Abstract
Forage quality and availability, climatic factors, and a wetland’s conservation status are expected to affect the densities of wetland birds. However, the conservation effectiveness is often poorly studied. Here, using twelve years’ census data collected from 78 wetlands in the Yangtze River floodplain, we aimed to understand the effect of these variables on five Anatidae species, and evaluate the effectiveness of the conservation measures by comparing population trends of these species among wetlands that differ in conservations status. We showed that the slope angle of a wetland and the variation thereof best explain the differences in densities of four species. We also found that the population abundances of the Anatidae species generally declined in wetlands along the Yangtze River floodplain over time, with a steeper decline in wetlands with a lower protection status, indicating that current conservation policies might deliver benefits for wintering Anatidae species in China, as population sizes of the species were buffered to some extent against decline in numbers in wetlands with a higher level protection status. We recommend several protection measures to stop the decline of these Anatidae species in wetlands along the Yangtze River floodplain, which are of great importance for the East Asian-Australasian Flyway.
Explaining and predicting animal distributions is one of the central objectives for ecologists and conservation biologists, as the species’ spatial distribution is a key variable in understanding population fluctuations1. Animal distribution is affected by a variety of ecological factors, such as habitat features, climatic factors and resource availability2. Understanding the effects of those factors on animals is still limited at a large scale where a network of wetlands that differ in suitability are included in the range that animals use. This may result in limited effectiveness of current protection measures. This issue is of great importance because the effectiveness of conservation measures along the East Asian-Australasian Flyway, especially in China, urgently needs attention because waterfowl population sizes are continuously declining3. Comparing population trends of a species over areas with different protection statuses can provide information with regard to the effectiveness of the protection measures. However, as long-term census data are often lacking, the effect of protection status on population trends has been poorly studied4 (but see work of Jesper Madsen and colleagues in Denmark5,6). Using census data of five common wintering herbivorous Anatidae species in 78 wetlands in the Yangtze River floodplain in China, we studied which factors affect Anatidae species population densities. We also analysed the species’ population trends and the effect of protection status using time series census data, available for a smaller subset of these lakes, evaluating the effectiveness of the different protection statuses in these wetlands.
Analysis of animal population trends is essential for understanding a species’ population status and, if required, for formulating protection strategies. For instance, population trends of waterbirds species in Europe indicated that loss of grassland feeding habitat negatively affected population sizes7,8. Habitat fragmentation negatively affected forest-nesting migratory birds in the United States9. However, an analysis which linked population trends to the effectiveness of current protection systems is generally lacking4, although conservation biologists and policymakers often assume to understand and address these relationships. Recently, Klein et al.10 found that conservation “paid off”, as waterbird species richness and abundance increased more rapidly in Ramsar wetlands than in unprotected wetlands in Morocco.
Many Anatidae species breed in the northern parts of Siberia, Europe and North America11. During the wintering period, eastern China is one of the hotspots for these migrating species in the world12. Eastern China supports around 1.1 million Anatidae birds and 80% of them use inland wetlands along the Yangtze River floodplain3,13. Meanwhile, these wetlands also offer food and raw materials for tens of millions of people. From 1990–2000, 30% of China’s natural wetlands have been lost due to various factors14. As a consequence, birds species richness in the Yangtze floodplains severely declined3.
In this paper, using systemic survey data from wetlands along the Yangtze River floodplain in 2004 and twelve years survey data (from 2001 to 2012) in four key wintering sites, we analysed the impact of abiotic and biotic factors on the densities of five Anatidae species to provide insight in the underlying causal factors for spatial and temporal changes in population trends, a prerequisite for effective conservation actions. Moreover, we tested the efficiency of conservation actions, and analysed whether the recent decline of Anatidae species is more severe in areas with a lower protection status compared to areas with a higher one. The species of interest were bean goose Anser fabialis, greater white-fronted goose Anser albifrons, lesser white-fronted goose Anser erythropus, swan goose Anser cygnoides and tundra swan Cygnus columbianus bewickii. The species selected are widely distributed in the wetlands in the Yangtze River floodplain with relatively large population sizes. Bean goose, greater and lesser white-fronted goose graze on recessional grassland, while swan goose and tundra swan mainly forage on submerged macrophytes, particularly the tubers of Vallisneria spiralis15,16. Hence, we expected that the grazing goose species would react to changes in e.g., grass availability, but that the tuber-feeding species would not be affected by this. Instead, the tuber-feeding species were expected to be sensitive to rainfall, which changes the availability of the tubers to geese through increasing water levels.
Results
Effect of the ecological variables on bird density
The distribution and abundance of the studied species is shown in Supplementary information Fig. S1–S5. The majority of the variables were not significant in the zero-inflated part of the Poisson model for all species (Table 1). For the Poisson part, most variables were significantly correlated with bird density, although the effects may not be in agreement with our predictions (Table 1).
Table 1. Predicted (H0) and observed effects (+: positive effect; −: negative effect; NS: no effect) of different variables on the bird density of five study species tested for each competing hypotheses using a zero-inflated Poisson regression model (b = regression coefficient, se = standard error, z = calculated z-value, p = significance, AICc = sample size corrected Akaike Information Criterion).
| Species | Model | Variables | H0 | Poisson model |
zero-inflated model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | se | z | p | b | se | z | p | AICC | ||||
| BG | Model I | LA | + | −0.007 | 0.001 | −11.3 | <0.001 | −0.023 | 0.012 | −1.913 | 0.056 | 8218 |
| Model II | TEMP | + | 1.271 | 0.038 | 33.53 | <0.001 | 0.161 | 0.397 | 0.406 | 0.685 | 6913 | |
| MP | + | 0.085 | 0.003 | 26.69 | <0.001 | −0.060 | 0.033 | −1.848 | 0.065 | |||
| Model III | NDVI | + | 3.817 | 0.313 | 12.190 | <0.001 | −0.990 | 4.498 | −0.220 | 0.826 | 8228 | |
| NPP | + | 0.007 | 0.001 | 5.311 | <0.001 | −0.041 | 0.018 | −2.230 | 0.026 | |||
| Model IV† | SLOPE | − | 0.951 | 0.031 | 30.33 | <0.001 | −0.524 | 0.498 | −1.053 | 0.293 | 6554 | |
| SLOPECV | − | −3.008 | 0.095 | −31.59 | <0.001 | −1.428 | 1.125 | −1.269 | 0.204 | |||
| Model V | NDVICV | − | −4.610 | 0.277 | −16.67 | <0.001 | −2.003 | 4.201 | −0.477 | 0.633 | 8124 | |
| GWFG | Model I | LA | + | −0.007 | 0.002 | −4.583 | <0.001 | 0.008 | 0.010 | 0.792 | 0.429 | 4157 |
| Model II | TEMP | + | 0.720 | 0.053 | 13.517 | <0.001 | −0.483 | 0.339 | −1.424 | 0.154 | 3922 | |
| MP | + | 0.016 | 0.004 | 4.156 | <0.001 | 0.091 | 0.038 | 2.379 | 0.017 | |||
| Model III | NDVI | + | 12.690 | 0.551 | 23.020 | <0.001 | −4.038 | 4.554 | −0.887 | 0.375 | 3611 | |
| NPP | + | 0.011 | 0.002 | 6.244 | <0.001 | −0.024 | 0.018 | −1.297 | 0.195 | |||
| Model IV† | SLOPE | − | 0.788 | 0.039 | 20.04 | <0.001 | 0.218 | 0.519 | 0.420 | 0.675 | 3453 | |
| SLOPECV | − | −3.124 | 0.164 | −19.04 | <0.001 | −0.067 | 1.079 | −0.062 | 0.950 | |||
| Model V | NDVICV | − | −4.999 | 0.568 | −8.801 | <0.001 | −0.317 | 4.331 | −0.073 | 0.942 | 4099 | |
| LWFG | Model I | LA | + | −0.008 | 0.001 | −7.065 | <0.001 | −0.013 | 0.008 | −1.558 | 0.119 | 2316 |
| Model II | TEMP | + | 2.907 | 0.189 | 15.40 | <0.001 | −1.421 | 0.998 | −1.424 | 0.154 | 1435 | |
| MP | + | 0.201 | 0.017 | 11.98 | <0.001 | −0.041 | 0.057 | −0.710 | 0.478 | |||
| Model III | NDVI | + | 0.543 | 0.820 | 0.662 | 0.508 | −18.11 | 6.676 | −2.713 | 0.007 | 1608 | |
| NPP | + | 0.080 | 0.004 | 22.023 | <0.001 | 0.011 | 0.028 | 0.400 | 0.689 | |||
| Model IV† | SLOPE | − | 2.261 | 0.074 | 30.51 | <0.001 | 0.184 | 0.723 | 0.254 | 0.800 | 630 | |
| SLOPECV | − | −2.431 | 0.200 | −12.17 | <0.001 | −0.264 | 1.465 | −0.180 | 0.857 | |||
| Model V | NDVICV | − | 2.381 | 0.655 | 3.633 | <0.001 | 12.719 | 6.744 | 1.886 | 0.059 | 2200 | |
| SG | Model I | WA | + | −0.012 | 0.001 | −17.37 | <0.001 | −0.006 | 0.004 | −1.445 | 0.148 | 5129 |
| Model II† | TEMP | + | −3.659 | 0.085 | −42.97 | <0.001 | 0.403 | 0.326 | 1.237 | 0.216 | 1563 | |
| MP | − | −0.134 | 0.004 | −34.90 | <0.001 | −0.080 | 0.036 | −2.228 | 0.026 | |||
| Model III | NDVI | NS | −3.617 | 0.382 | −9.461 | <0.001 | 4.368 | 5.557 | 0.786 | 0.432 | 5613 | |
| NPP | NS | 0.002 | 0.002 | 0.870 | 0.384 | −0.031 | 0.021 | −1.474 | 0.140 | |||
| Model IV | SLOPE | − | −3.184 | 0.131 | −24.37 | <0.001 | −0.485 | 0.670 | −0.723 | 0.470 | 3591 | |
| SLOPECV | − | 5.183 | 0.146 | 35.53 | <0.001 | −1.590 | 1.270 | −1.253 | 0.210 | |||
| Model V | NDVICV | NS | 0.185 | 0.287 | 0.645 | 0.519 | 1.461 | 5.023 | 0.291 | 0.771 | 5708 | |
| TS | Model I | WA | + | −0.024 | 0.001 | −20.58 | <0.001 | −0.010 | 0.008 | −1.294 | 0.196 | 5027 |
| Model II | TEMP | + | 0.571 | 0.041 | 13.807 | <0.001 | 0.207 | 0.272 | 0.761 | 0.446 | 5546 | |
| MP | − | −0.002 | 0.003 | −0.627 | 0.531 | −0.015 | 0.029 | −0.514 | 0.607 | |||
| Model III | NDVI | NS | −4.993 | 0.436 | −11.44 | <0.001 | 2.869 | 4.724 | 0.607 | 0.544 | 5370 | |
| NPP | NS | −0.030 | 0.002 | −18.94 | <0.001 | −0.037 | 0.019 | −1.981 | 0.048 | |||
| Model IV† | SLOPE | − | −2.057 | 0.076 | −27.12 | <0.001 | −0.754 | 0.527 | −1.432 | 0.152 | 4782 | |
| SLOPECV | − | 2.359 | 0.118 | 19.96 | <0.001 | −0.101 | 1.094 | −0.092 | 0.926 | |||
| Model V | NDVICV | NS | −0.722 | 0.364 | −1.983 | 0.047 | 2.099 | 4.368 | 0.481 | 0.631 | 5832 | |
BG: bean goose; GWFG: greater white-fronted goose; LWFG: lesser white-fronted goose; SG: swan goose; TS: tundra swan. For variable abbreviation see Table 4.
†best competing model.
A negative individual-area relationship was found for all studied species (Table 1). Climate (temperature, rainfall) and vegetation availability (NDVI, NPP) variables had positive effects on the grazing birds. NDVI together with its square term yielded significant unimodal models for all grazing species as all these latter models had a positive main term and a negative squared term for these three species (see Supplementary Table S1 online), so a higher bird density was found at intermediate NDVI values. The effects of climate and vegetation availability on tuber-feeding birds were general negative, except for temperature that had a positive effect on tundra swan density. Slope angle variables affected bird densities differently. Slope angle was positively correlated with the grazing bird density, but negatively correlated with that of tuber-feeding birds. In contrast, the coefficient of variance of slope (SLOPECV) negatively affected grazing bird density and positively affected that of tuber-feeding birds. The spatial heterogeneity (NDVICV) negatively influenced the densities of bean goose and greater white-fronted goose, but a positive correlation was found for lesser white-fronted goose. For tuber-feeding birds, there was no effect of spatial heterogeneity on swan goose density, but a marginally significant negative effect was found on densities of tundra swan (Table 1).
According to the AICc values, the slope model was the best model explaining differences in densities of all grazing birds and tundra swans. However, the climate model best explained the density of swan goose (Table 1).
When comparing all subset models, the most parsimonious model (△AICC ≤ 2) was often the most extensive model, including most of the predictor variables (see Supplementary Table S2, S3 online). For each species, the effects of the predictor variables sometimes changed, but were generally in line with our individual predictions (see Supplementary Table S3 online). For example, not in line with our predictions, the model averaging procedure showed that both climate variables had a negative effect on the density of the greater white-fronted goose. The results showed that different mechanisms influence the bird densities of studied species simultaneously.
Species population trends and the effect of the protection status
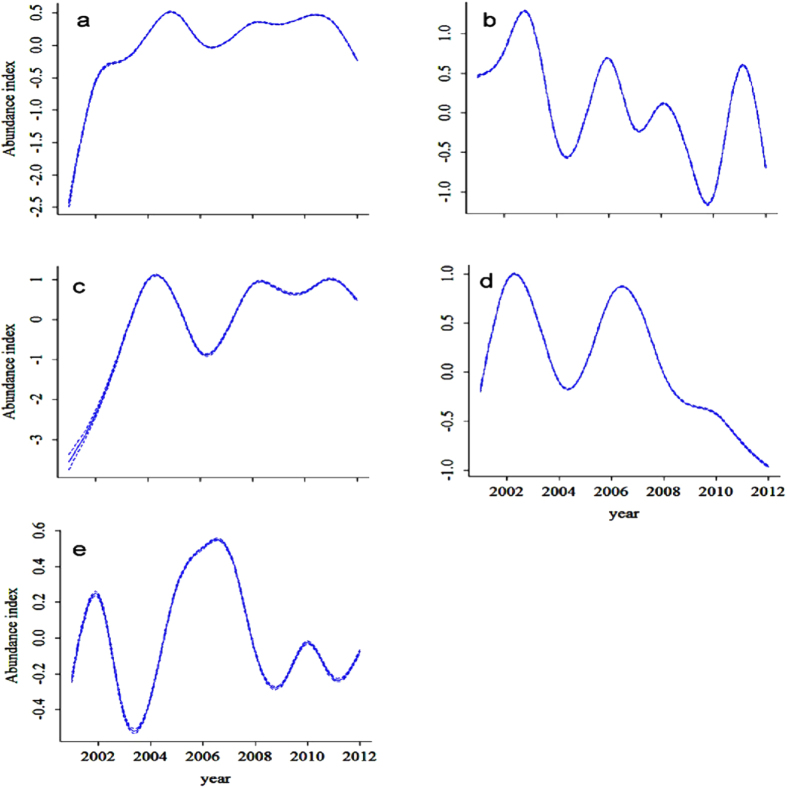
The overall population abundance indices from 2001 to 2012 for the five species varied strongly according to the GAMM-results (Fig. 1). The model yielded a deviance varying from 12.5% (greater white-fronted goose) to 24.9% (bean goose). For all species, year was found to have a smoothing term significantly different from zero (Table 2). The abundance of bean goose and lesser white-fronted goose first showed an increasing trend at the beginning of the decade and then remained stable (Fig. 1a,c). The population size of the greater white-fronted goose fluctuated more and showed an overall decreasing trend (Fig. 1b). Both swan goose and tundra swan numbers decreased, especially in recent years (Fig. 1d,e).
Figure 1. Estimated changes in population sizes of five Anatidae species from 2001 to 2012 in the Yangtze floodplain using Generalized Additive Mixed Models (GAMM).
The solid line shows the population abundance index of each species and the broken lines show the 95% confidence intervals (barely visible, due to small confidence intervals). (a) bean goose; (b) greater white-fronted goose; (c) lesser white-fronted goose; (d) swan goose; (e) tundra swan.
Table 2. Results of the Generalized Additive Mixed Model (GAMM) analysing the overall changes in population sizes of five Anatidae species from 2001 to 2012 in wetlands of the Yangtze floodplain.
| Species | Smooth terms |
Explanatory variables | ||||
|---|---|---|---|---|---|---|
| UBRE | Deviance explained (%) | edf | χ2 | p | site | |
| BG | 5321 | 24.9 | 8.945 | 40391 | <0.001 | <0.001 |
| GWFG | 5574 | 12.5 | 8.976 | 97537 | <0.001 | <0.001 |
| LWFG | 2155 | 15.6 | 8.973 | 33465 | <0.001 | <0.001 |
| SG | 7137 | 20.7 | 8.924 | 223695 | <0.001 | <0.001 |
| TS | 4615 | 12.7 | 8.938 | 49992 | <0.001 | <0.001 |
BG: bean goose; GWFG: greater white-fronted goose; LWFG: lesser white-fronted goose; SG: swan goose; TS: tundra swan. UBRE: Un-Biased Risk Estimator; edf: effective degrees of freedom (n = 78).
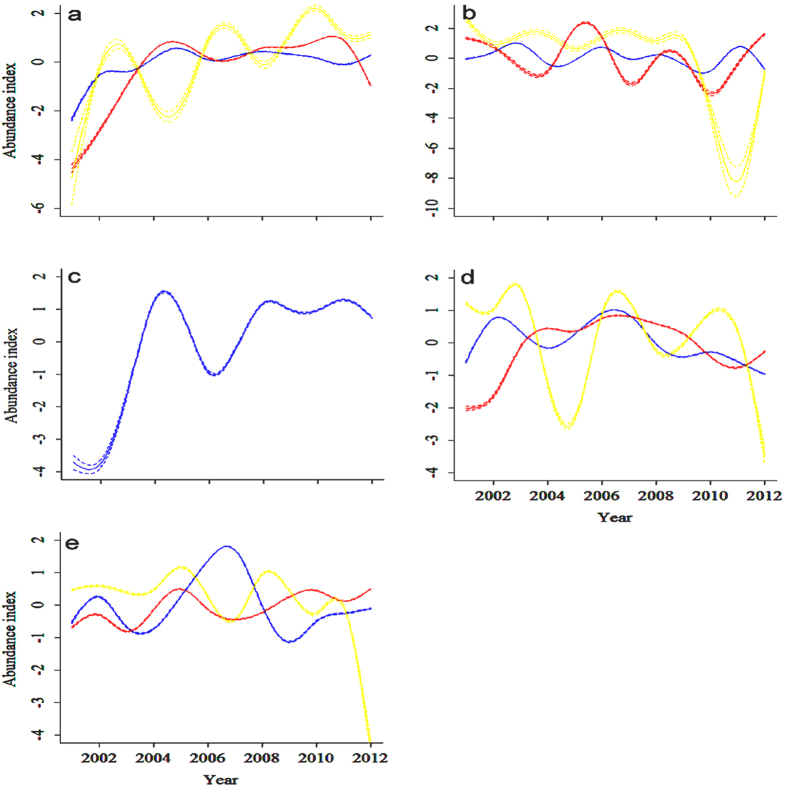
When analysing the effect of protection status, we found that bean goose and greater white-fronted goose showed a similar pattern over the three classes (i.e., national, provincial, and county nature reserve), but fluctuations were larger in reserves under a lower protection status (Fig. 2a,b). Moreover, the decreasing trends of the tuber-feeding birds in recent years in county nature reserves seemed relatively more rapid compared to the trends in national and provincial nature reserves in the floodplains of the Yangtze River (Fig. 2d,e; Table 3).
Figure 2. Population abundance indices of five Anatidae species from 2001 to 2012 in the 25 wetlands differing in protection status in the Yangtze floodplain using Generalized Additive Mixed Models (GAMM). Blue line: wetlands designated as national nature reserves; red line: provincial nature reserves; yellow line: county nature reserves.
The solid line shows the population abundance index of each species and the broken lines show the 95% confidence intervals (barely visible, due to small confidence intervals). (a) bean goose; (b) greater white-fronted goose; (c) lesser white-fronted goose; (d) swan goose; (e) tundra swan. As lesser white-fronted goose was only counted in the national nature reserves, there are no population trends shown in provincial and county nature reserves for this species.
Table 3. Results of the Generalized Additive Model (GAMM) analysing the changes in population sizes of five Anatidae species from 2001 to 2012 in 25 wetlands with different protection statuses in the Yangtze floodplain.
| Species | Smooth terms |
Explanatory variable | |||||
|---|---|---|---|---|---|---|---|
| UBRE | Deviance explained (%) | edf | χ2 | p | site | ||
| National nature reserve (n = 6) | BG | 6735 | 22.2 | 8.976 | 20598 | <0.001 | <0.001 |
| GWFG | 7570 | 43.7 | 8.992 | 121895 | <0.001 | <0.001 | |
| LWFG | 4488 | 17.5 | 8.964 | 22898 | <0.001 | <0.001 | |
| SG | 9486 | 51.5 | 8.978 | 174450 | <0.001 | <0.001 | |
| TS | 3849 | 54.2 | 8.980 | 115464 | <0.001 | <0.001 | |
| Provincial nature reserve (n = 11) | BG | 6655 | 13.8 | 8.922 | 50873 | <0.001 | <0.001 |
| GWFG | 354 | 45.5 | 8.987 | 11794 | <0.001 | <0.001 | |
| LWFG | 39 | 46.1 | 8.888 | 805 | <0.001 | <0.001 | |
| SG | 3352 | 11.5 | 8.959 | 33164 | <0.001 | <0.001 | |
| TS | 4388 | 10.7 | 8.971 | 20799 | <0.001 | <0.001 | |
| County nature reserve (n = 8) | BG | 286 | 22.9 | 8.932 | 4616 | <0.001 | <0.001 |
| GWFG | 339 | 24.9 | 8.988 | 5505 | <0.001 | <0.001 | |
| LWFG | 22 | 58.3 | 6.746 | 253 | <0.001 | <0.001 | |
| SG | 957 | 24.3 | 8.983 | 13950 | <0.001 | <0.001 | |
| TS | 2725 | 11.1 | 8.979 | 13923 | <0.001 | <0.001 | |
BG: bean goose; GWFG: greater white-fronted goose; LWFG: lesser white -fronted goose; SG: swan goose; TS: tundra swan. UBRE: Un-Biased Risk Estimator; edf: effective degrees of freedom.
Discussion
In this study we demonstrated that various ecological variables affected the densities of Anatidae species and the most important variables were slope and climate variables. However, these ecological variables also operated at the same time, as illustrated by the model averaging procedures. Three out of five studied species showed declining population trends with a steep decrease in recent years. Comparing the population trends among wetlands with a different protection status suggested that the largest recent declines in Anatidae species population abundances were mainly recorded from wetlands with a lower level protection status, suggesting that the current conservation policy in national nature reserves might not halt the decline in bird abundance. A larger conservation effort seems required to maintain the Anatidae population, especially for wetlands with a lower level protection status.
Our results showed that majority of the potential ecological variables significantly affected the density of Anatidae species in wetlands along the Yangtze River, although the effects sometimes were contrary to our predictions (Table 1). Slope features best explained differences in densities of all studied species except for swan goose. Partly in agreement with our hypotheses, littoral slopes had a negative effect on tuber-feeding bird density, but a positive effect on the densities of all grazing species (Table 1). Slope has a negative effect on aquatic vegetation occurrence and biomass17 and therefore probably negatively affected density of tuber-feeding birds. However, grazing birds on recessional grasslands may benefit from a gentle slope. For example, a gentle slope is important for an optimal habitat of Canada goose18. A gentle slope may also offer adequate drainage19, which is advantageous to littoral vegetation growth in wetland. The littoral slope in the studied wetlands was relatively flat and gentle (ranging from only 0.00 ~ 2.75°), which may explain the positive effect on grazing bird densities. However, if the range in slope angles would have been larger, we expect to find dome-shaped relationships. The coefficient of variance of these littoral slopes had a negative effect on the density of all grazing birds, but was positively correlated with that of tuber-feeding birds. Lakes with larger variation in slopes had a larger proportion of the area covered by aquatic vegetation20. Swan goose and tundra swan mainly forage on submerged vegetation16, which may explain this positive correlation.
In line with our hypothesis, mean precipitation had a positive effect on grazing bird density and a negative effect on swan goose density, but no effect was found on tundra swans. Also other studies found positive effects of precipitation on bird habitat use and density21. Grassland bird density increased with increasing precipitation22. Higher precipitation increased food availability and resulted in an increase in wintering snow goose (Anser caerulescens) in the USA23. However, a higher precipitation may also result in increasing water levels in wetlands, which decreases the food accessibility for tuber-feeding birds24. The found negative effect of precipitation on swan goose density is therefore expected to come from a reduction in availability of submerged vegetation. Precipitation had no effect on tundra swan density, probably because tundra swans have longer necks and hence have a higher forage availability compared to swan geese.
As predicted, temperature had a positive effect on grazing bird and tundra swan densities (Table 1). Wintering birds tend to select warmer sites to reduce the cost of thermoregulation25. In addition, plant primary productivity is positively correlated with temperature in grassland26. Unexpectedly, we found that temperature negatively influenced densities of swan goose, suggesting that densities of swan goose might be higher in higher latitude areas where temperatures are lower. However, interference competition might also play an important role in determining the distribution of herbivores27, and is mediated by body size28. Both swan goose and tundra swan are tuber-feeding birds, and when these two species forage together, interference competition may occur. Tundra swan, having a larger body size and longer necks, is expected to be the superior species, outcompeting swan goose. Another explanation for the negative effect of temperature on swan goose may be climate warming. Climate warming was a good predictor for a northward shifts in several bird species29,30,31. The reproductive success of waterbirds can be negatively influenced by the long distance migration from their wintering grounds to their breeding grounds32. As the temperatures were relatively high during the survey period, swan goose might decide to winter at higher latitude wetlands, and thereby minimize their migration distance.
Not in accordance with our predictions and former studies33 was that area was negatively correlated with the bird densities for all studied species, resulting in lower bird densities in lakes with larger areas available for foraging. Human activities in larger lakes may play an important role in affecting bird densities. For example, sand mining decreased food availability for birds34 and thereafter the density of birds in larger wetlands. It is also possible that population sizes of studied species was relatively low, resulting in lower densities in larger wetlands. For tuber-feeding species, the negative relation between area and birds densities may be partly explained by the uneven distribution of submerged aquatic vegetation among and within wetlands.
NPP had a positive effect on grazing bird densities (Table 1). NDVI yielded significant unimodal models for all three grazing bird species (see Supplementary Table S1 online). Following the forage maturation hypothesis35, the densities of these grazing birds first increased with increasing resource availability to a maximum level and then decreased. However, for tuber-feeding birds, NDVI and NPP had negative effects. Carex spp., perennial sedges that occur in dense patches, are the dominate species of these recessional wetlands in winter. In summer, Carex spp. beds are flooded while the roots remain buried in the soil, which may prohibit the establishment and development of V. spiralis, explaining the negative correlation of NDVI and NPP on densities of tuber-feeding birds.
As expected, habitat spatial heterogeneity (NDVICV) had a negative effect on bird densities of bean goose and greater white-fronted goose and no effect on the densities of both tuber-feeding species. The positive effect on lesser white-fronted goose is probably influenced by its restricted distribution range, because the majority of lesser white-fronted goose was counted in East Dongting Lake National Reserve36 (Supplementary information, Fig. S3), biasing our analysis.
The results of model averaging showed that the most parsimonious model was often the most extensive model, indicating that different response variables influence bird densities at the same time (see Supplementary Table S2, S3 online). The derived correlation coefficients were generally similar between the single term models and the parsimonious multiple variables models. So, when testing several competing hypotheses, the interdependencies of those predictions should also be considered.
The recent decline of Anatidae species was more severe in areas with a lower protection status compared to areas with a higher one, which is in agreement with our expectations. Our results indicated that current conservation policies might deliver benefits for wintering Anatidae species in China, as population sizes of the studied species were buffered to some extent against a decline in numbers in wetlands with a higher level protection status. The funding that national nature reserves receive is twice as large as that of local nature reserves and the staff working in the national nature reserves have better training opportunities comparing to staff of local nature reserves37. Reserve staff are able to take action when more funding is received, e.g., to improve wildlife protection. For example, in some national nature reserves, extra food is provided during periods when animals face food shortages. Reserves with more funding and/or a higher protection status also initiate community programs and contribute to increase the local community’s awareness, enhancing their sense of responsibility and acceptation of protection actions. In contrast, insufficient funding often leads to increased economic activities within reserves, such as the exploitation of natural resources and tourism activities34,37.
Our results, together with the studies in Europe38,39 and Africa10, generate a preliminary framework to evaluate the effectiveness of conservation policies. However, our analyses also had limitations as our census data were all collected from protected areas. Because of land use changes, wild birds can change their wintering site and select protected conservation areas over unprotected areas40. Hence, survey efforts should be broadened to cover both protected and unprotected areas in order to acquire a better understanding of the effectiveness of conservation policies.
Application
In China, a comprehensive understanding of the spatial differences in the densities of wintering waterfowl under influence of ecological variables is still missing, reducing efficiency of protection actions. Based on our study, we suggest that hydrological regimes should be optimized to provide forage during the entire wintering period for migratory herbivorous Anatidae species. The majority of lakes along the Yangtze is connected to the Yangtze river through sluices so that management of water level heights for conservation actions is feasible. For example, through hydrological regulation, the areas of recessional grasslands for wintering birds during certain periods of the year can be increased. Water level regulation can facilitate Anatidae species grazing and regrazing by carefully timing the moment of exposure of these recessional wetlands. A sudden increase in suitable habitat will only provide preferred food in a short period, after which a “grass-sea” takes over, i.e., a wetland with a large proportion of tall and lower quality sedges. Hence, a collaborating, multidisciplinary conservation network should be built in order to formulate a scientific sound basis for protection strategies for migrating Anatidae species over a network of wetlands.
To better evaluate the effectiveness of the protection actions, a systematic annual waterbirds survey should be carried out both in protected and unprotected areas by Chinese government departments such as the state forest bureaus in collaboration with scientists, and the data should be freely available. For example, the North American Breeding Bird Survey (BBS) was initiated in 1966 and the survey is conducted every year. The main objective is to track the status and trends of North American bird populations and data can be retrieved freely from a public website. In the Netherlands, SOVON started in 1973, carrying out standardised annual national bird surveys. We strongly advocate that China starts an annual wintering birds survey, offering a basis for current and future conservation work.
Furthermore, we suggest that it is time to involve birdwatchers and volunteers in China’s conservation network. Larger survey projects can strongly benefit from contributions from birdwatchers and volunteers. Birdwatchers and volunteers are often highly motivated and skilled, and can contribute to surveys. For example, thousands of volunteer birdwatchers participated in the Breeding Bird Survey in the UK. Nowadays, the number of birdwatchers is increasing in China and they can contribute to the necessary bird surveys.
Finally, we claim that nature reserves with a lower protection status should also be given more attention in terms of investment, local community education and research efforts. Some lower protection status wetlands, such as the Anhui Anqing Yangtze Riverine Provincial Nature Reserve, could be upgraded to a national nature reserve to increase the conservation efforts in this important wetland. Moreover, even the national nature reserves are apparently not sufficient to stop the decline of the Anatidae birds, and thus additional measures are required. We therefore call for an in-depth investigation into the decline of Anatidae species in the East Asian-Australasian Flyway, as contrasted to the successes of the American and European counterparts.
Methods
Census data
Data from the studied five Anatidae species was obtained from the middle-lower Yangtze River floodplain survey carried out in February 2004, the first comprehensive survey in this area41. All selected species are herbivorous birds wintering in the wetlands in the Yangtze River floodplain13. We only selected data from lakes, whereas estuaries and shoals were excluded from the analysis. The whole dataset included 78 lakes over 5 provinces (see Supplementary Table S4 online). Another dataset was obtained from a systematic survey in four nature reserves (Poyang Hu, Dongting Hu, Shengjin Hu and Anqing lakes) of waterbirds in the winters from 2000/1 through 2011/12 (see Supplementary Table S5 online). The “look-see” counting method is commonly used to count waterbirds42 and was used for all surveys. The“look-see” counting method required the observers to be familiar with the species involved and their habitat-preferences42. Multiple methods were used to access the wetlands and birds, but in most cases cars were employed to reach the target areas as close as possible and then the observers proceeded on foot. Most Anatidae often gather in large visible flocks during the wintering season, making them easy to locate and count43. The surveys were conducted by staff of the nature reserve and by the authors using the same survey methods; detailed survey methods are described in Barter et al.41.
Variables
Lake land and water area
Previous studies have pointed out that habitat area positively affects bird density33,44. Grazing Anatidae species wintering in the Yangtze River floodplain mainly feed on recessional grasslands. The size of the grassland that is exposed, and hence available to grazing birds for foraging, increases with decreasing lake water levels and thereby affects the density of these birds. We related the density of tuber-feeding birds to lake water area as they mainly forage on submerged V. spiralis tubers15,16. For tuber-feeding birds a similar positive relationship was expected, although the size of the lake area is positively correlated to height of the water level, and therefore maybe negatively with the accessibility of the tubers24. We measured lake land and water area of the studied 78 wetlands during the wintering survey in 2004 using satellite images. The data description is shown in Table 4, with detailed methods available in the Supplementary information Appendix S1.
Table 4. Potential predictor variables, abbreviations, data sources and resolutions used to analyse differences in species abundance in wetlands of the Yangtze River floodplain.
| Variables | Abbreviation | Unit | Range | Source | Resolution |
|---|---|---|---|---|---|
| Lake land area | LA | km2 | 0.20 ~ 216.04 | landsat TM/ETM+ | 30 m |
| Water area | WA | km2 | 0.13 ~ 1612.16 | landsat TM/ETM+ | 30 m |
| February mean air temperature | TEMP | °C | 7.30 ~ 11.20 | http://www.cma.gov.cn/2011qxfw/2011qsjgx/ | 0.5° × 0.5° |
| Mean January precipitation | MP | mm | 3.70 ~ 158.60 | http://www.cma.gov.cn/2011qxfw/2011qsjgx/ | 0.5° × 0.5° |
| Littoral slopes | SLOPE | ° | 0.00 ~ 2.75 | http://srtm.csi.cgiar.org | 90 m |
| Coefficient of variance of littoral slopes | SLOPECV | no unit | 0.00 ~ 1.49 | http://srtm.csi.cgiar.org | 90 m |
| Normalized difference vegetation index | NDVI | no unit | 0.20 ~ 0.43 | landsat TM/ETM+ | 30 m |
| Net primary productivity | NPP | g/m2 month−1 | 52.00 ~ 98.60 | http://neo.sci.gsfc.nasa.gov/ | 0.1° × 0.1° |
| Habitat heterogeneity | NDVICV | no unit | 0.08 ~ 0.35 | landsat TM/ETM+ | 30 m |
Littoral slopes
Vegetation growth is often affected by lake morphology such as littoral slopes. Littoral slopes negatively affect vegetation occurrence and biomass17 and thereby also the densities of herbivorous Anatidae species18. A gentle slope is therefore more suitable for vegetation development in wetlands20,45. Thus, we predict that Anatidae species densities will be negatively correlated with the mean littoral slope angle. In addition, variation of the wetlands’ littoral slope angles may also affect vegetation growth, with highest growth rates and biomass often found on gentle slopes46. We hence predicted a negative effect of the coefficient of variation (CV) of littoral slope angles on bird densities. We calculated the average and CV of littoral slope angles of each lake using Shuttle Radar Topography Mission (SRTM) digital elevation data from February 2000 (Table 4) as topography changes were negligible from 2000 to 2004.
Climate data
Weather conditions can affect bird distribution and density through changing temperatures and precipitation47. The abundance of wintering birds normally decreased with decreasing temperatures in winter47 (but see Ridgill & Fox48). Root49 suggested that this could be explained by the species’ energy expenditure. Moreover, plant primary productivity is positively correlated with temperature. We therefore expected that bird densities will be positively correlated with temperature. Precipitation positively affects plant primary productivity26, but these effects often have a time lag in influencing vegetation availability of about a month50. We therefore also related mean January (i.e. the previous month for the surveys) precipitation to the densities of grazing birds, expecting a positive effect. However, lake water level increases with increasing precipitation, and the food accessibility for tuber-feeding birds, which is dependent on water depth and the bird’s neck length, therefore decreases24. Hence, we predicted that densities of tuber-feeding birds will be negatively correlated with mean precipitation. Monthly mean air temperatures and precipitation were obtained from the China Meteorological Administration (Table 4).
Normalized Difference Vegetation Index (NDVI) and Net Primary Productivity (NPP)
Forage quantity is an important variable in determining animal distribution51,52. So NDVI and NPP were used as predictors in the density analyses of grazing birds. As functional response curves suggest that animal densities are correlated to forage biomass through a unimodal relationship52, we hence included its square terms, NDVI2 and NPP2, in the analysis. For tuber-feeding birds, we expected that NDVI and NPP have no effect on bird density as these species mainly forage on tubers but not on grass. We calculated the mean NPP (Table 4) per lake, and the mean NDVI for only recessional grasslands per lake using the satellite images (see Supplementary Table S6 online). The detailed image processing methods are available in the Supplementary information, Appendix S1.
Habitat heterogeneity
Studies showed that habitat heterogeneity can decrease foraging efficiency of grazers by increasing searching and handling times53. Intake rates of herbivores are generally lower while feeding on heterogeneous swards compared to homogenous swards, such as shown for several overwintering waterbird species (e.g., Anser spp., Anas spp.)52 and habitat heterogeneity is therefore expected to affect grazing bird density negatively, but not affect tuber-feeding bird density. We calculated the CV of NDVI from the different pixels in the same period (see above) as an index of the spatial heterogeneity in forage availability at these recessional grasslands for each lake, expecting a negative correlation with bird density (Table 4).
Protection status
Establishing protected area is a cornerstone for maintaining the global biodiversity54. Birds species benefitted from various conservation measures in Europe38,39. Moreover, waterbirds increased more rapidly in Ramsar-designated wetlands in Morocco compared to unprotected wetlands10.
China’s protected area system includes national, provincial, city and county nature reserves, with some wetlands designated as Ramsar sites. Provincial, city and county nature reserves are often poorly managed because of reduced funding compared to national nature reserves37. As city nature reserves were not available in our second dataset, we therefore categorized our research lakes into national, provincial, and county nature reserve according to the list of China’s nature reserves (State Ministry of Environmental Protection 2012). We predicted that national reserves would have a stronger positive effect on population trends compared to the wetlands with a lower protection status.
Statistical analysis
Following the above reasoning we formulated a set of working hypotheses. Model I represents the effect of habitat area on the bird density of Anatidae species (Individual-area relationship). Model II, III, IV, V represent effect of climate, vegetation availability, slope and spatial heterogeneity respectively (Table 5).
Table 5. Theoretical models expected to affect the densities of Anatidae species in wetlands.
| Theoretical model | LA/WA | TEMP | MP | SLOPE | SLOPECV | NDVI | NPP | NDVICV |
|---|---|---|---|---|---|---|---|---|
| Model I | ||||||||
| Individual-area relationship | X | |||||||
| Model II | ||||||||
| Climate | X | X | ||||||
| Model III | ||||||||
| Slope | X | X | ||||||
| Model IV | ||||||||
| Vegetation availability | X | X | ||||||
| Model V | ||||||||
| Spatial heterogeneity | X |
Count data often include many zero observations. Poisson regression can be used to model the relationship between species abundance and environmental variables, but zero-inflated Poisson models often perform better than Poisson models or zero-inflated negative binomial models55. Hence, a zero-inflated Poisson model was applied to analyse the effects of different ecological variables on bird densities. A zero-inflated Poisson model includes two parts: a Poisson model and a zero-inflated model. The zero-inflated part provides insight on variables influencing the species’ presence/absence while the Poisson part provides insight on the variables affecting the species’ density. We performed a zero-inflated Poisson regression analysis for each of the hypotheses. The Akaike Information Criterion (AIC), adjusted for small sample sizes (AICC), was used to rank the competing models. Before fitting the zero-inflated Poisson models, we assessed the multi-collinearity by examining the Variance Inflation Factor (VIF) of the candidate variables, by including all candidate variables as independent variables in a regression model with animal density as response variable. VIF values of all variables were less than 4 (see Supplementary Table S7 online), indicating that there was no multi-collinearity problem56.
Furthermore, different mechanisms may influence the density of each species at the same time, but distinguishing their independent effect is a challenging task57. Hence, zero-inflated Poisson models were also used to test for the combined and independent influence of the predictor variables on the densities of each of the species. All possible subset models were ranked according to △AICc and Akaike weights (ωi) were calculated to estimate the likelihood of each model58. Model averaging was used to obtain parameter estimates for these variables. The model averaging calculation was done on the most parsimonious models using a cut-off △AICC ≤ 258.
To analyse population trends for each of the five waterbird species, a Generalized Additive Mixed Model (GAMM) was applied using the time series survey data (2001–2012) from 25 wetlands in the four nature reserves where birds counts were carried out annually, with province as random factor. The GAMM model accommodates for smooth, nonlinear changes over time in population size59. In the model (Eq. 1), yij is the expected bird count at site i and year j. The expected count therefore depends on the site effect ai and the smoother s(j). The analysis was done in two parts: we first analysed the overall population trends of each species in these wetlands. Then another GAMM was applied for each species but separately for the wetlands with a different protection status (national, provincial, and county). We used a GAMM with a Poisson distribution and a log link function (Eq. 2).
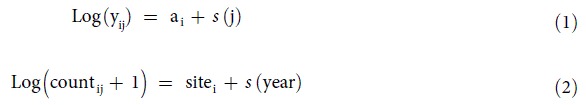 |
Spatial autocorrelation is a potential problem when analysing ecological data and should be properly accounted for. We therefore explored whether there was spatial autocorrelation in birds abundances over different wetlands by calculating the Moran’s I index of the residuals for each species. We found little evidence for spatial autocorrelation of studied species (all |Moran’s I| < 0.05) which suggested that spatial autocorrelation was not a point of concern in our analysis. All statistical analyses were conducted in R 2.13.060 with the package pscl, MuMIn, mgcv and ape.
Additional Information
How to cite this article: Zhang, Y. et al. Effect of conservation efforts and ecological variables on waterbird population sizes in wetlands of the Yangtze River. Sci. Rep. 5, 17136; doi: 10.1038/srep17136 (2015).
Supplementary Material
Acknowledgments
We thank Dr. David Kleijn (Wageningen University) for the discussions and comments on this study. We thank the staff from Poyang Lake National Nature Reserve, East Dongting Lake National Nature Reserve, Shengjin Lake National Nature Reserve and Anhui Anqing Yangtze Riverine Provincial Nature Reserve for sharing annual census data with us. The comprehensive survey in 2004 and 2005 was funded by WWF. We also want to acknowledge the late Mark Barter, and thank Prof. Anthony David Fox (Aarhus University), Peihao Cong, Xin Wang, Meijuan Zhao, Qiang Jia, Yuzhan Yang, Yan Chen and Qing Zhu for helping us to carry out the waterbirds census in East Dongting Lake, Shengjin Lake and Anqing Lake. Yong Zhang gratefully acknowledges the support from the CAS-KNAW Joint PhD Training Programme.
Footnotes
Author Contributions Conceived and designed the study: Y.Z., L.C., W.F.D.B. and H.H.T.P. Performed the study: Y.Z, Q.J. and L.C. Analyzed the data: Y.Z. and Q.J. Wrote the paper: Y.Z., Q.J., W.F.D.B. and H.H.T.P. All authors reviewed the manuscript.
References
- Hanski I. Metapopulation ecology. (Oxford University Press 1999). [Google Scholar]
- De Boer W. F. et al. Understanding spatial differences in African elephant densities and occurrence, a continent-wide analysis. Biol. Conserv. 159, 468–476 (2013). [Google Scholar]
- De Boer W. F. et al. Comparing the community composition of European and Eastern Chinese waterbirds and the influence of human factors on the China waterbird community. Ambio 40, 68–77 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn D., Rundlof M., Scheper J., Smith H. G. & Tscharntke T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 26, 474–481 (2011). [DOI] [PubMed] [Google Scholar]
- Madsen J., Pihl S. & Clausen P. Establishing a reserve network for waterfowl in Denmark: a biological evaluation of needs and consequences. Biol. Conserv. 85, 241–255 (1998). [Google Scholar]
- Madsen J. Experimental refuges for migratory waterfowl in Danish wetlands. I. Baseline assessment of the disturbance effects of recreational activities. J. Appl. Ecol. 35, 386–397 (1998). [Google Scholar]
- Musilová Z., Musil P., Zouhar J. & Romportl D. Long-term trends, total numbers and species richness of increasing waterbird populations at sites on the edge of their winter range: cold-weather refuge sites are more important than protected sites. J. Ornithol. 156, 923–932 (2015). [Google Scholar]
- Duncan P. et al. Long-term changes in agricultural practices and wildfowling in an internationally important wetland, and their effects on the guild of wintering ducks. J. Appl. Ecol. 36, 11–23 (1999). [Google Scholar]
- Donovan T. M. & Flather C. H. Relationships among north American songbird trends, habitat fragmentation, and landscape occupancy. Ecol. Appl. 12, 364–374 (2002). [Google Scholar]
- Kleijn D., Cherkaoui I., Goedhart P. W., van der Hout J. & Lammertsma D. Waterbirds increase more rapidly in ramsar designated wetlands than in unprotected wetlands. J. Appl. Ecol. 51, 289–298 (2014). [Google Scholar]
- Kear J. Ducks, geese and swans. (Oxford University Press, 2005). [Google Scholar]
- Dalby L., McGill B. J., Fox A. D. & Svenning J. C. Seasonality drives global-scale diversity patterns in waterfowl (Anseriformes) via temporal niche exploitation. Global Ecol. Biogeogr. 23, 550–562 (2014). [Google Scholar]
- Cao L., Zhang Y., Barter M. & Lei G. Anatidae in eastern China during the non-breeding season: Geographical distributions and protection status. Biol. Conserv. 143, 650–659 (2010). [Google Scholar]
- Gong P. et al. China’s wetland change (1990–2000) determined by remote sensing. Sci. China-Earth Sci. 53, 1036–1042 (2010). [Google Scholar]
- Zhang Y. et al. Changing distribution and abundance of Swan Goose Anser cygnoides in the Yangtze River floodplain: the likely loss of a very important wintering site. Bird Conserv. Int. 21, 36–48 (2010). [Google Scholar]
- Fox A. D. et al. Declines in the tuber-feeding waterbird guild at Shengjin Lake National Nature Reserve, China - a barometer of submerged macrophyte collapse. Aquat. Conserv. Mar. Freshwat. Ecosyst. 21, 82–91 (2011). [Google Scholar]
- Partanen S., Luoto M. & Hellsten S. Habitat level determinants of emergent macrophyte occurrence, extension and change in two large boreal lakes in Finland. Aquat. Bot. 90, 261–268 (2009). [Google Scholar]
- Dunton E. M. & Combs D. L. Movements, habitat selection, associations, and survival of giant Canada goose broods in central Tennessee. Hum-Wild. Interact. 4, 192–201 (2010). [Google Scholar]
- Wisz M. et al. Modelling pink-footed goose (Anser brachyrhynchus) wintering distributions for the year 2050: potential effects of land-use change in Europe. Divers. Distrib. 14, 721–731 (2008). [Google Scholar]
- Kolada A. The effect of lake morphology on aquatic vegetation development and changes under the influence of eutrophication. Ecol. Indicators 38, 282–293 (2014). [Google Scholar]
- Tingley M. W., Monahan W. B., Beissinger S. R. & Moritz C. Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci. USA 106, 19637–19643 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Duarte A. et al. Factors influencing habitat use by migratory grassland birds in the State of Chihuahua, Mexico. Auk 126, 896–905 (2009). [Google Scholar]
- Hobaugh W. C. Habitat use by Snow Geese wintering in southeast Texas. J. Wildl. Manage. 48, 1085–1096 (1984). [Google Scholar]
- Nolet B. A., Fuld V. N. & van Rijswijk M. E. C. Foraging costs and accessibility as determinants of giving-up densities in a swan-pondweed system. Oikos 112, 353–362 (2006). [Google Scholar]
- Villen-Perez S., Carrascal L. M. & Seoane J. Foraging patch selection in winter: A balance between predation risk and thermoregulation benefit. PLoS One 8, e6844 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milchunas D. G. & Lauenroth W. K. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 63, 327–366 (1993). [Google Scholar]
- Kristiansen J. N. & Jarrett N. S. Inter-specific competition between Greenland White-fronted Geese Anser albifrons flavirostris and Canada Geese Branta canadensis interior moulting in West Greenland: Mechanisms and consequences. Ardea 90, 1–13 (2002). [Google Scholar]
- Smith R. D., Ruxton G. D. & Cresswell W. Dominance and feeding interference in small groups of Blackbirds. Behav. Ecol. 12, 475–481 (2001). [Google Scholar]
- Jensen R. A. et al. Prediction of the distribution of Arctic-nesting pink-footed geese under a warmer climate scenario. Glob. Chang. Biol. 14, 1–10 (2008). [Google Scholar]
- Lehikoinen A. et al. Rapid climate driven shifts in wintering distributions of three common waterbird species. Glob. Chang. Biol. 19, 2071–2081 (2013). [DOI] [PubMed] [Google Scholar]
- La Sorte F. A. & Thompson F. R. Poleward shifts in winter ranges of North American birds. Ecology 88, 1803–1812 (2007). [DOI] [PubMed] [Google Scholar]
- Alerstam T., Christie D. & Ulfstrand A. Bird migration. (Cambridge University Press, 1990). [Google Scholar]
- Connor E. F., Courtney A. C. & Yoder J. M. Individuals-area relationships: The relationship between animal population density and area. Ecology 81, 734–748 (2000). [Google Scholar]
- Wu G. F., De Leeuw J., Skidmore A. K., Prins H. H. T. & Liu Y. L. Concurrent monitoring of vessels and water turbidity enhances the strength of evidence in remotely sensed dredging impact assessment. Water Res. 41, 3271–3280 (2007). [DOI] [PubMed] [Google Scholar]
- Hassall M., Riddington R. & Helden A. Foraging behaviour of Brent Geese, Branta b. bernicla, on grasslands: effects of sward length and nitrogen content. Oecologia 127, 97–104 (2001). [DOI] [PubMed] [Google Scholar]
- Wang X., Fox A. D., Cong P. H. & Cao L. Food constraints explain the restricted distribution of wintering Lesser White-fronted Geese Anser erythropus in China. Ibis 155, 576–92 (2013). [Google Scholar]
- Liu J. G. et al. Protecting China’s biodiversity. Science 300, 1240–1241 (2003). [DOI] [PubMed] [Google Scholar]
- Donald P. F. et al. International conservation policy delivers benefits for birds in Europe. Science 317, 810–813 (2007). [DOI] [PubMed] [Google Scholar]
- Hiley J. R., Bradbury R. B., Holling M. & Thomas C. D. Protected areas act as establishment centres for species colonizing the UK. Proc. R. Soc. B-Biol. Sci. 280(1760), 20122310 (2013). [DOI] [PMC free article] [PubMed]
- Pouzols F. M. et al. Global protected area expansion is compromised by projected land-use and parochialism. Nature 516, 383–386 (2014). [DOI] [PubMed] [Google Scholar]
- Barter M., Cao L., Chen L. & Lei G. Results of a survey for waterbirds in the lower Yangtze floodplain, China, in January-February 2004. Forktail 21, 1–7 (2005). [Google Scholar]
- Bibby C. J., Burgess N. D., Hill D. A. & Mustoe M. Bird census techniques. (Academic press, 2000). [Google Scholar]
- Wood K. A. et al. The role of season and social grouping on habitat use by Mute Swans (Cygnus olor) in a lowland river catchment. Bird Study 60, 229–237 (2013). [Google Scholar]
- Zhang Y., Jia Q., Prins H. H., Cao L. & de Boer W. F. Individual-area relationship best explains goose species density in wetlands. PLoS One 10, e0124972 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudds T. D., Elmberg J., Sjoberg K., Poysa H. & Nummi P. Ecomorphology in breeding Holarctic dabbling ducks: the importance of lamellar density and body length varies with habitat type. Oikos 91, 583–588, (2000). [Google Scholar]
- Zohary T. & Gasith A. The littoral zone in Lake Kinneret (ed. Zohary et al. ) 517–532 (Springer, 2014). [Google Scholar]
- Meehan T. D., Jetz W. & Brown J. H. Energetic determinants of abundance in winter landbird communities. Ecol. Lett. 7, 532–537 (2004). [Google Scholar]
- Ridgill S. & Fox A. D. Cold weather movements of waterfowl in western Europe . IWRB Special Publication (1990). [Google Scholar]
- Root T. Energy constraints on avian distributions and abundances. Ecology 69, 330–339 (1988). [Google Scholar]
- Bayliss P. Population-dynamics of Magpie Geese in relation to rainfall and density - Implications for harvest models in a fluctuating environment. J. Appl. Ecol. 26, 913–924 (1989). [Google Scholar]
- Van Gils J. A., Gyimesi A. & Van Lith B. Avian herbivory: An experiment, a field test, and an allometric comparison with mammals. Ecology 88, 2926–2935 (2007). [DOI] [PubMed] [Google Scholar]
- Heuermann N., van Langevelde F., van Wieren S. E. & Prins H. H. T. Increased searching and handling effort in tall swards lead to a Type IV functional response in small grazing herbivores. Oecologia 166, 659–669 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley L. A. The influence of bite size on foraging at larger spatial and temporal scales by mammalian herbivores. Oikos 116, 1964–1974 (2007). [Google Scholar]
- Gaston K. J., Jackson S. E., Cantu-Salazar L. & Cruz-Pinon G. The Ecological Performance of Protected Areas. Annu. Rev. Ecol. Evol. Syst. 39, 93–113 (2008). [Google Scholar]
- Joseph L. N., Elkin C., Martin T. G. & Possingham H. P. Modeling abundance using N-mixture models: the importance of considering ecological mechanisms. Ecol. Appl. 19, 631–642 (2009). [DOI] [PubMed] [Google Scholar]
- O’Brien R. M. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity 41, 673–690 (2007). [Google Scholar]
- Currie D. J. et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (2004). [Google Scholar]
- Burnham K. P. & Anderson D. R. Model selection and multi-model inference: a practical information-theoretic approach. (Springer-Verlag 2002). [Google Scholar]
- Fewster R. M., Buckland S. T., Siriwardena G. M., Baillie S. R. & Wilson J. D. Analysis of population trends for farmland birds using generalized additive models. Ecology 81, 1970–1984 (2000). [Google Scholar]
- Core Team R. (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.