Abstract
Type II heat-labile enterotoxins (HLTs) constitute a promising set of adjuvants that have been shown to enhance humoral and cellular immune responses when coadministered with an array of different proteins, including several pathogen-associated antigens. However, the adjuvant activities of the four best-studied HLTs, LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc, have never been compared side by side. We therefore conducted immunization studies in which LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc were coadministered by the intradermal route to mice with two clinically relevant protein subunit vaccine antigens derived from the enzymatic A subunit (RTA) of ricin toxin, RiVax and RVEc. The HLTs were tested with low and high doses of antigen and were assessed for their abilities to stimulate antigen-specific serum IgG titers, ricin toxin-neutralizing activity (TNA), and protective immunity. We found that all four HLTs tested were effective adjuvants when coadministered with RiVax or RVEc. LT-IIa was of particular interest because as little as 0.03 μg when coadministered with RiVax or RVEc proved effective at augmenting ricin toxin-specific serum antibody titers with nominal evidence of local inflammation. Collectively, these results justify the need for further studies into the mechanism(s) underlying LT-IIa adjuvant activity, with the long-term goal of evaluating LT-IIa's activity in humans.
INTRODUCTION
The absence of effective adjuvants constitutes a critical bottleneck to the development of protein subunit vaccines for biodefense and emerging infectious diseases (1–3). RiVax, for example, is a recombinant, attenuated derivative of the enzymatic subunit of ricin toxin (RTA), which has been deemed to be safe for human use (4, 5). Unfortunately, phase I clinical trials indicated that even repeated high-dose immunizations were relatively poor at stimulating ricin-specific IgG antibodies and toxin-neutralizing activity (TNA) in sera of volunteers. Adsorption of RiVax to aluminum salts adjuvant only marginally improved the antibody response to the vaccine antigen, indicating that the future success of RiVax and other ricin toxin subunit vaccine antigens under investigation, like RVEc, may depend on the identification of more-potent adjuvants (6).
The type I and type II bacterial heat-labile enterotoxins (HLTs) are among the most potent adjuvants described to date. The HLTs are AB5 subunit toxins, consisting of a single ADP-ribosylating (“A”) subunit joined noncovalently to pentamers of ganglioside-binding (“B5”) subunits. The well-characterized type I HLTs include cholera toxin (CT), expressed by Vibrio cholerae, and LT enterotoxin, expressed by enterotoxigenic strains of Escherichia coli. The type II enterotoxins, on the other hand, are less well characterized and more diverse than CT or LT (7, 8). The best-studied type II HLTs are LT-IIa, LT-IIb, and LT-IIc, each of which has been shown to enhance antigen-specific immune responses when coadministered with a model antigen like ovalbumin (OVA) (9). Adjuvant activities of the type I and II HLTs are due to a combination of the A subunit's ability to stimulate intracellular cyclic AMP (cAMP) levels through ADP-ribosylation of Gsα regulatory proteins and the pentameric B subunit's tropism for specific cell types. CT and LT each bind ganglioside GM1 and therefore have similar cell tropisms, including intestinal epithelial cells. The type II HLTs LT-IIa, LT-IIb, and LT-IIc, on the other hand, recognize an array of gangliosides and, therefore, target a broader array of cell types, including lymphocytes, dendritic cells, and other professional antigen-presenting cells. Each member of the type II HLTs stimulates a different pattern of cytokines and different degrees of lymphocyte activation, resulting in potentially differential adjuvant activities (7). Four of the type II HLTs, LT-IIa, LT-IIb, and LT-IIc and a genetically detoxified mutant of LT-IIb known as LT-IIbT13I, have been tested as adjuvants in conjunction with a variety of antigens, including OVA (7, 9, 10), AgI/II from Streptococcus mutans (10), and ESAT-6 from Mycobacterium tuberculosis antigen (11). However, a systematic side-by-side comparison among the HLTs has never been done. It is therefore not possible to rank order them based on adjuvant activity. Nor have dose-response studies been conducted, so the actual lowest dose of type II HLTs that retains adjuvanticity has not been determined.
Therefore, the goal of the current study was to evaluate the adjuvant activities of LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc side by side in a mouse intradermal (i.d.) immunization model. We chose to use RiVax and RVEc for these studies, because they are each well-characterized investigational vaccine antigens that have been deemed safe in phase I clinical trials (4, 5). Both are recombinant, nontoxic derivatives of ricin's enzymatic subunit, RTA. RiVax is a full-length (267-residue) variant of RTA with two attenuating point mutations at residues Y80 and V76, while RVEc lacks RTA's C terminus (residues 199 to 267) as well as a small hydrophobic loop in the N terminus (residues 34 to 43). Despite their different physical makeups, RiVax and RVEc were reported to be indistinguishable in terms of stimulating protective immunity to ricin in mice (12). Moreover, we have previously examined the potential of LT-IIb and LT-IIbT13I to serve as adjuvants for RiVax when administered to mice via the intradermal (i.d.) and intranasal (i.n.) routes (13). LT-IIb and LT-IIbT13I each significantly enhanced the onset and magnitude of RiVax-specific serum IgG levels and protective immunity in mice, demonstrating the general compatibility of RiVax with the HLT adjuvants. We now report a systematic comparison of the adjuvant activities of LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc when administered to mice by the i.d. route in conjunction with RiVax and RVEc. The results of the study indicate that while all four HLTs tested were effective adjuvants, LT-IIa proved the most promising because of its effectiveness even at subinflammatory doses.
MATERIALS AND METHODS
Chemicals, reagents, and cell lines.
Ricin was purchased from Vector Laboratories (Burlingame, CA) and dialyzed against phosphate-buffered saline (PBS) at 4°C in 10,000-molecular-weight (MW)-cutoff Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL) prior to use in cytotoxicity and mouse studies. RiVax (lot 190-100L-GLP-FF-090105) in 144 mM NaCl, 10 mM histidine, pH 6.0, in 50% glycerol was obtained from Soligenix, Inc. RVEc (lot 011314a) in 20 mM sodium succinate, 100 mM NaCl, 0.12% Tween 20 at pH 6.5 was obtained from Leonard Smith and Ralph Tammariello at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID; Fort Detrick, MD). Recombinant His-tagged LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc were purified from E. coli (10). Alhydrogel aluminum adjuvant (batch 4772) was obtained from Brenntag (Mülheim an der Ruhr, Germany). Vero cells were purchased from the American Type Culture Collection (Manassas, VA). Cell lines were maintained in a humidified incubator at 37°C with 5% CO2. Unless noted specifically, all other chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Immunization protocols.
Female BALB/c mice 7 to 12 weeks of age were obtained from Taconic Laboratories (Hudson, NY) or Harlan Laboratories (Madison, WI). Animals were housed under conventional, specific-pathogen-free conditions and were treated in compliance with the approval of the local Institutional Animal Care and Use Committee (IACUC) guidelines at the Wadsworth Center and the University at Buffalo. Immunization studies were conducted with 5 to 10 mice per group, as specified in Results and figure legends. RiVax or RVEc was mixed with LT-II adjuvants or adsorbed to Alhydrogel (0.85 mg/ml) for 2 h just prior to immunization. Intradermal immunizations were conducted as described previously, with each injection containing a 30-μl total volume (13). The specific vaccination regimens are described in Results, but as a rule mice were primed on day 0 and boosted on days 10 and 20. Blood was collected via the lateral tail vein on days 17 and 27.
Ricin toxin challenge studies.
Mice were challenged by intraperitoneal (i.p.) injection with 10 50% lethal doses (LD50s) of ricin (∼2 μg/mouse). Survival was monitored over a 7-day period. Hypoglycemia was used as a surrogate marker of ricin intoxication (14). Mice were euthanized when they became overtly moribund and/or blood glucose levels fell below 25 mg/dl.
Analysis of skin inflammation.
Mice were immunized by the i.d. route with RiVax and LT-II adjuvants and were observed daily for reactogenicity at the site of immunization. For gross morphological analysis, edema was measured using digital calipers. Edema was determined by taking two orthogonal measurements (M1 and M2, M1 > M2) of the edema diameter and then multiplied by an estimated depth, which was arbitrarily set at M2 because of the difficulty in accurately measuring the depth of swelling, especially at early time points. Hence, edema volume was calculated as M1 × M2 × M2, and values were reported as cubic millimeters as done previously (9).
ELISA.
Nunc 96-well plates were coated overnight at 4°C with ricin (1 μg/ml) and then blocked for 2 h with 2% goat serum in 0.1% PBS-Tween (PBST). Twofold serial dilutions of serum, starting from 1:100, were then applied in duplicate for 1 h, washed, and detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG for 1 h. After another wash, the reaction was developed with SureBlue tetramethylbenzidine (TMB) (KPL, Gaithersburg, MD), and quenched with 1 M phosphoric acid before being scanned at 450 nm on a VersaMax microtiter plate spectrophotometer (Molecular Devices, Sunnydale, CA). The endpoint titer, determined by direct ricin toxin enzyme-linked immunosorbent assay (ELISA), was defined as the lowest dilution whose absorbance (450 nm) was >3 times background. Seroconversion was defined as an endpoint titer of ≥1:100. Geometric mean titers (GMTs) were calculated from the endpoint titers. The endpoint titer for any mouse that had not seroconverted was set to 1 for GMT calculations.
Ricin toxin neutralization assays.
Vero cells were trypsinized, adjusted to ∼5 × 104 cells/ml, seeded (100 μl/well) into white-bottom 96-well plates (Corning Life Sciences, Corning, NY), and allowed to adhere overnight. The cells were treated with ricin (10 ng/ml), ricin-serum mixtures, or culture medium (negative control) for 2 h at 37°C. Cells were then washed to remove noninternalized ricin or ricin-serum mixtures and incubated for 48 h at 37°C. Cell viability was assessed using CellTiter-Glo (Promega, Madison, WI). All treatments were performed in triplicate, and 100% viability was defined as the average value obtained from wells in which cells were treated with culture medium only. The percent viability was defined as the percentage of Vero cells that were protected from the effects of ricin (10 ng/ml) at a given serum dilution (1:50 or 1:100).
Statistical analysis and software.
Statistical analysis was carried out with GraphPad Prism 5 (GraphPad Software, San Diego, CA). Endpoint titers were log transformed prior to statistical analysis. Endpoint and neutralizing titers were compared using 1-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. Edema measurements were compared with a 1-way ANOVA, followed by the Newman-Keuls multiple comparison test. Survival data were tested using the log rank Mantel-Cox test. In all cases, the significance threshold was set at P < 0.05.
RESULTS
LT-II adjuvants stimulate ricin-specific serum IgG and toxin-neutralizing titers when coadministered with RiVax or RVEc.
We first compared the adjuvant activities of the four different LT-II enterotoxins, LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc, each combined with high (5-μg) or low (0.5-μg) doses of RiVax and administered to BALB/c mice by the i.d. route. Mice were primed on day 0, boosted on days 10 and 20, and then challenged with 10 LD50s of ricin toxin on day 34. Sera were collected from the mice on days 17 and 27 and examined for toxin-specific serum IgG levels and TNA.
Analysis of sera collected on day 17 from animals that received high- or low-dose RiVax without adjuvant indicated seroconversion rates of ~60% and extremely low GMTs (30 to 47) (Table 1; Fig. 1). In contrast, groups of mice that received high-dose RiVax plus one of the LT-II adjuvants had a seroconversion rate of 100% and GMTs ranging from 5,400 (in the case of LT-IIbT13I) to 47,000 (in the case of LT-IIa) (Table 1; Fig. 1). Mice that received low-dose RiVax plus one of the LT-II adjuvants also demonstrated 100% seroconversion, although GMTs were considerably lower (range, 1,200 to 12,800). By day 27, all mice that received high- or low-dose RiVax even without adjuvant had seroconverted. In the high-dose RiVax group, the GMT increased ∼360-fold (GMT of 16,890) compared to day 17, while the low-dose group GMT increased 100-fold (GMT of 3,940). On day 27, the GMTs of mice that received high- or low-dose RiVax plus any one of the LT-II adjuvants were significantly higher than GMTs of mice that received RiVax only, underscoring that fact that the LT-II adjuvants not only accelerated the onset of detectable ricin-specific serum IgG titers but ultimately increased the absolute magnitude of the antigen-specific antibody responses. All animals that were vaccinated with RiVax (irrespective of the presence or absence of adjuvant) survived ricin toxin challenge administered on day 34 (Table 1).
TABLE 1.
Adjuvant activities associated with LT-II toxins when coadministered with RiVax and RVEc
| LT-II enterotoxin | RiVax (5 μg)a |
RiVax (0.5 μg) |
RVEc (0.5 μg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GMTb (day) |
% Viabc | Survivald | GMT (day) |
% Viab | Survival | GMT (day) |
% Viab | Survival | ||||
| 17 | 27 | 17 | 27 | 17 | 27 | |||||||
| LT-IIae | 47,771 | 95,543 | 45f | 10/10 | 12,800 | 27,437 | 23f | 10/10 | 10,397 | 31,517 | 13f | 10/10 |
| LT-IIb | 11,143 | 38,802 | 17 | 10/10 | 4,525 | 33,779 | 32f | 10/10 | 7,352 | 29,407 | 39 f | 10/10 |
| LT-IIb(T13I) | 5,486 | 44,572 | 20 | 10/10 | 3,430 | 19,401 | 21 | 10/10 | 6,400 | 19,401 | 20 | 10/10 |
| LT-IIc | 15,759 | 38,802 | 23 | 10/10 | 1,213 | 19,401 | 11 | 10/10 | 2,599 | 14,703 | 3 | 10/10 |
| - | 47 | 16,890 | 10 | 10/10 | 30 | 3,940 | 4 | 10/10 | 36 | 2,263 | 1 | 10/10 |
Ricin subunit antigen and dose used in the experiment.
Geometric mean endpoint titer.
Mean cell viability achieved by a 1:50 dilution of serum collected on day 27 for groups receiving 0.5 μg RiVax or RVEc or 1:100 for serum collected on day 27 from mice that received 5 μg RiVax.
Number of mice to survive a 10 LD50 ricin challenge on day 34 out of 10 total mice. Sham-immunized mice (not shown) all succumbed to ricin challenge, as shown in the accompanying figures.
Adjuvant in a given experiment at a dose of 1 μg.
P < 0.05 vs RiVax or RVEc without adjuvant.
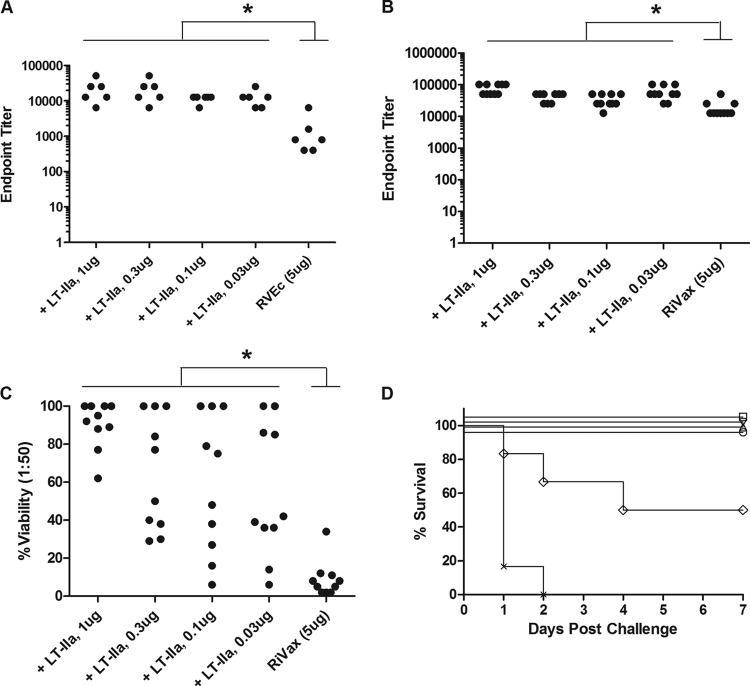
FIG 1.
Effects of LT-II adjuvants on ricin toxin-specific serum IgG levels when coadministered with two different candidate ricin subunit vaccine antigens. As indicated in Materials and Methods, groups of BALB/c mice (n = 10 per group) were immunized by the i.d. route on days 0, 10, and 20 with high- or low-dose RiVax (A and B) or RVEc (C) with indicated LT-II adjuvants. Sera were collected on day 17 (left panels) or 27 (right panels) and analyzed for ricin-specific serum IgG by ELISA. The dash (-) on the far right of each x axis indicates no adjuvant addition. Seroconversion was defined as an endpoint titer of ≥1:100. Mice that had not seroconverted were arbitrarily assigned a titer of 1 to enable determination of GMT. *, P < 0.05.
Among the four adjuvants tested, LT-IIa tended to elicit the highest ricin-specific endpoint titers on day 17. For example, in conjunction with high-dose RiVax, LT-IIa elicited a GMT after a single prime-boost regimen that was ∼3-fold higher than the response elicited by prime and two boosts with RiVax alone (Table 1). Furthermore, a prime-boost regimen of low-dose RiVax with LT-IIa elicited ricin-specific GMTs that were roughly equivalent to those elicited by prime and two boosts with high-dose RiVax (Table 1).
To assess the “quality” of the serum antibody responses elicited by RiVax with LT-II adjuvants, sera were evaluated for TNA. We and others have recently reported that toxin-neutralizing titers generally lag behind total ricin-specific IgG levels by 1 to 2 weeks, a phenomenon that we note in Discussion (15, 16). It was, therefore, not surprising that serum samples from day 17 were devoid of detectable in vitro TNA (data not shown). However, by day 27, TNAs were evident in all groups, with the lowest values associated with mice that received RiVax without adjuvant (Table 1; Fig. 2). The highest TNA values were associated with mice that received RiVax plus LT-IIa or LT-IIb (Table 1; Fig. 2).
FIG 2.
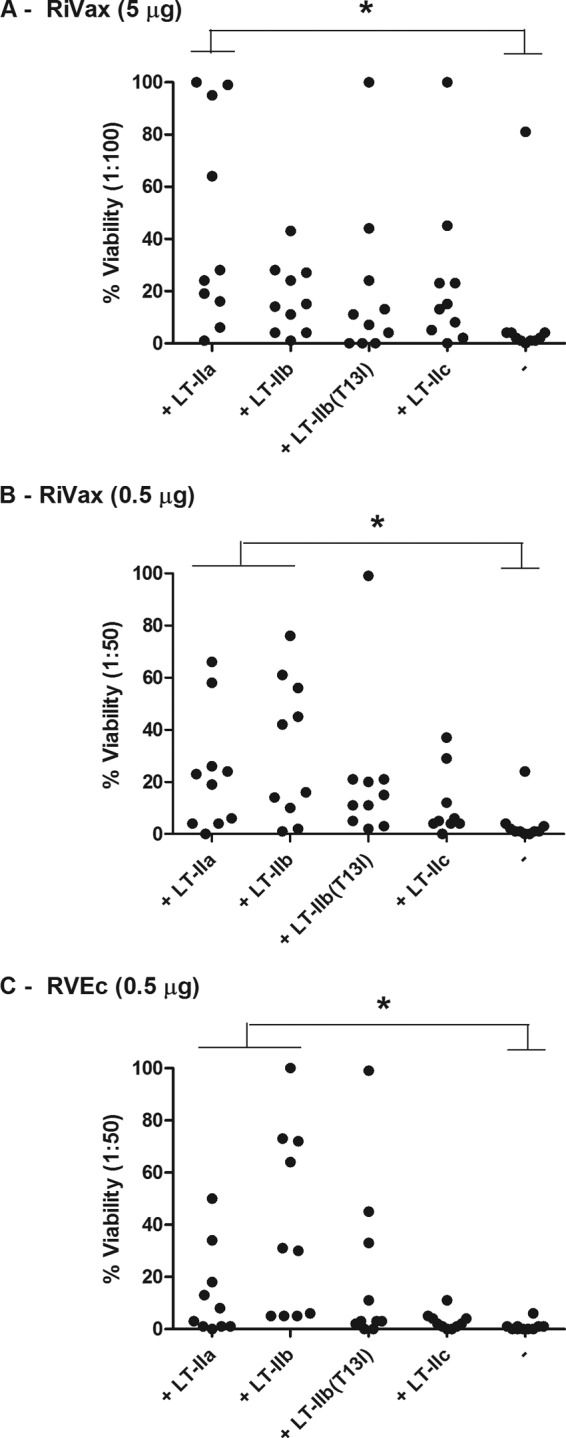
Effects of LT-II adjuvants on ricin toxin-neutralizing activity in sera of mice following immunization with two different candidate ricin subunit vaccine antigens. Groups of BALB/c mice (n = 10 per group) were immunized by the i.d. route on days 0, 10, and 20 with RiVax (A and B) or RVEc (C) with indicated LT-II adjuvants, as described in the legend to Fig. 1. The dash (-) on the far right of each x axis indicates no adjuvant addition. Sera were collected on day 27 and tested for toxin-neutralizing activity in a standard Vero cell cytotoxicity assay. Serum samples were diluted 1:100 (A) or 1:50 (B and C), mixed with ricin, and applied to Vero cells in triplicate in microtiter plates. Shown is the percent cell viability. *, P < 0.05 versus antigen alone.
We next evaluated the degree to which the individual LT-II enterotoxins enhanced the immune response to RVEc, a truncated RTA-based subunit vaccine antigen (12, 17). Low-dose RVEc (0.5 μg) was mixed with each of the four different LT-II enterotoxins and administered to groups of BALB/c mice by the i.d. route. Analysis of sera collected on days 17 and 27 indicated that ricin-specific GMTs were significantly higher in LT-II-treated groups of mice than in mice that received only RVEc (Table 1; Fig. 1). The highest TNA values were again associated with the sera from animals immunized with LT-IIa or LT-IIb (Table 1; Fig. 2). Collectively, these data demonstrate that all four LT-II enterotoxins have adjuvant activity when combined with RiVax or RVEc, with the most potent being LT-IIa and LT-IIb.
Local inflammation associated with LT-II adjuvants.
We determined the acute inflammatory response associated with each of the LT-II adjuvants when administered in conjunction with RiVax to mice by the i.d. route. We previously reported that LT-IIb is relatively inflammatory, as evidenced by significant and persistent edema at the site of injection that was characterized by extensive fluid accumulation and cellular infiltration (13). LT-IIbT13I, on the other hand, was deemed largely nonreactogenic. As described in Materials and Methods, each of the LT-II enterotoxins (1 μg) was mixed with RiVax (5 μg) and delivered to mice by the i.d. route, after which edema was scored daily for up to 10 days. We found that LT-IIb was the most inflammatory, with a maximal edema volume of about 2,500 mm3 3 days after administration, while LT-IIbT13I was the least inflammatory (Fig. 3A). LT-IIa and LT-IIc elicited intermediate levels of inflammation, at least when administered at 1 μg.
FIG 3.

Effects of LT-II adjuvants on local inflammatory response following intradermal immunization. Groups of BALB/c mice (n = 10 per group) were given a primary immunization with RiVax alone or RiVax with LT-II adjuvant by the i.d. route. Edema volume was measured at the injection sites daily for up to 10 days. (A) LT-II adjuvants (1 μg) induce differential amounts of inflammation, as measured by edema volume at the injection site over a 10-day period. (B) Lowering the dose of LT-IIa significantly reduces the associated inflammation at the injection site. *, P < 0.05; **, P < 0.01; ***, P < 0.001, versus RiVax on that particular day.
Adjuvant activity of LT-IIa at nominally inflammatory levels.
Although LT-IIa was the most effective among the four LT-II enterotoxins at serving as an adjuvant for RiVax and RVEc, it demonstrated intermediate levels of inflammation when administered at the standard dose of 1 μg per injection. We therefore sought to examine whether subinflammatory or minimally inflammatory amounts of LT-IIa still retained significant adjuvant activity. We first performed a dose-response study in which mice received 3-fold dilutions of LT-IIa (range, 1.0 to 0.03 μg) mixed with RiVax (5 μg) by the i.d. route. Edema was measured over a 10-day period. As shown in Fig. 3B, inflammation associated with LT-IIa was indeed dose dependent. The smallest amount of LT-IIa tested, 0.03 μg, showed no evidence of edema compared to RiVax except for a slight increase on day 2. For that reason, we considered 0.03 μg LT-IIa to be nominally inflammatory.
To determine if the low doses of LT-IIa are sufficient to enhance antigen-specific immune responses, different amounts of LT-IIa (range, 1.0 to 0.03 μg) were combined with RiVax and RVEc and administered to mice by the i.d. route. Analysis of sera collected 7 days after a prime and one (Fig. 4A) or two (Fig. 4B) boosts indicated that even the smallest amount of LT-IIa tested (0.03 μg) gave rise to ricin-specific serum IgG GMTs that were significantly higher than those from mice that received RiVax or RVEc without adjuvant (Table 2; Fig. 4A and B). Low-dose LT-IIa (0.03 μg) was also sufficient to elicit ricin toxin-neutralizing antibodies to levels significantly above those elicited by antigen alone and roughly half of that elicited by high-dose LT-IIa (1.0 μg) (Table 2; Fig. 4C). Finally, mice immunized with a prime and a single boost of low-dose LT-IIa (0.03 μg) in conjunction with RVEc were fully protected against 10-LD50 ricin challenge (Fig. 4D).
FIG 4.
Dose effects of LT-IIa on ricin toxin-specific serum IgG levels, TNA, and protection against ricin challenge. Groups of BALB/c mice were immunized by the i.d. route on days 0, 10, and 20 with indicated amounts of LT-IIa (1.0 to 0.03 μg) combined with RVEc or RiVax. (A and B) Ricin-specific GMT on day 17 following RVEc immunizations (A) or on day 27 following RiVax immunizations (B). (C) Toxin-neutralizing activity in sera collected on day 27 following a prime and two boosts with LT-IIa and RiVax. *, P < 0.05. (D) Mice immunized with RVEc without (diamonds) or with LT-IIa at 1 μg (squares), 0.3 μg (inverted triangles), 0.1 μg (triangles), or 0.03 μg (circles) were challenged with ricin on day 22 after a prime-boost regimen. Naive control mice are indicated by the × symbol. When two or more lines on the graph overlapped because of identical survival patterns (e.g., 100% survival), the “nudge” option was applied in GraphPad to make each line visible. There were six animals per group (n = 6) for experiments presented in panels A and D and 10 (n = 10) for experiments presented in panels B and C.
TABLE 2.
Effect of LT-IIa on ricin-specific GMT and TNA when combined with RiVax or RVEc
| RiVax (2 or 3×)a |
||||||
|---|---|---|---|---|---|---|
| LT-IIa (μg) | RVEc (2×) on day 17a |
Day 17 |
Day 27 |
|||
| GMT | % Viabb | GMT | % Viabb | GMT | % Viabb | |
| 1 | 18,102 | 1 | 33,779 | 4 | 72,408 | 90 |
| 0.3 | 18,102 | 1 | 15,759 | 2 | 41,587 | 65 |
| 0.1 | 11,404 | 0 | 5,198 | 1 | 31,517 | 59 |
| 0.03 | 11,404 | 2 | 7,352 | 2 | 54,875 | 54 |
| 0 | 1,008 | 0 | 159 | 1 | 16,890 | 9 |
Antigen and number of immunizations. Both antigens were administered at 5 μg.
Mean Vero cell viability with sera diluted 1:50, as described in Materials and Methods.
Adjuvant activity of LT-IIa compared to Alhydrogel.
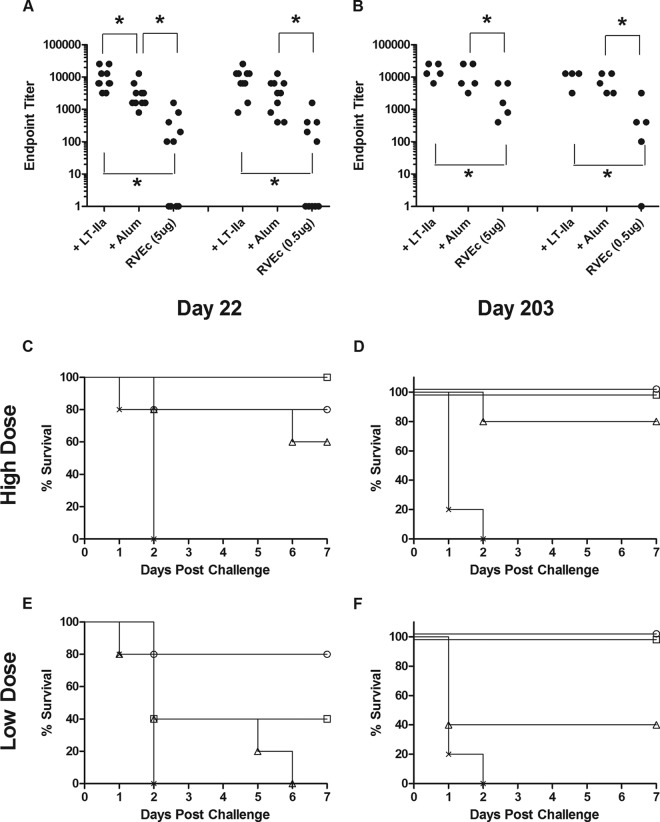
Although aluminum salt is not an adjuvant normally administered via the i.d. route, it does constitute the standard against which new adjuvants are generally compared (1). For this reason, we chose to perform a comparative vaccination study between Alhydrogel (0.85 mg/ml) and LT-IIa (0.03 μg) in conjunction with high- and low-dose RVEc. Groups of mice were immunized by the i.d. route on days 0 and 10. On day 17, each group was divided into two subgroups: one subgroup was challenged with ricin on day 22, while the other was monitored over a 6-month period for the onset of ricin-specific GMT and TNA before being challenged with ricin on day 203. LT-IIa and Alhydrogel were virtually identical with respect to their abilities to adjuvant RVEc, as evidenced by nominal differences in GMT, TNA, and survival on days 22 and 203 (Fig. 5; also see Tables S1 and S2 in the supplemental material). Taken together, these results demonstrated that LT-IIa was as effective as Alhydrogel in serving as an adjuvant for RVEc when delivered i.d.
FIG 5.
Comparison between LT-IIa and Alhydrogel as adjuvants in conjunction with RVEc. Groups of BALB/c mice (n = 10 per group) were immunized by the i.d. route on days 0 and 10 with low- or high-dose RVEc adsorbed to Alhydrogel (Alum) or combined with LT-IIa (0.03 μg), as described in Materials and Methods. On day 22, each group of animals was divided into two groups of five. Groups of mice were challenged with ricin on day 22 or 203. (A and B) Ricin-specific GMTs in sera collected on day 17 (n = 10) (A) or 199 (n = 5) (B). (C to F) Survival curves for groups of animals (n = 5/group) that received high- (C and D) or low-dose (E and F) RVEc and were challenged on day 22 (C and E) or 203 (D and F). Symbols: circles, LT-IIa; squares, Alhydrogel; triangles, RVEc; crosses, control mice. When two or more lines on the graph overlapped because of identical survival patterns (e.g., 100% survival), the “nudge” option was applied in GraphPad to eliminate overlap and make each line visible. *, P < 0.05.
In a separate but related study, we investigated the potential of LT-IIa to work in concert with Alhydrogel. In this experiment, groups of mice received i.d. injections of low-dose RVEc plus LT-IIa, Alhydrogel, or a combination of the two on days 0 and 10. We also evaluated LT-IIa in combination with LT-IIbT13I using the same regimen. Analysis of serum antibody responses on day 17 demonstrated that all three adjuvants administered individually enhanced ricin toxin-specific serum IgG levels over those observed in RVEc-vaccinated mice but that no additional benefit was observed by combining adjuvants (see Fig. S1 in the supplemental material). Thus, the adjuvant effects of LT-IIa are not additive to those observed with aluminum salts or LT-IIbT13I.
DISCUSSION
The type II heat-labile enterotoxins constitute a promising set of adjuvants that have been shown to enhance systemic and mucosal humoral and cellular responses to model antigens and several pathogen-associated antigens (7). In this study, we compared the four best-studied type II HLTs, namely, LT-IIa, LT-IIb, LT-IIbT13I, and LT-IIc, for their abilities to serve as adjuvants for two different clinically relevant protein subunit antigens, RiVax and RVEc, in a mouse model. While all four enterotoxins tested had significant adjuvant activity and are worthy of further investigation, LT-IIa was of particular interest in that extremely low doses of LT-IIa proved sufficient to enhance the onset and magnitude of ricin-specific serum IgG levels with only nominal local inflammatory responses. The fact that the adjuvant and inflammatory activities can be uncoupled increases the likelihood that LT-IIa will be amenable for use in humans (1).
The immunomodulatory properties of LT-IIa are distinct from those elicited by CT, LT-I, or the other type II HLTs. LT-IIa, originally identified from a strain of E. coli isolated from water buffalo, has a unique ganglioside binding profile summarized as binding avidly to GD1b and secondarily to GD1a, GM1, and others (18, 19). When administered intranasally to mice, LT-IIa induce a balanced Th1 and Th2 cytokine profile with subsequent enhancement of IgG1, IgG2a, IgG2b, and mucosal IgA responses (20). When coadministered with OVA to mice via the i.d. route, LT-IIa promoted OVA-specific serum IgG titers and induced OVA-specific CD8+ T cells (21). Mathias-Santos and colleagues tested LT-IIa's adjuvant activity at only a single dose (0.5 μg). It would be interesting to examine antigen-specific CD8+ T cell responses with low-dose LT-IIa in light of our finding that LT-IIa retains adjuvant activity (e.g., induction of serum IgG) in the low-nanogram level.
It has been reported that ADP-ribosylating toxins like CT and LT, when administered as adjuvants to rodents and humans by the transcutaneous route, stimulate the production of antigen-specific mucosal antibody responses (22–24). By extension, one might expect that LT-II enterotoxins administered i.d. might also induce antigen-specific secretory IgA (SIgA) antibody responses. Eliciting mucosal immune responses to ricin toxin is obviously beneficial, considering its toxicity following inhalation or ingestion (25–27). In the current study, we did assess, using protocols previously established in our laboratory (28), ricin-specific IgA antibodies in fecal pellets from mice immunized with RiVax and the four different LT-II adjuvants. However, in no instance did we detect levels of IgA above background (data not shown), suggesting that the four LT-II adjuvants tested here do not communicate with the gastrointestinal mucosal immune system following i.d. delivery. We did not extend our studies to include lung or bronchial alveolar lavage.
Results presented in the current study extend a previous report from our laboratory indicating that RiVax and RVEc are effectively indistinguishable with respect to their ability to elicit ricin-specific serum IgG titers, toxin-neutralizing activity, and protective immunity to ricin (12). In the previous study, RiVax and RVEc were adsorbed onto Alhydrogel and then administered to mice by subcutaneous (s.c.) injection. We have now demonstrated that RiVax and RVEc are indeed identical to each other even when administered to mice with type II HLT adjuvants rather than aluminum salts and by i.d. immunization rather than s.c. immunization.
Another point worth noting is that the onset of TNA following immunization with RiVax and RVEc, even with the addition of LT-IIa, generally lagged well behind peak or near-peak ricin-specific GMTs. This lag in TNA relative to total toxin-specific antibody levels has been noted in previous studies conducted in mice (16) and, very recently, in rhesus macaques (15). In the former study, groups of mice received RiVax, a modified anthrax protective antigen (PA), or a combination of the two. By day 20 following a prime-boost regimen, we noted that 19/20 PA-immunized mice had anthrax lethal toxin-neutralizing antibodies, whereas 1/20 RiVax-immunized mice had ricin-specific TNA. We speculated in that study that it may be intrinsically more difficult to neutralize ricin than other toxins because of a limited number of neutralizing B cell epitopes on RTA (29, 30). As a result, potent ricin-neutralizing antibodies may not arise until a certain threshold of specificity and/or avidity is achieved that is above that for other protein toxins. We can conclude from our current study that the observed lag in TNA following RiVax and RVEc vaccination is not limited to the use of aluminum salts adjuvant as we previously had suggested, since the same effects were observed with LT-II enterotoxins.
ACKNOWLEDGMENTS
We thank Soligenix, Inc. (Princeton, NJ) for providing us with RiVax and Len Smith (USAMRIID) for providing RVEc used in these studies. We also thank Erin Sully and Greta Van Slyke for technical assistance.
The work described here was supported by contract HDTRA1-13C-0094 from the Defense Threat Reduction Agency (DTRA).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00402-15.
REFERENCES
- 1.Alving CR, Peachman KK, Rao M, Reed SG. 2012. Adjuvants for human vaccines. Curr Opin Immunol 24:310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitz SM, Golenbock DT. 2012. Beyond empiricism: informing vaccine development through innate immunity research. Cell 148:1284–1292. doi: 10.1016/j.cell.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, Schindler J. 2006. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A 103:2268–2273. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitetta ES, Smallshaw JE, Schindler J. 2012. Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol 19:1697–1699. doi: 10.1128/CVI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe DN, Florence W, Bryant P. 2013. Current biodefense vaccine programs and challenges. Hum Vaccin Immunother 9:1591–1597. doi: 10.4161/hv.24063. [DOI] [PubMed] [Google Scholar]
- 7.Hajishengallis G, Connell TD. 2013. Type II heat-labile enterotoxins: structure, function, and immunomodulatory properties. Vet Immunol Immunopathol 152:68–77. doi: 10.1016/j.vetimm.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jobling MG, Holmes RK. 2012. Type II heat-labile enterotoxins from 50 diverse Escherichia coli isolates belong almost exclusively to the LT-IIc family and may be prophage encoded. PLoS One 7:e29898. doi: 10.1371/journal.pone.0029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu JC, Mathias-Santos C, Greene CJ, King-Lyons ND, Rodrigues JF, Hajishengallis G, Ferreira LC, Connell TD. 2014. Intradermal administration of the type II heat-labile enterotoxins LT-IIb and LT-IIc of enterotoxigenic Escherichia coli enhances humoral and CD8+ T cell immunity to a co-administered antigen. PLoS One 9:e113978. doi: 10.1371/journal.pone.0113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nawar HF, Arce S, Russell MW, Connell TD. 2005. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect Immun 73:1330–1342. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, Khader SA. 2013. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol 6:972–984. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hara JM, Brey RN III, Mantis NJ. 2013. Comparative efficacy of two leading candidate ricin toxin A subunit vaccines in mice. Clin Vaccine Immunol 20:789–794. doi: 10.1128/CVI.00098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene CJ, Chadwick CM, Mandell LM, Hu JC, O'Hara JM, Brey RN III, Mantis NJ, Connell TD. 2013. LT-IIb(T13I), a non-toxic type II heat-labile enterotoxin, augments the capacity of a ricin toxin subunit vaccine to evoke neutralizing antibodies and protective immunity. PLoS One 8:e69678. doi: 10.1371/journal.pone.0069678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pincus SH, Eng L, Cooke CL, Maddaloni M. 2002. Identification of hypoglycemia in mice as a surrogate marker of ricin toxicosis. Comp Med 52:530–533. [PubMed] [Google Scholar]
- 15.Roy CJ, Brey RN, Mantis NJ, Mapes K, Pop IV, Pop LM, Ruback S, Killeen SZ, Doyle-Meyers L, Vinet-Oliphant HS, Didier PJ, Vitetta ES. 2015. Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: epitope-specific neutralizing antibodies correlate with protection. Proc Natl Acad Sci U S A 112:3782–3787. doi: 10.1073/pnas.1502585112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vance DJ, Rong Y, Brey RN III, Mantis NJ. 2015. Combination of two candidate subunit vaccine antigens elicits protective immunity to ricin and anthrax toxin in mice. Vaccine 33:417–421. doi: 10.1016/j.vaccine.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meagher MM, Seravalli JG, Swanson ST, Ladd RG, Khasa YP, Inan M, Harner JC, Johnson SK, Van Cott K, Lindsey C, Wannemacher R, Smith LA. 2011. Process development and cGMP manufacturing of a recombinant ricin vaccine: an effective and stable recombinant ricin A-chain vaccine-RVEc. Biotechnol Prog 27:1036–1047. doi: 10.1002/btpr.631. [DOI] [PubMed] [Google Scholar]
- 18.Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun 56:1748–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawar HF, Arce S, Russell MW, Connell TD. 2007. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect Immun 75:621–633. doi: 10.1128/IAI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. 2000. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect Immun 68:281–287. doi: 10.1128/IAI.68.1.281-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathias-Santos C, Rodrigues JF, Sbrogio-Almeida ME, Connell TD, Ferreira LC. 2011. Distinctive immunomodulatory and inflammatory properties of the Escherichia coli type II heat-labile enterotoxin LT-IIa and its B pentamer following intradermal administration. Clin Vaccine Immunol 18:1243–1251. doi: 10.1128/CVI.00012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glenn GM, Flyer DC, Ellingsworth LR, Frech SA, Frerichs DM, Seid RC, Yu J. 2007. Transcutaneous immunization with heat-labile enterotoxin: development of a needle-free vaccine patch. Expert Rev Vaccines 6:809–819. doi: 10.1586/14760584.6.5.809. [DOI] [PubMed] [Google Scholar]
- 23.Scharton-Kersten T, Yu J, Vassell R, O'Hagan D, Alving CR, Glenn GM. 2000. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect Immun 68:5306–5313. doi: 10.1128/IAI.68.9.5306-5313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Cassels F, Scharton-Kersten T, Hammond SA, Hartman A, Angov E, Corthesy B, Alving C, Glenn G. 2002. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect Immun 70:1056–1068. doi: 10.1128/IAI.70.3.1056-1068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sully EK, Whaley KJ, Bohorova N, Bohorov O, Goodman C, Kim DH, Pauly MH, Velasco J, Hiatt E, Morton J, Swope K, Roy CJ, Zeitlin L, Mantis NJ. 2014. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin Vaccine Immunol 21:777–782. doi: 10.1128/CVI.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smallshaw JE, Richardson JA, Vitetta ES. 2007. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 25:7459–7469. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoder JM, Aslam RU, Mantis NJ. 2007. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect Immun 75:1745–1750. doi: 10.1128/IAI.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal LM, McCarthy EA, Morris CR, Mantis NJ. 2011. Vaccine-induced intestinal immunity to ricin toxin in the absence of secretory IgA. Vaccine 29:681–689. doi: 10.1016/j.vaccine.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hara JM, Mantis NJ. 2013. Neutralizing monoclonal antibodies against ricin's enzymatic subunit interfere with protein disulfide isomerase-mediated reduction of ricin holotoxin in vitro. J Immunol Methods 395:71–78. doi: 10.1016/j.jim.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. 2014. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA). Immunol Lett 158:7–13. doi: 10.1016/j.imlet.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]