2427–2432. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT, Kennett GA. (2001a) Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacology 41:200–209. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. (2001b) Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav 69:643–652. [DOI] [PubMed] [Google Scholar]