Abstract
No consistent definition exists for energy products in the United States. These products have been marketed and sold as beverages (conventional foods), energy shots (dietary supplements), and in pill or tablet form. Recently, the number of available products has surged, and formulations have changed to include caffeine. To help characterize the use of caffeine-containing energy products in the United States, three sources of data were analyzed: sales data, data from federal sources, and reports from the Drug Abuse Warning Network. These data indicate that sales of caffeine-containing energy products and emergency room visits involving their consumption appear to be increasing over time. Data from the National Health and Nutrition Examination Survey (NHANES) 2007–2010 indicate that 2.7% [standard error (SE) 0.2%] of the US population ≥1 year of age used a caffeine-containing energy product, providing approximately 150–200 mg/day of caffeine per day in addition to caffeine from traditional sources like coffee, tea, and colas. The highest usage of these products was among males between the ages of 19 and 30 years (7.6%, SE 1.0). Although the prevalence of caffeine-containing energy product use remains low overall in the US population, certain subgroups appear to be using these products in larger amounts. Several challenges remain in determining the level of caffeine exposure from and accurate usage patterns of caffeine-containing energy products.
Keywords: beverages, caffeine, dietary supplements, energy, energy drinks
Introduction
Caffeine (1,3,7-trimethylpurine-2,6-dione) has more than 250 different common names (e.g., guaranine, thein, methyltheobromine, cafeina, koffein, mateina, and “cafipel”). Caffeine is a naturally occurring alkaloid found in the seeds, leaves, and fruit of coffee, tea, cocoa, maté, guarana, kola nuts, yerba maté, and more than 60 other plants.1,2 Caffeine is also added to a number of products for its known stimulant effects.
Regulations of the US Food and Drug Administration specify that caffeine can be added to cola-type beverages (conventional foods) at up to 200 parts per million (or 0.02% [weight %]) and to other foods if it is an approved food additive or a GRAS (generally recognized as safe) ingredient.1,3 In dietary supplements, caffeine and caffeine sources are regulated as dietary ingredients. Caffeine is also permitted as an active ingredient in over-the-counter and prescription drugs.1 Because caffeine occurs naturally and also as an added ingredient in conventional foods, dietary supplements, and drugs, potential caffeine exposure can be quite high. A product's caffeine content does not always appear on its label; declarations on labels of the amount per serving or dose depend on both the formulation and the regulatory category into which the product falls. The Nutrition Facts Panel on food labels is required to include recommended dietary information only for nutrients, and caffeine is not a nutrient.4 This poses a challenge for researchers in determining the caffeine content of many products and, thus, in estimating caffeine intakes.
In US law, no consistent definition presently exists for energy products, but products in this category often contain caffeine and have been marketed and sold as beverages, energy shots, gels, and in pill or tablet forms as dietary supplements. Energy products are generally described as containing stimulants, like caffeine, at levels higher than those found in cola-type beverages and are distinguished from sports drinks that typically contain electrolytes and other ingredients but may or may not contain caffeine or other stimulants. However, some companies, in response to safety concerns, now offer caffeine-free versions of their energy products, and as these brands grow, other line extensions are being introduced.
Although caffeine from tea and coffee has been consumed for centuries, energy drinks and dietary supplements are a relatively new source of caffeine in the diet and the number of caffeine-containing energy products has increased dramatically in recent years. Changes to product formulations have also been made to include caffeine and other caffeine-containing ingredients such as guarana or green tea, as well as other bioactive ingredients such as taurine, inositol, and B vitamins in a variety of products. However, the marketer's option to declare (or not declare) the amount of caffeine, or other non-nutrient ingredients, on product labels makes it difficult to discern a product's actual content. Moreover, some products that were previously marketed as dietary supplements are now marketed as beverages.3 These factors, together with inconsistent definitions of what constitutes an energy product, from a regulatory standpoint, make estimating dietary intakes and trends of consumption challenging. Therefore, a variety of approaches are necessary to estimate the use of caffeine-containing energy products in the United States.
Evaluation of Sales Data
According to a variety of trade reports, sales of caffeine-containing energy drinks continue to grow. One estimate claims that sales of energy drinks and shots grew 60% from 2008 to 2012, and that sales totalled $12.5 billion in 2012.5 Energy drinks accounted for 78% of the sales in 2012, followed by energy shots, at about 18%, and energy drink mixes, at roughly 4%.5 Despite recent negative media attention6 and increased scrutiny by the US Food and Drug Administration in 2013, sales of caffeine-containing energy drinks grew 6.7% over the previous year.7,8 Although these numbers appear impressive, they are less so in the context of the overall landscape for these products; caffeine-containing energy drinks have the lowest consumption rates of any ready-to-drink beverage and account for only a small percentage of total dietary supplement sales.8,9 According to the 2012 State of the Industry Report, caffeine-containing energy drinks outsold bottled water for the first time in 2012 and sales were more than $6.9 billion (19.4%) over 2011 sales, while sales of bottled water were $6.7 billion (3.4%) higher than 2011 sales.8
Sales of caffeine-containing energy drinks marketed as dietary supplements are more difficult to characterize. According to the 2013 Supplement Business Report, sales of these product types, which are grouped under “hardcore drinks,” were estimated at $329 million in 2012, which was up from $128 million in 2002.9 However, these numbers do not include some common products (e.g., 5-hour Energy), which are thought of as energy drinks but sold as dietary supplements. Launched in 2003, 5-hour Energy sales are now estimated to be $1 billion and the product has the third highest sales number in the category, following Monster and Red Bull.7,10 Total sales of energy shots for 2012 were reported to be $1.1 billion.8
Evaluation of National Data
National Health and Nutrition Examination Survey
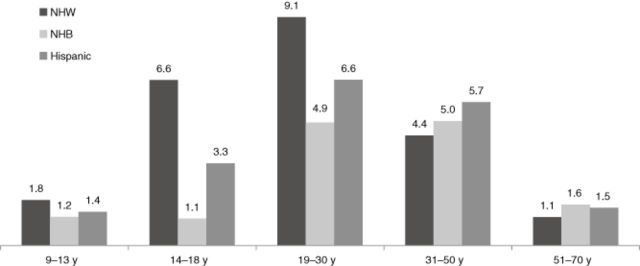
Publically available data from the National Health and Nutrition Examination Survey (NHANES) 2007–2010 includes dietary intake data collected on US residents aged 1 year and older (n = 19,142) including the use of dietary supplements and “energy drinks” (either marketed as foods or supplements). Overall, approximately 3% of Americans were classified as “users” of these products (Table 1); a user was defined as someone who reported the use of a supplement containing caffeine in the previous 30 days or use of an energy drink containing caffeine in one of two 24-hour dietary recalls. Males tended to report higher use of caffeine-containing energy products than females, and exhibited a differential race/ethnic pattern (Figure 1). Among those using caffeine-containing energy products, average daily caffeine intake was approximately 160 mg/day, but intakes varied somewhat within gender and age groups (Table 2).
Table 1.
Prevalence of use of caffeine-containing energy products in the National Health and Nutrition Examination Survey, 2007–2010
| Sex and age group | No. surveyed | % (SE) |
|---|---|---|
| Total | 19,142 | 2.7 (0.2) |
| 1–3 years | 1,602 | 0 (0) |
| 4–8 years | 2,047 | 0.1 (0.1) |
| 9–13 years | 1,822 | 1.0 (0.4) |
| 14–18 years | 1,594 | 3.9 (0.1) |
| 19–30 years | 2,389 | 5.8 (0.7) |
| 31–50 years | 4,022 | 3.6 (0.3) |
| 51–70 years | 3,678 | 1.3 (0.3) |
| 70+ years | 1,988 | 0.2 (0.1) |
| Male | 9,568 | 3.4 (0.3) |
| 1–3 years | 836 | 0 (0) |
| 4–8 years | 1,082 | 0.1 (0.1) |
| 9–13 years | 906 | 1.6 (0.6) |
| 14–18 years | 838 | 4.8 (1.0) |
| 19–30 years | 1,170 | 7.6 (1.0) |
| 31–50 years | 1,924 | 4.7 (0.5) |
| 51–70 years | 1,853 | 1.3 (0.4) |
| 70+ years | 959 | 0.0 (0.0) |
| Female | 9,574 | 1.9 (0.2) |
| 1–3 years | 766 | 0 (0) |
| 4–8 years | 965 | 0 (0) |
| 9–13 years | 916 | 0.3 (0.2) |
| 14–18 years | 756 | 2.8 (0.8) |
| 19–30 years | 1,219 | 3.9 (0.8) |
| 31–50 years | 2,098 | 2.6 (0.4) |
| 51–70 years | 1,825 | 1.3 (0.4) |
| 70+ years | 1,029 | 0.3 (0.2) |
Prevalence of use (% [SE]) of caffeine-containing energy products of males by age group and race/ethnicity in the National Health and Nutrition Examination survey, 2007–2010.
Abbreviations: NHB, Non-Hispanic black; NHW, Non-Hispanic white.
Table 2.
Mean daily caffeine intakes (mg/day) among users of caffeine-containing energy products in the National Health and Nutrition Examination Survey, 2007–2010
| Sex and age group | No. surveyed | Mean intake (SE)a |
|---|---|---|
| Total | 300 | 159.8 (6.4) |
| 9–13 years | 15 | 181.8 (60.1) |
| 14–18 years | 41 | 141.2 (16.5) |
| 19–30 years | 91 | 161.4 (11.1) |
| 31–50 years | 117 | 165.2 (12.9) |
| 51–70 years | 33 | 154.3 (12.8) |
| Male | 212 | 173.9 (8.2) |
| 9–13 years | 13 | 196.6 (72.4) |
| 14–18 years | 25 | 153.3 (14.7) |
| 19–30 years | 64 | 175.3 (15.0) |
| 31–50 years | 86 | 180.7 (13.5) |
| 51–70 years | 22 | 158.4 (17.6) |
| Female | 88 | 127.6 (11.5) |
| 14–18 years | 16 | 119.5 (40.5) |
| 19–30 years | 27 | 134.9 (16.5) |
| 31–50 years | 31 | 114.6 (21.4) |
| 51–70 years | 11 | 148.2 (21.1) |
Very small sample sizes exist for population subgroups and inferences drawn from this data should be limited to the data with genders combined. Subgroups with very small sample sizes (n < 10) are omitted. These estimates do not include caffeine from foods and beverages that are not marketed as dietary supplement.
Caffeine content of energy drinks in food composition tables
Data on the caffeine content of energy drinks are not readily available in standard food or dietary supplement composition databases. The U.S. Department of Agriculture (USDA) publishes food composition data that include levels of caffeine, but only for foods consumed in dietary studies.11 The Food and Nutrient Database for Dietary Studies is designed for the coding and analysis of food consumption data in the United States. Additionally, several independent (and commissioned) sources of food composition data are available, which are compiled primarily from label information, information published on company websites, and, to a lesser extent, from analytic data.
According to an analysis reported in Consumer Reports, caffeine levels in 27 of the most popular caffeine-containing energy drinks and supplements on the market ranged from 6 mg (a decaffeinated product) to 242 mg per serving.12 Eleven of the products did not have a labelled amount for the caffeine. Of the 16 products that listed the amount of caffeine on the label, the actual level in 5 products was more than 20% higher than the labelled amount and the remaining products were within a 20% deviation.12
Dietary Supplement Ingredient Database
The Dietary Supplement Ingredient Database (DSID) is a federal database developed by the USDA's Agricultural Research Service and the National Institutes of Health's Office of Dietary Supplements. DSID provides analytically derived estimates of the nutrient content of select ingredients in dietary supplements. Ingredients are prioritized for study based on the existence of information on safety concerns, health benefits, public exposure, federal research priorities, and the availability of validated analytical methods and analytical reference materials. Laboratory analyses conducted in 2005 by the DSID investigators found that the caffeine content of 53 dietary supplement products (tablets only) ranged from 0.07 mg to 307 mg caffeine per tablet.2 Twenty-eight of the 53 products provided the amount of caffeine listed on the product label, and 89% of these had analytic values that fell within 16% of the labeled amount.
Dietary Supplement Label Database
The Dietary Supplement Label Database (DSLD) is another public use database that is a collaborative effort of the National Institutes of Health, USDA, and other federal health agencies. DSLD contains complete label information for many dietary supplements sold in the United States. Of the =30,000 products currently in the database, 157 have “energy” in the product name. Of these, 126 are in non-liquid forms (mainly pills) and 31 are in liquid form (serving size =1 fluid ounce). Of the nonliquid forms, 52 (41%) of 126 declared caffeine on the label and 43 (83%) of the 52 provided the amount of caffeine. The corresponding numbers for the liquid forms, 30 (97%) of the 31 product labels declared caffeine and 9 (30%) of the 30 products provided the amount of caffeine.
In contrast to conventional foods, levels of caffeine in dietary supplements can be declared within the Supplement Facts Panel, because caffeine and caffeine sources are regulated as dietary ingredients.13 However, since caffeine is often added as a blend of caffeine-containing botanicals such as guarana, green tea, yerba maté, and kola nut, few labels actually declare the total amount of caffeine. Energy shots generally carry a label claim comparing the caffeine content with the amount of caffeine in a cup of the leading premium brand of coffee (approximately 200 mg), although the specific amount is not declared on product labels.
Evaluation of Drug Abuse Warning Network Public Health Surveillance Data
Another approach to identifying energy drink consumption patterns is to extrapolate information from data provided by the Drug Abuse Warning Network, which is a public health surveillance system. According to the most recent figures, the number of emergency department visits involving energy drinks doubled from 10,068 visits in 2007 to 20,783 in 2011.14 Energy drink–related emergency department visits involving “misuse or abuse” of these drinks also nearly doubled from 3,060 visits in 2007 to 6,090 visits in 2011, accounting for approximately 60% of the visits.14 Males account for approximately two-thirds or more of these visits. Thus, these figures from emergency room visits track with the sales trends mentioned above, which indicate a dramatic uptick in the use of energy drinks in the United States.
Other Sources of Data on Energy Drink Use
According to data compiled by Packaged Facts, energy drink consumers are typically men between the ages of 18 and 34 years.5 This report indicates 5% of adults consume energy drinks at least 5–7 times per month, while less than 2% of adults are defined as heavy users, consuming energy drinks 10 or more times per month.5 Users of these products were more likely to be Hispanic, from the Pacific region of the United States, and adults with children in the household.5
Future Directions
There are numerous challenges to estimating caffeine intakes and usage patterns for caffeine-containing energy products in this rapidly growing and evolving market. Caffeine intake from energy drinks and supplements should be combined with dietary intake estimates from traditional sources to assess the total amount of caffeine in the diet. This dietary assessment of caffeine intake should then be combined with biomarkers of caffeine metabolite excretion to best assess total exposure.
A consistent definition of “energy products” is also needed; while products in this category are generally described as beverages containing caffeine at levels higher than those found in cola-type beverages, and are distinguished from sports drinks as typically containing stimulants like caffeine in addition to other ingredients, not all energy products fit these descriptions. The lack of a consistent definition results in highly variable estimates of sales and exposure to energy products. A need consequently exists to expand federal resources to address potential safety issues with energy drinks. Resources for compiling products in the USDA food composition databases are presently insufficient to keep pace with the rapid growth.
Mandatory declaration of total caffeine levels on product labels is needed to reflect the ever-evolving landscape of the marketplace and to facilitate the necessary updating of food composition information. Updating this information is especially time consuming and expensive when data on caffeine content are not readily available on the products.
Conclusion
The limited data available indicate that a wide variety of caffeine-containing energy products exist in the US marketplace, and sales of these products have been increasing over time; nevertheless, use of caffeine-containing energy products in the overall US population remains low. One important caveat about the NHANES 2007–2010 data reviewed here is that, by design, it reflects a representative sample of the entire US population and is, thus, unlikely to represent those individuals in the highest usage groups (i.e., athletes); another is that it is arguably older than the sales data and may not reflect the most current usage patterns. In addition, the estimates presented in this summary from NHANES do not include caffeine from traditional sources like coffee, tea, and colas, so it is estimated that users of caffeine-containing energy products add approximately 150–200 mg of caffeine per day to their diet from these products alone. From all data sources, it appears that males (∼18–50 years) are the highest users of caffeine-containing energy products.
Acknowledgments
Funding
This work was fully funded by the Office of Dietary Supplement at the National Institutes of Health. , National Institutes of Health
Declarations of interest
Dr Dwyer is a public trustee of the North America branch of the International Life Sciences Institute and has stock in Kraft Foods, Mondelez, Pepsico and Coca Cola companies. None of the other authors have relevant interests to declare.
References
- PubChem Compound. Caffeine. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=2519. Accessed November 1, 2013.
- Andrews KW, Schweitzer A, Zhao C, et al. The caffeine contents of dietary supplements commonly purchased in the US: Analysis of 53 products with caffeine-containing ingredients. Anal Bioanal Chem. 2007;389:231–239. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Guidance for Industry: Factors that Distinguish Liquid Dietary Supplements from Beverages, Considerations Regarding Novel Ingredients, and Labeling for Beverages and Other Conventional Foods. Draft Guidance. December 2009; Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm192702.htm. Accessed November 1, 2013.
- US Food and Drug Administration. Guidance for Industry: A Food Labeling Guide/. 1994 Revised 2013; Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm2006828.htm. Accessed November 1, 2013.
- Packaged Facts. Energy drinks and shots: US market trends. Rockville, MD: Packaged Facts; 2013.
- Haiken M. Can energy drinks kill? The FDA investigates, consumers worry, a business under fire. Forbes. October 23, 2012; Available at: http://www.forbes.com/sites/melaniehaiken/2012/10/23/can-energy-drinks-kill-the-fda-investigates. Accessed November 1, 2013.
- Caffeine Informer. The Top 15 Energy Drink Brands. 2013; Available at: http://www.energyfiend.com/the-15-top-energy-drink-brands. Accessed November 1, 2013.
- Beverage Industry. 2012 State of the Industry: Energy Drinks. July 18, 2012; Available at: http://www.bevindustry.com/articles/85655-consumers-seek-out-energy-boosts. Accessed November 1, 2013.
- Nutrition Business Journal. 2013 Supplement Business Report. 2013; Available at: http://newhope360.com/nbj-2013-supplement-business-report. Accessed November 1, 2013.
- O'Connor C. The mystery monk making billions with 5-hour energy. Forbes. February 8, 2012; Available at: http://www.forbes.com/sites/clareoconnor/2012/02/08/manoj-bhargava-the-mystery-monk-making-billions-with-5-hour-energy/. Accessed November 1, 2013.
- US Department of Agriculture, Agricultural Research Service. What is the USDA Food and Nutrient Database for Dietary Studies (FNDDS)? Available at: http://www.ars.usda.gov/Services/docs.htm?docid=12072#description. Accessed November 1, 2013.
- Consumer Reports magazine. The Buzz on Energy-Drink Caffeine. Caffeine Levels per Serving for the 27 Products We Checked Ranged from 6 Milligrams to 242 Milligrams per Serving.December 2012; Available at: http://www.consumerreports.org/cro/magazine/2012/12/the-buzz-on-energy-drink-caffeine/index.htm. Accessed November 1, 2013.
- US Food and Drug Administration. Dietary Supplement Labeling Guide. April 2005; Available at: http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/dietarysupplements/ucm070597.htm. Accessed November 1, 2013.
- Substance Abuse and Mental Health Service Administration (SAMHSA). The DAWN Report. January 10, 2013; Available at: http://www.samhsa.gov/data/2k13/DAWN126/sr126-energy-drinks-use.pdf. Accessed November 1, 2013.