Alopecia is a frequent adverse event of targeted therapies, and can significantly impair patients’ health-related quality of life. The incidence and risk with targeted agents is significantly higher than a placebo but lower than with chemotherapy. Our findings may facilitate decisions on therapy, pre-therapy counseling, evidence-based management strategies and identification of risk factors.
Keywords: alopecia, adverse events, antineoplastic agents, drug-induced alopecia, hair loss, targeted therapies
Abstract
Background
The introduction of molecularly targeted anticancer therapies presents new challenges, among which dermatologic adverse events are noteworthy. Alopecia in particular is frequently reported, but the true incidence is not known.
Patients and methods
We sought to ascertain the incidence and risk of developing alopecia during treatment with approved inhibitors of oncogenic pathways and molecules [anaplastic lymphoma kinase, breakpoint cluster region-abelson, B-rapidly accelerated fibrosarcoma, Bruton's tyrosine kinase, cytotoxic T-lymphocyte antigen-4, epidermal growth factor receptor, human epidermal growth factor receptor-2, Janus kinase, MAPK/ERK (extracellular signal-regulated kinase) Kinase, mammalian target of rapamycin, smoothened, vascular endothelial growth factor, vascular endothelial growth factor receptor, platelet derived growth factor receptor; proteasomes; CD20, CD30, CD52]. Electronic database (PubMed, Web of Science) and ASCO meeting abstract searches were conducted to identify clinical trials reporting alopecia. Meta-analysis was conducted utilizing fixed- or random-effects models.
Results
The calculated overall incidence of all-grade alopecia was 14.7% [95% confidence interval (CI) 12.6% to 17.2%]—lowest with bortezomib, 2.2% (95% CI 0.4% to 10.9%), and highest with vismodegib, 56.9% (95% CI 50.5% to 63.1%). There was an increased risk of all-grade alopecia [relative risk (RR), 7.9 (95% CI 6.2–10.09, P ≤ 0.01)] compared with placebo, but when compared with chemotherapy, the risk was lower [RR, 0.32 (95% CI 0.2–0.55, P ≤ 0.01)].
Conclusions
Targeted therapies are associated with an increased risk of alopecia.
introduction
Significant advances in the field of cancer biology have spurred the development of several molecularly targeted anticancer therapies, which have shown impressive clinical benefit in terms of efficacy and survival rates. As a result, several agents have received marketing approval over the last decade for the treatment of various cancers. Unlike conventional cytotoxic chemotherapeutic agents, these drugs selectively target pro-oncogenic pathways/molecules crucial to tumor growth and survival. Although this action circumvents the severe adverse events (AEs) associated with conventional chemotherapies (e.g. myelosuppression, nausea, vomiting), a wide range of other AEs that affect nearly all organ systems continue to be increasingly recognized.
Among these, dermatologic manifestations are most noteworthy and include rashes, xerosis, pruritus, paronychia, mucositis, and hair disorders [1]. The latter comprise alopecia, textural changes, trichomegaly, and hair dyspigmentation, reported primarily with the epidermal growth factor receptor (EGFR) inhibitors [2, 3]. Although these alterations may not be dose-limiting or life-threatening, the impact on psychosocial well-being and body image, and the resulting anxiety and distress bear the potential to impair patients' health-related quality of life (HRQoL).
This aspect is well recognized in patients with chemotherapy-induced alopecia (CIA), which has an estimated overall incidence of 65% [4]. For example, women with breast cancer may find alopecia very traumatizing and distressing, and subsequently refuse treatment—some women describe the experience as more difficult than even losing a breast [5–7]. With similar studies and estimates lacking in patients receiving targeted therapies, the clinical scenario of alopecia remains poorly characterized in this setting. This is of significant concern because the therapeutic armamentarium and indications for use of these drugs are fast expanding, and patients are being treated for increasingly longer periods of time. Therefore, we performed a systematic review and meta-analysis of published clinical trials, to determine the incidence and risk of alopecia.
patients and methods
data sources and search strategy
We accessed the United States Food and Drug Administration website [8], to identify systemic targeted anticancer agents approved for marketing in the United States (as of December 2013) (supplementary Table S1, available at Annals of Oncology online). We searched the PubMed and Thomson-Reuters' Web of Science databases using the drug's generic name (e.g. afatinib), the operator ‘AND’ and ‘Phase II OR phase III’, to identify human-only studies (1 January 1960–31 May 2014). Abstracts from the American Society of Clinical Oncology's annual and thematic meetings were also searched.
study selection and screening process
We included all phase II and III oncology trials utilizing a targeted agent and reporting clear safety data on ‘alopecia’ or ‘hair loss’ (Figure 1). We reviewed only the most updated full-text English versions and discarded duplicates. Phase I or I/II trials (involving multiple dosings and dose escalations) and combination trials with other agents/modalities were excluded.
Figure 1.
Flow diagram showing the selection process for studies included in the final analysis.
data extraction and clinical end points
We extracted the name of the first author, year of publication, clinical trial design, enrollment number, treatment arms (experimental/control) and their sample sizes, number of patients with all-grade and grade 2 alopecia in each arm, the underlying cancer diagnosis, and the AE severity grading system used. In addition, the Clinicaltrials.gov website was searched utilizing the indexed ‘NCT’ number (if published in the manuscript) and any updated study results were ascertained [9].
The safety profile of each clinical trial was examined for the clinical end points. Over the years, the Common Terminology Criteria for Adverse Events (CTCAE) issued by the National Cancer Institute has evolved (versions 2.0, 3.0, 4.0) (supplementary Appendix S2, available at Annals of Oncology online) [10].
meta-analytic strategy
All statistical analyses were carried out using version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, NJ) [11]. The total number of patients with all-grade alopecia was extracted from selected trials, as delineated above. For each clinical trial, the incidence of alopecia was calculated, and the 95% confidence interval (CI) derived. The relative risk (RR) of alopecia among patients assigned to the targeted agent was calculated and compared only with those assigned to control treatment in the same trial. Forest plots were constructed.
For the meta-analysis, both the fixed-effects model (weighted with inverse variance) and the random-effects model were considered [12]. For each meta-analysis, Cochran's Q statistic was first calculated to assess the heterogeneity of the included trials. For P value <0.1, the assumption of homogeneity was deemed invalid, and the random-effects model was used [13]. Otherwise, results from both the fixed-effects model and the random-effects model were evaluated, and if they were similar, only fixed-effects model results were reported. A two-tailed P value <0.05 was considered as statistically significant.
results
search results
We identified a total of 54 322 potentially relevant records, of which 119 clinical trials were retained for statistical analysis (phase II = 89; phase III = 30) (Figure 1). Of these, 113 trials investigated a targeted agent in solid organ malignancies and 6 trials involved hematologic malignancies. This discrepancy is because most agents have been tried and/or are approved in the treatment of solid tumors.
incidence of all-grade alopecia
Using the random-effects model, the calculated overall incidence of alopecia in our meta-analysis (heterogeneity: Q = 1872, I2 = 93, P ≤ 0.001) was 14.7% (95% CI 12.6% to 17.2%), and was lowest for bortezomib, 2.2% (95% CI 0.4% to 10.9%) and highest for vismodegib, 56.9% (95% CI 50.6% to 63.1%) (Table 1; supplementary Table S1, available at Annals of Oncology online).
Table 1.
Incidence of alopecia with approved targeted agents in monotherapya
| # | Class of targeted therapy | Targeted agent | All-grade alopecia (95% CI) | Rank by incidence |
|---|---|---|---|---|
| 1 | SMO inhibitor | Vismodegib | 56.9% (50.6% to 63.1%) | 1 |
| 2 | VEGFR inhibitor(s) | Sorafenib | 29% (23.9% to 34.7%) | 2 |
| 3 | Regorafenib | 23.5% (9.7% to 46.7%) | 4 | |
| 4 | Cabozantinib | 16.4% (12.0% to 21.9%) | 6 | |
| 5 | Pazopanib | 12.3% (9.0% to 16.6%) | 10 | |
| 6 | Axitinib | 7.5% (4.4% to 12.7%) | 17 | |
| 7 | Sunitinib | 6.9% (4.9% to 9.6%) | 18 | |
| 8 | EGFR/VEGFR inhibitor | Vandetanib | NR | NA |
| 9 | BRAF inhibitor(s) | Vemurafenib | 23.7% (9.6% to 47.5%) | 3 |
| 10 | Dabrafenib | 18.9% (10.5% to 31.5%) | 5 | |
| 11 | Bcr-abl inhibitor(s) | Nilotinib | 15.9% (12.4% to 20.1%) | 7 |
| 12 | Dasatinib | 7.8% (3.0% to 19.1%) | 16 | |
| 13 | Imatinib | 6.6% (3.9% to 10.9%) | 19 | |
| 14 | Bosutinib | NR | NA | |
| 15 | Ponatinib | NR | NA | |
| 16 | Anti-CD30 mAb | Brentuximab | 14.0% (9.9% to 19.4%) | 8 |
| 17 | MEK inhibitor | Trametinib | 13.3% (6.2% to 26.4%) | 9 |
| 18 | EGFR inhibitor(s) | Afatinib | 11.9% (9.1% to 15.4%) | 11 |
| 19 | Cetuximab | 8.9% (2.2% to 29.7%) | 13 | |
| 20 | Erlotinib | 8.9% (5.4% to 14.4%) | 13 | |
| 21 | Panitumumab | NR | NA | |
| 22 | VEGF inhibitor | Bevacizumab | 10% (3.3% to 26.8%) | 12 |
| 23 | ALK inhibitor | Crizotinib | 8.1% (4.9% to 13.2%) | 15 |
| 24 | mTOR inhibitor(s) | Everolimus | 5.3% (1.9% to 13.2%) | 20 |
| 25 | Temsirolimus | 5.2% (0.9% to 25.9%) | 22 | |
| 26 | Anti-CD52 mAb | Alemtuzumab | 5.3% (0.7% to 29.4%) | 20 |
| 27 | CTLA-4 inhibitor | Ipilimumab | 5.1% (1.3% to 18.3%) | 23 |
| 28 | HER2 inhibitor(s) | Ado-trastuzumab emtansine | 4.3% (1.4% to 12.6%) | 24 |
| 29 | Trastuzumab | NR | NA | |
| 30 | Proteasome inhibitor(s) | Bortezomib | 2.2% (0.4% to 10.9%) | 25 |
| 31 | Carfilzomib | NR | NA | |
| 32 | BTK inhibitor | Ibrutinib | NR | NA |
| 33 | JAK inhibitor | Ruxolitinib | NR | NA |
| 34 | Anti-CD20 mAbs | Ofatumumab | NR | NA |
| 35 | Rituximab | NR | NA |
The top five agents with the highest incidence of alopecia appear in bold.
aThe full bibliography for this table is provided in supplementary Appendix S1, available at Annals of Oncology online.
NA, not applicable; NR, not reported; SMO, smoothened; VEGFR, vascular endothelial growth factor receptor; EGFR, epidermal growth factor receptor; BRAF, B-rapidly accelerated fibrosarcoma; Bcr-abl, breakpoint cluster region-abelson; MEK, MAPK/ERK (extracellular signal-regulated kinase) Kinase; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; ALK, anaplastic lymphoma kinase; mTOR, mammalian target of rapamycin; CTLA-4, cytotoxic T-lymphocyte antigen-4; HER2, human epidermal growth factor receptor; BTK, Bruton's tyrosine kinase; JAK, Janus kinase.
When individual trials of different drugs were analyzed, the incidence ranged between 0.25% and 80%. The lowest incidence was noted with erlotinib [0.25% (95% CI 0.02% to 3.9%)] in a phase III trial of non-small cell lung cancer patients [14], while the highest incidence was noted with sorafenib, 80% (95% CI 57.2% to 92.2%), in a phase II clinical trial (n = 21) involving patients with metastatic medullary thyroid cancer [15].
relative risk of developing alopecia: targeted therapies versus placebo
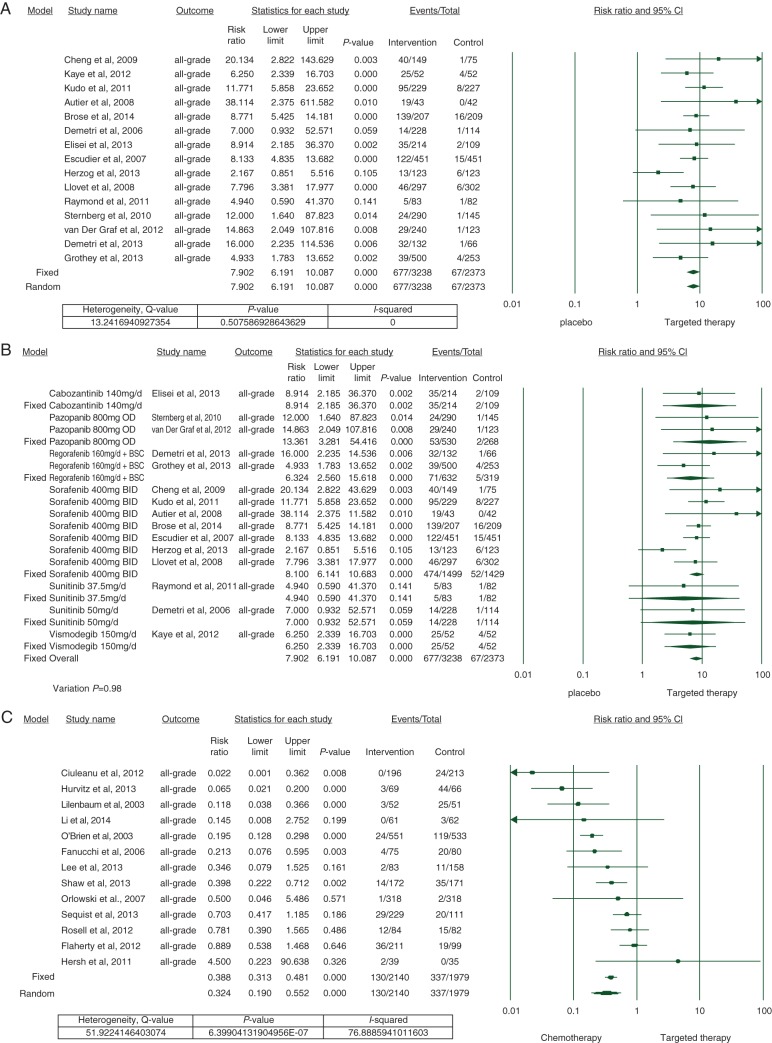
To calculate the risk of developing alopecia, we carried out a meta-analysis on all the available 15 randomized, controlled trials (RCTs) involving cabozantinib [16], pazopanib [17, 18], regorafenib [19, 20], sorafenib [21–27], sunitinib [28, 29], vismodegib [30], and a placebo. All-grade alopecia was noted in 677/3238 of patients treated with the targeted therapies, presenting an overall RR of 7.9 (95% CI 6.2–10.09) when compared with placebo (67/2373 patients); according to both the fixed- and random-effects models (Figure 2A). Statistical heterogeneity was not noted (Q = 13.2, I2 = 0, P = 0.5).
Figure 2.
Forest plot corresponding to the main random-effects meta-analysis, including risk estimates quantifying the relationship between treatment with targeted agents and the development of all-grade alopecia: (A) targeted therapies versus placebo, (B) individual agent versus placebo, (C) targeted therapies versus chemotherapy. The size of the square box represents each risk estimate, and is proportional to the weight that the risk estimate contributed to the summary risk estimate (diamond symbol). Filled square box, risk estimate in each trial; horizontal lines, 95% CI; filled diamond, summary risk estimate.
Among individual drugs, the risk was lowest with sunitinib at 37.5 mg [RR 4.9 (95% CI 0.6–41.4, P = 0.14)] and highest with pazopanib 800 mg [RR 13.4 (95% CI 3.3–54.4, P ≤ 0.001)] (Figure 2B).
relative risk of developing alopecia: targeted therapies versus chemotherapy
To calculate the risk of developing alopecia in patients receiving targeted therapies versus chemotherapy, we meta-analyzed 13 RCTs [14, 31–42]. Since we observed significant heterogeneity (Q = 51.9, I2 = 76.8, P ≤ 0.01) among these studies, a random-effects model was utilized to combine the effect of studies included (Figure 2C). All-grade alopecia was noted in 130/2140 of patients treated with the targeted therapies, presenting an overall RR of 0.32 (95% CI 0.2–0.55, P ≤ 0.001) when compared with chemotherapy (337/1979 patients).
discussion
Our findings suggest that a majority of these drugs (∼70%) are associated with alopecia, albeit to varying degrees. Inhibitors of the Sonic Hedgehog (Shh), vascular endothelial growth factor receptor (VEGFR), and mitogen-activated protein kinase (MAPK) signaling pathways are among the most commonly associated, with vismodegib exhibiting the highest incidence (56.9%). Overall, it appears that the risk of developing all-grade alopecia with targeted therapies is higher than with a placebo, but lower than with chemotherapy.
In our study, the incidence of alopecia was 14.7%, which is lower than the rates seen with CIA (65%) [4]. This may be attributed to the targeted action of these agents and molecular prescreening to select patient population, which is in contrast to cytotoxic chemotherapy where rapidly proliferating cells (both normal and tumoral) are inhibited indiscriminately and patients are not prescreened for determining treatment eligibility. Likewise, in our analysis, the overall risk of developing alopecia was also threefold lower than with chemotherapy. Besides, it appears that the event itself may be dose-dependent, as evinced in a limited number of sunitinib trials (37.5 mg: RR, 4.94 versus 50 mg: RR, 7.0) (Figure 2B). Notwithstanding, alopecia is not considered a dose-limiting AE in clinical oncology practice.
The mechanisms underlying this event remain poorly understood. These drugs are designed to selectively target various oncogenic molecules/pathways (e.g. SMO, VEGFR, MAPK) critical to the growth and survival of tumors. Intriguingly, alopecia may be noted with the blocking of a variety of such (distinct) targets and with different drugs (Tables 1 and 2). The incidence tends to vary even among drugs acting on the same primary molecular target [e.g. vascular endothelial growth factor receptor: sorafenib (29%) versus sunitinib (6.9%)]. However, it must be acknowledged that each of these drugs often target multiple other pathways [e.g. Raf, fibroblast growth factor receptor (FGFR), PDGFR, c-MET, c-KIT], and besides, the spectrum of inhibition and receptor affinity might vary. This suggests that various pathogenic mechanisms may be involved.
Table 2.
Alopecia with approved targeted agents in monotherapy
| Agents that USUALLY cause alopecia (incidence >15%) | Agents that INFREQUENTLY cause alopecia (incidence 5%–15%) | Agents that RARELY cause alopecia (incidence <5%, or NR) |
|---|---|---|
| Vismodegib | Brentuximab | Ado-trastuzumab emtansine |
| Sorafenib | Trametinib | Bortezomib |
| Vemurafenib | Pazopanib | |
| Regorafenib | Afatinib | |
| Dabrafenib | Bevacizumab | Vandetanib (NR) |
| Cabozantinib | Cetuximab, erlotinib | Bosutinib (NR) |
| Nilotinib | Crizotinib | Ponatinib (NR) |
| Dasatinib | Panitumumab (NR) | |
| Axitinib | Trastuzumab (NR) | |
| Sunitinib | Carfilzomib (NR) | |
| Imatinib | Ibrutinib (NR) | |
| Everolimus, alemtuzumab | Ruxolitinib (NR) | |
| Temsirolimus | Ofatumumab (NR) | |
| Ipilimumab | Rituximab (NR) |
NR, not reported.
The role of the Shh and EGFR pathways in hair follicle biology and epidermal homeostasis is well established. Murine studies have shown that Shh pathway inhibition in the skin can lead to (reversible) alopecia and arrest of hair growth in the telogen phase [43], which explains the occurrence of alopecia with vismodegib. Inhibition of the EGFR, located in the outer root sheath of hair follicle [44] and crucial to anagen–catagen transition [45], can lead to follicular disintegration accompanied by inflammation [46, 47]. The large number of case reports describing folliculitis and folliculitis decalvans attest to these findings. On the other hand, studies in mice have shown that fibroblast growth factor (FGF) signaling may stimulate anagen hair growth [48]. The anagen inner root sheath and telogen bulge of hair follicles (FGF) and the hair follicle matrix cells (FGFR) appear to be active regions. Also, PDGF signaling has been found to play an important role in the induction and maintenance of the anagen phase in hair follicles [49]. Hence, the primary target inhibited, type of the drug (e.g. mAb, tyrosine kinase inhibitor), variations in the target spectrum of inhibition, molecular cross-talk between pathways, and finally, the inherent role of these molecules in hair follicle biology may all play important roles in the pathogenesis of alopecia. This is in contrast to conventional chemotherapy where the mechanism is predominantly nonselective cytotoxicity [50].
The onset and pattern of alopecia are not recorded by oncologists, with published case reports/series and personal experience (SW, KJB, MEL) offering some insights. The alopecia may be a frontal (androgenetic-like), diffuse, or patchy, with some slowing of hair growth. It is generally nonscarring [51, 52], and may be accompanied by pruritus. In some cases, scarring alopecia/folliculitis decalvans with pain and associated infection may develop, especially with erlotinib [53, 54]. The onset after initiation of treatment may range from a couple of weeks to months, with resolution 1–6 months after drug discontinuation; the quality of hair and rate of regrowth, however, may be affected [55]. Jaber et al. reported widespread alopecia (scalp, eyebrows, face, pubic region, and trunk) with ipilimumab, which mimicked alopecia areata both clinically and histologically [56]. Other abnormalities include but are not limited to trichomegaly, textural abnormalities (fine, brittle and curly hair), dyspigmentation (brown to orange/red, hypo- to depigmentation), and facial hypertrichosis [57]. The histopathology and a management algorithm for alopecia being used in the author's practice (M.E.L.) are discussed (supplementary Appendix S3, available at Annals of Oncology online).
Our study has some limitations. First, the reporting of safety data is inconsistent in clinical trials [58, 59]. Second, the data available/extracted represent only the summary results. Third, trials report AEs encountered at a certain frequency (e.g. >10%); any AEs below these cutoffs therefore would not have been captured. We attempted to minimize this issue by additionally searching the ClinicalTrials.gov website. Fourth, oncologists' expertise in diagnosing alopecia may vary (interobserver bias). In addition, telogen effluvium is common in patients stressed with the diagnosis of cancer and the prospect of receiving anticancer therapy. The actual incidence in general population and cancer patients is not known; however, some degree of alopecia occurs in up to 40% of females and 70% of men aged above 60 years [60–62]. Lastly, the CTCAE definitions include only grades 1 and 2 for alopecia—the data for the latter were not reported in some trials, while others erroneously reported grade 3 alopecia, thus precluding the estimation of high-grade alopecia. In summary, our findings may be an underestimation of the true incidence and severity.
conclusion
With the expanding indications for targeted agents (including off-label use), there is an urgent need for prospective studies and investigation into the mechanistic basis of this distressing condition (alopecia) and design of evidence-based management strategies. This is also crucial to direct supportive care efforts, ensure consistent dosing, treatment compliance, and maintain patients' HRQoL.
funding
This work was supported by the RJR Oncodermatology Fund and funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
disclosure
VRB, KM, CE, LG, TP, PAG, and KJB have no conflicts of interest to declare; SW has a speaking arrangement with Novartis, Bayer-Onyx, Pfizer, and Mediavation. MEL has a speaking, consultant or advisory role with Advancell, Amgen, AstraZeneca, Augmentium, Aveo, Bayer, Berg Pharma, Biopharm Communications, Boehringer Ingelheim, Brickell Biotech, Bristol-Myers Squibb, Clinical Assistance Programs, Clinical Care Options, EMD Serono, Envision Communications, Foamix, Galderma, Genentech, GlaxoSmithKline, Helsinn, Institute for Medical Education and Research, Integro-MC, Lindi Skin, Medscape, Medtrend International, Merck, Nerre Therapeutics, Novartis, Novocure, Oncology Specialty Group, OSI Pharmaceuticals, Permanyer, Physicians Education Resource, Pierre Fabre, Pfizer, Reata Pharmaceuticals, Roche, Sandoz, Sanofi Aventis, and Threshold Pharmaceuticals. The contents of this manuscript have not been presented earlier, and are not under consideration for publication elsewhere.
Supplementary Material
acknowledgements
We thank the Information Systems' data delivery group (DataLine) at the Memorial Sloan Kettering Cancer Center, especially Dwayne Long (Data Administrator) and Stuart Gardos (Project Manager), for their swift assistance with the complex task of medical data acquisition and electronic medical record search. The role of Isla Otap (Pathology Office Assistant) in retrieving the pathology slides for review is also greatly appreciated. Lastly, we thank Bernadette Murphy (Medical Photographer) for the clinical photography.
references
- 1.Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. J Support Oncol 2010; 8(4): 149–161. [PubMed] [Google Scholar]
- 2.Robert C, Soria JC, Spatz A et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 2005; 6(7): 491–500. [DOI] [PubMed] [Google Scholar]
- 3.Agero AL, Dusza SW, Benvenuto-Andrade C et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermat 2006; 55(4): 657–670. [DOI] [PubMed] [Google Scholar]
- 4.Trueb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg 2009; 28(1): 11–14. [DOI] [PubMed] [Google Scholar]
- 5.Choi EK, Kim IR, Chang O et al. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psychooncology 2014; 23(10): 1103–1110. [DOI] [PubMed] [Google Scholar]
- 6.Freedman TG. Social and cultural dimensions of hair loss in women treated for breast cancer. Cancer Nurs 1994; 17(4): 334–341. [PubMed] [Google Scholar]
- 7.Browall M, Gaston-Johansson F, Danielson E. Postmenopausal women with breast cancer: their experiences of the chemotherapy treatment period. Cancer Nurs 2006; 29(1): 34–42. [DOI] [PubMed] [Google Scholar]
- 8.United States Food and Drug Administration [Internet]. Silver Springs: The Organization (cited 12 July 2014). Drugs. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm (10 July 2014, date last accessed).
- 9.US National Library of Medicine [Internet]. Bethesda: The Organization (cited 12 July 2014). Clinical Trial Registry Numbers in MEDLINE®/PubMed® Records. http://www.nlm.nih.gov/bsd/policy/clin_trials.html (21 February 2014, date last accessed)
- 10.National Cancer Institute [Internet]. Bethesda: The Organization (cited 12 July 2014). Cancer Therapy Evaluation Program: Protocol Development. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (20 March 2013, date last accessed).
- 11.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2, Biostat, Englewood, 2005. [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 13.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Int Med 1997; 127(9): 820–826. [DOI] [PubMed] [Google Scholar]
- 14.Ciuleanu T, Stelmakh L, Cicenas S et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012; 13(3): 300–308. [DOI] [PubMed] [Google Scholar]
- 15.Lam ET, Ringel MD, Kloos RT et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010; 28(14): 2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elisei R, Schlumberger MJ, Muller SP et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013; 31(29): 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternberg CN, Davis ID, Mardiak J et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010; 28(6): 1061–1068. [DOI] [PubMed] [Google Scholar]
- 18.van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012; 379(9829): 1879–1886. [DOI] [PubMed] [Google Scholar]
- 19.Demetri GD, Reichardt P, Kang YK et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381(9863): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381(9863): 303–312. [DOI] [PubMed] [Google Scholar]
- 21.Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med 2007; 356(2): 125–134. [DOI] [PubMed] [Google Scholar]
- 22.Autier J, Escudier B, Wechsler J et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermat 2008; 144(7): 886–892. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med 2008; 359(4): 378–390. [DOI] [PubMed] [Google Scholar]
- 24.Cheng AL, Kang YK, Chen Z et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1): 25–34. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M, Imanaka K, Chida N et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011; 47(14): 2117–2127. [DOI] [PubMed] [Google Scholar]
- 26.Herzog TJ, Scambia G, Kim BG et al. A randomized phase II trial of maintenance therapy with sorafenib in front-line ovarian carcinoma. Gynecol Oncol 2013; 130(1): 25–30. [DOI] [PubMed] [Google Scholar]
- 27.Brose MS, Nutting CM, Jarzab B et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014; 384(9940): 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demetri GD, van Oosterom AT, Garrett CR et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006; 368(9544): 1329–1338. [DOI] [PubMed] [Google Scholar]
- 29.Raymond E, Dahan L, Raoul JL et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl J Med 2011; 364(6): 501–513. [DOI] [PubMed] [Google Scholar]
- 30.Kaye SB, Fehrenbacher L, Holloway R et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin Cancer Res 2012; 18(23): 6509–6518. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien SG, Guilhot F, Larson RA et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. New Engl J Med 2003; 348(11): 994–1004. [DOI] [PubMed] [Google Scholar]
- 32.Flaherty KT, Robert C, Hersey P et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. New Engl J Med 2012; 367(2): 107–114; Updated/Expanded results: https://clinicaltrials.gov/ct2/show/results/NCT01245062?term=NCT&rank=1§=X40156#othr (15 May 2014, date last accessed). [DOI] [PubMed] [Google Scholar]
- 33.Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31(27): 3327–3334; Updated/Expanded results available from: https://clinicaltrials.gov/ct2/show/results/NCT00949650?term=NCT.&rank=1§=X40156#othr (15 May 2014, date last accessed)]. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Lee JS, Kim SW et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer 2013; 49(15): 3111–3121. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Ou W, Yang H et al. A randomized phase 2 trial of erlotinib versus pemetrexed as second-line therapy in the treatment of patients with advanced EGFR wild-type and EGFR FISH-positive lung adenocarcinoma. Cancer 2014; 120(9): 1379–1386. [DOI] [PubMed] [Google Scholar]
- 36.Lilenbaum R, Axelrod R, Thomas S et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008; 26(6): 863–869. [DOI] [PubMed] [Google Scholar]
- 37.Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13(3): 239–246. [DOI] [PubMed] [Google Scholar]
- 38.Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New Engl J Med 2013; 368(25): 2385–2394. [DOI] [PubMed] [Google Scholar]
- 39.Hersh EM, O'Day SJ, Powderly J et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs 2011; 29(3): 489–498. [DOI] [PubMed] [Google Scholar]
- 40.Hurvitz SA, Dirix L, Kocsis J et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2013; 31(9): 1157–1163. [DOI] [PubMed] [Google Scholar]
- 41.Fanucchi MP, Fossella FV, Belt R et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2006; 24(31): 5025–5033. [DOI] [PubMed] [Google Scholar]
- 42.Orlowski RZ, Nagler A, Sonneveld P et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol 2007; 25(25): 3892–3901. [DOI] [PubMed] [Google Scholar]
- 43.Wang LC, Liu ZY, Gambardella L et al. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol 2000; 114(5): 901–908. [DOI] [PubMed] [Google Scholar]
- 44.Nanney LB, Magid M, Stoscheck CM, King LE Jr.. Comparison of epidermal growth factor binding and receptor distribution in normal human epidermis and epidermal appendages. J Invest Dermatol 1984; 83(5): 385–393. [DOI] [PubMed] [Google Scholar]
- 45.Philpott MP, Kealey T. Effects of EGF on the morphology and patterns of DNA synthesis in isolated human hair follicles. J Invest Dermatol 1994; 102(2): 186–191. [DOI] [PubMed] [Google Scholar]
- 46.Hansen LA, Alexander N, Hogan ME et al. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am J Pathol 1997; 150(6): 1959–1975. [PMC free article] [PubMed] [Google Scholar]
- 47.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 2006; 6(10): 803–812. [DOI] [PubMed] [Google Scholar]
- 48.Kawano M, Komi-Kuramochi A, Asada M et al. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol 2005; 124(5): 877–885. [DOI] [PubMed] [Google Scholar]
- 49.Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci 2006; 43(2): 105–115. [DOI] [PubMed] [Google Scholar]
- 50.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol 2013; 14(2): e50–e59. [DOI] [PubMed] [Google Scholar]
- 51.Robert C, Mateus C, Spatz A et al. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol 2009; 60(2): 299–305. [DOI] [PubMed] [Google Scholar]
- 52.Osio A, Mateus C, Soria JC et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol 2009; 161(3): 515–521. [DOI] [PubMed] [Google Scholar]
- 53.Hepper DM, Wu P, Anadkat MJ. Scarring alopecia associated with the epidermal growth factor receptor inhibitor erlotinib. J Am Acad Dermatol 2011; 64(5): 996–998. [DOI] [PubMed] [Google Scholar]
- 54.Hoekzema R, Drillenburg P. Folliculitis decalvans associated with erlotinib. Clin Exp Dermatol 2010; 35(8): 916–918. [DOI] [PubMed] [Google Scholar]
- 55.Lacouture ME, Anadkat MJ, Bensadoun RJ et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011; 19(8): 1079–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaber SH, Cowen EW, Haworth LR et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol 2006; 142(2): 166–172. [DOI] [PubMed] [Google Scholar]
- 57.Cohen PR, Escudier SM, Kurzrock R. Cetuximab-associated elongation of the eyelashes: case report and review of eyelash trichomegaly secondary to epidermal growth factor receptor inhibitors. Am J Clin Dermatol 2011; 12(1): 63–67. [DOI] [PubMed] [Google Scholar]
- 58.Seruga B, Sterling L, Wang L, Tannock IF. Reporting of serious adverse drug reactions of targeted anticancer agents in pivotal phase III clinical trials. J Clin Oncol 2011; 29(2): 174–185. [DOI] [PubMed] [Google Scholar]
- 59.Pitrou I, Boutron I, Ahmad N, Ravaud P. Reporting of safety results in published reports of randomized controlled trials. Arch Int Med 2009; 169(19): 1756–1761. [DOI] [PubMed] [Google Scholar]
- 60.Norwood OT. Male pattern baldness: classification and incidence. South Med J 1975; 68(11): 1359–1365. [DOI] [PubMed] [Google Scholar]
- 61.Malkud S. A hospital-based study to determine causes of diffuse hair loss in women. J Clin Diag Res 2015; 9(8): WC01–WC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su LH, Chen HH. Androgenetic alopecia in policemen: higher prevalence and different risk factors relative to the general population (KCIS no. 23). Arch Dermatol Res 2011; 303(10): 753–761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.