Abstract
Previous studies have highlighted the efficacy of tumor necrosis factor alpha (TNF-α) inhibitors, including monoclonal antibodies and soluble receptors, in the treatment and management of intestinal bowel disease (IBD). However, because of the immunogenicity of xenogeneic TNF-α inhibitors, antidrug antibodies (ADAs) can be triggered after repeated administration. An alternative way to target TNF-α is active immunization to elicit the production of high titers of neutralizing antibodies. In this study, we prepared a xenogeneic TNF-α protein vaccine and studied the protective effects in experimental colitis models. The xenogeneic TNF-α protein vaccine could overcome self-tolerance and induce TNF-α-specific neutralizing antibody. Moreover, the xenogeneic TNF-α protein vaccine could protect mice from acute and chronic colitis induced by dextran sodium sulfate (DSS). One possible explanation for this protective effect is the production of TNF-α-specific neutralizing antibody, which absorbed the biological activity of mouse TNF-α (mTNF-α) and failed to induce T lymphocyte apoptosis. In summary, use of the xenogeneic TNF-α protein vaccine may be a potent therapeutic strategy for IBD.
INTRODUCTION
Intestinal bowel disease (IBD), characterized as chronic relapsing inflammatory disorders of the gastrointestinal tract (1), is primarily a syndrome of the developed world (2). However, an increasing rate of prevalence has been observed in traditionally low-incidence regions such as Asia, South America, and southern and eastern Europe (3). The conventional treatments are limited to anti-inflammatory drugs and immune-suppressive medications. However, their application has been restricted by problems with long-term efficacy and safety issues (4).
Previous studies have highlighted the efficacy of tumor necrosis factor alpha (TNF-α) inhibitors, including monoclonal antibodies (MAbs) and soluble receptors, in the treatment and management of IBD, especially in patients who are refractory to or intolerant of the conventional treatment regimens (5). TNF-α, a pleiotropic proinflammatory cytokine, shows increased expression in the mucosa of inflamed intestine (6–8). However, because of the immunogenicity of the xenogeneic TNF-α inhibitors, antidrug antibodies (ADAs) can be triggered after repeated administration, leading to treatment resistance (9). The reported rates of loss of response (LOR) ranged between 11% and 48% (10). Furthermore, these therapeutic approaches are expensive and cumbersome. These limitations prompted investigations of alternative strategies, including active anti-TNF-α immunization.
However, because of immune tolerance, immunity to self-antigens is difficult to elicit. Our previous studies have explored the feasibility of immunotherapy of tumors by treatment with xenogeneic homologous molecules as vaccines against those on autologous cells in a cross-reaction between the xenogeneic homologous and self-molecules (11–14). However, this xenogeneic vaccination strategy has not yet been tested in inflammatory diseases. In this study, we prepared a xenogeneic TNF-α protein vaccine and studied the protective effects in a mouse IBD model.
MATERIALS AND METHODS
Experimental mice.
Male 6-to-8-week-old C57BL/6 mice were bred and kept under pathogen-free conditions. All animal protocols were approved by the Animal Care and Use Committee of State Key Laboratory of Biotherapy.
Plasmid construction.
Human TNF-α (hTNF-α) and mouse TNF-α (mTNF-α) open reading frames (ORF) were purchased from InvivoGen (San Diego, CA, USA). cDNA fragments coding soluble mTNF-α (residues 80 to 235) and hTNF-α (residues 77 to 233) were amplified by Ex Taq DNA polymerase (TaKaRa Biotechnology, Dalian, China). Primers used in this study were as follows: for human TNF-α, 5′-GGGGTACCGACGACGACGACAAGGTCAGATCATCTTCTCGAAC-3′ (sense primer) and 5′-CCGCTCGAGTCACAGGGCAATGATCCCA-3′ (antisense primer); and for mouse TNF-α, 5′-GGGGTACCGACGACGACGACAAGCTCAGATCATCTTCTCAAAATTC-3′ (sense primer) and 5′-CCGCTCGAGTCACAGAGCAATGACTCCAAAG-3′ (antisense primer). The KpnI site is underlined, the XhoI site is shown in bold, and the enterokinase site coding sequence is shown in italics. The PCR product was digested with KpnI and XhoI (NEB, Ipswich, MA, USA) and was subcloned into a pET32a(+) vector (Novagen, Madison, WI, USA). The resultant recombinant protein consisted of thioredoxin (Trx), His tag, the cleavage sequence of thrombin, S tag, and soluble TNF-α.
Protein expression and purification.
The Escherichia coli BL21(DE3) strain bearing the expression plasmids was induced with IPTG (isopropyl-β-d-thiogalactopyranoside) for protein production. The bacteria were lysed using a high-pressure homogenizer (GEA Niro Soavi, Parma, Italy). mTNF-α or hTNF-α protein was purified via four processing steps, which included Ni-chelating Sepharose affinity chromatography (GE Healthcare, Piscataway, NJ, USA), excision of the Trx-His6 tag by enterokinase, removal of the Trx-His6 tag with secondary Ni-chelating Sepharose affinity chromatography, and the use of HiTrap Q HP ion exchange columns (GE Healthcare). The protein concentration was estimated by the use of a protein assay reagent (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as a standard, and the purity was estimated by SDS-PAGE and high-performance liquid chromatography (HPLC) analysis. Both proteins were negative for endotoxin contamination in the limulus amebocyte lysate (LAL) test. Moreover, the recombinant proteins were characterized by Western blotting assay with rabbit monoclonal anti-TNF-α antibodies (CST, Danvers, MA, USA), and peptide mass fingerprinting were determined by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analysis as described before (15, 16).
TNF-α bioassay and the neutralizing activity of TNF-α-specific Abs.
The activity of the recombinant proteins was determined using TNF-α-sensitive L929 fibroblasts as described before (17). L929 cells were treated with serial dilutions of recombinant TNF-α for 24 h in the presence of actinomycin D (Sigma-Aldrich, St. Louis, MO, USA, 1 μg/ml). The viable cells were identified by crystal violet staining.
The neutralizing activity of TNF-α-specific Abs was similarly determined after incubation with serially diluted sera.
Immunizations.
Male C57BL/6 mice were randomized into 3 groups and immunized subcutaneously with 10 μg mTNF-α or hTNF-α in a total volume of 100 μl in combination with Alhydrogel (Brenntag Biosector, Frederikssund, Denmark) as the adjuvant six times at 1-week intervals. As a negative control, mice in the third group (phosphate buffer [PB] group) were immunized with 100 μl of PB in a combination with Alhydrogel. Sera were collected 5 days after immunization and at death. Moreover, weight loss, ruffling of fur, behavior, and feeding data were recorded twice a week during TNF-α vaccination.
Anti-hTNF-α Ab titer assay.
Specific anti-mTNF-α antibody titers in sera of immunized and control mice were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well polystyrene plates were precoated with 1 μg/well mTNF-α in coating buffer (carbonate-bicarbonate; pH 9.6) overnight at 4°C. After being washed and blocked, the plates were incubated with serial dilutions of sera from immunized and control mice for 2 h at 37°C. The plates were washed and incubated with 100 μl horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10,000 dilution) for 1 h at 37°C followed by 100 μl/well Sureblue TMB (3,3′,5,5′-tetramethylbenzidine) substrate (KPL, Inc., Gaithersburg, MD, USA) for 15 min. The color development was stopped by adding 1 M H2SO4 (50 μl/well), and the optical density (OD) value was measured at 450 nm using a Multiskan MK3 microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The results were considered positive when the ratio of absorbency in the antiserum group versus the control serum group was greater than 2.1.
Induction of colitis.
Male C57BL/6 mice were allowed free access to a solution of 2.5% DSS (MP Biomedicals, LLC, Eschwege, Germany) (dissolved in sterile, distilled water; molecular weight, 36,000 to 50,000 [wt/vol]) as drinking water for 7 days. Chronic colitis was induced by three cycles of administration of 2% DSS for 5 days, alternating with DSS-free drinking water for 5 days.
Determination of DAI score.
Animal body weight, stool consistency, and the presence of occult or gross blood were recorded using a scale to evaluate clinical disease severity, as described previously (1, 18). The scores were defined as follows: change in body weight (0, none; 1, 1% to 5%; 2, 5% to 10%; 3, 10% to 15%; 4, >15%), change in stool consistency (0, normal; 2, loose stool; 4, diarrhea), and change in stool blood status (0, negative; 2, positive; 4, gross bleeding). The clinical disease activity index (DAI) score, ranging from 0 to 4, represented the sum of scores for these parameters divided by 3.
Histological scoring.
After mice were sacrificed, the entire colon was removed and fixed in 10% buffered formalin for histological analysis. Sections (4-μm thick) were prepared and subjected to staining with hematoxylin and eosin (H&E). Histological scoring was performed using a previously described scoring system by two experienced pathologists in a blind fashion (1, 19). Three independent parameters were measured (severity of inflammation, depth of injury, and crypt damage) and scored from 0 (normal) to 3 (1, severe; 2, transmural injury; 3, inflammation with entire crypt and epithelium loss). The score of each parameter was multiplied by a factor reflecting the percentage of tissue involvement (1, 0% to 25%; 2, 26% to 50%; 3, 51% to 75%; 4, 76% to 100%), and the scores were summed to obtain a histological injury score.
Furthermore, liver, kidney, lung, spleen, or heart tissues were removed, and pathological studies were tested to investigate potential adverse effects.
Adoptive transfer of immune sera.
Serum from mice vaccinated with hTNF-α or mTNF-α protein was obtained 1 week after the last immunization and stored at 4°C until use. Acute colitis was induced in mice as described above, and the mice were injected intraperitoneally (i.p.) with 0.2 ml of the serum from vaccinated mice on days 0, 2, 4, and 6 after DSS treatment. Animals treated with serum from control mice served as controls.
Cytokine quantification.
Serum mTNF-α levels were measured with a commercially available ELISA kit (Neobioscience, Shenzhen, China) according to the manufacturer's instructions.
Detection of apoptosis in vitro.
To measure the effects of mTNF-α-specific antiserum on apoptosis of T lymphocytes, splenocytes were dissociated by crushing the mouse spleen with a 2-ml syringe piston on a 70-μm-pore-size filter (cell strainer; BD Biosciences, San Jose, CA, USA) and were then were separated by the use of Ficoll solution according to the manufacturer's protocol. Purified lymphocytes were incubated in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum in the presence of 2 μg/ml of anti-CD3 antibody (BD Biosciences) for 48 h. Activated T lymphocytes were washed and resuspended in culture medium in the presence of sera at a dilution of 1:100. After 24 h, cells were doubly stained with annexin V staining and propidium iodide (PI) to detect apoptosis. Data were acquired using a Novocyte flow cytometer (ACEA Biosciences, San Diego, CA, USA).
Statistical analysis.
Parametric data were analyzed statistically with one-way analysis of variance (ANOVA) followed by Tukey's multicomparison test when appropriate. In all cases, a P value of 0.05 was determined to be significant.
RESULTS
Expression, purification, and characterization of recombinant proteins.
hTNF-α and mTNF-α recombinant proteins were expressed in the E. coli expression system and purified as described above. The purified proteins were analyzed by SDS-PAGE and HPLC, and the results indicated that the purity of both recombinant proteins was above 90% of the total protein amount (Fig. 1A and B). The recombinant proteins were recognized specifically by the anti-TNF-α antibodies, as shown by the results of a Western blot assay (Fig. 1C). Moreover, both recombinant proteins were characterized by MALDI-TOF analysis (data not shown).
FIG 1.
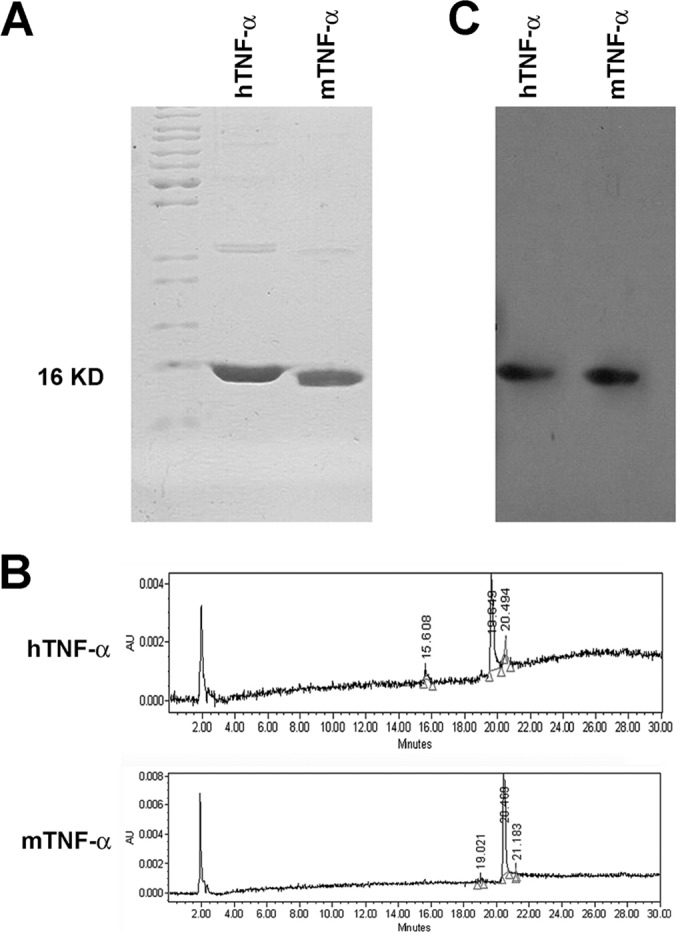
Characterization of recombinant hTNF-α and mTNF-α proteins. (A and B) The purified proteins were analyzed by SDS-PAGE (A) and HPLC (B). (C) Western blot showing that the recombinant proteins were recognized specifically by the anti-TNF-α antibodies. AU, arbitrary units.
hTNF-α vaccine induces anti-TNF-α Abs.
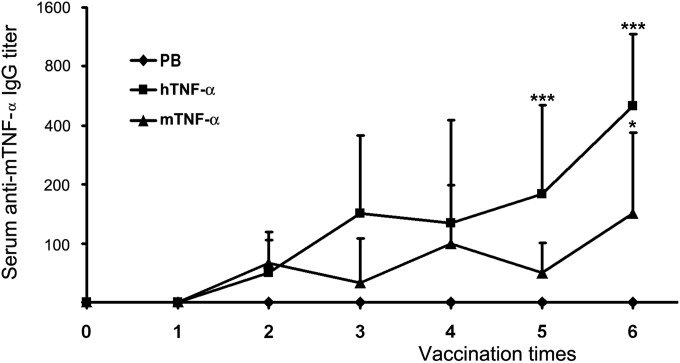
C57BL/6 mice were immunized subcutaneously with 10 μg mTNF-α or hTNF-α in a total volume of 100 μl in combination with Alhydrogel as the adjuvant. As shown in Fig. 2, after the last vaccination, hTNF-α vaccine could overcome self-tolerance and induce mTNF-α-specific Abs in all mice, whereas immunization with mTNF-α elicited much lower levels of anti-mTNF-α Abs in only 4 of 6 mice (P = 0.023).
FIG 2.
mTNF-α-specific IgG responses after TNF-α protein vaccination. C57BL/6 mice were immunized subcutaneously with mTNF-α or hTNF-α proteins in combination with 2% Alhydrogel as the adjuvant 6 times at 1-week intervals. Sera were collected 5 days after immunization and at death. Data represent geometric mean antigen-specific IgG titers ± standard deviations (SD) for n = 6 mice/group. *, P < 0.05; ***, P < 0.001 (compared with the results from the PB control group).
hTNF-α vaccination protects mice from development of colitis.
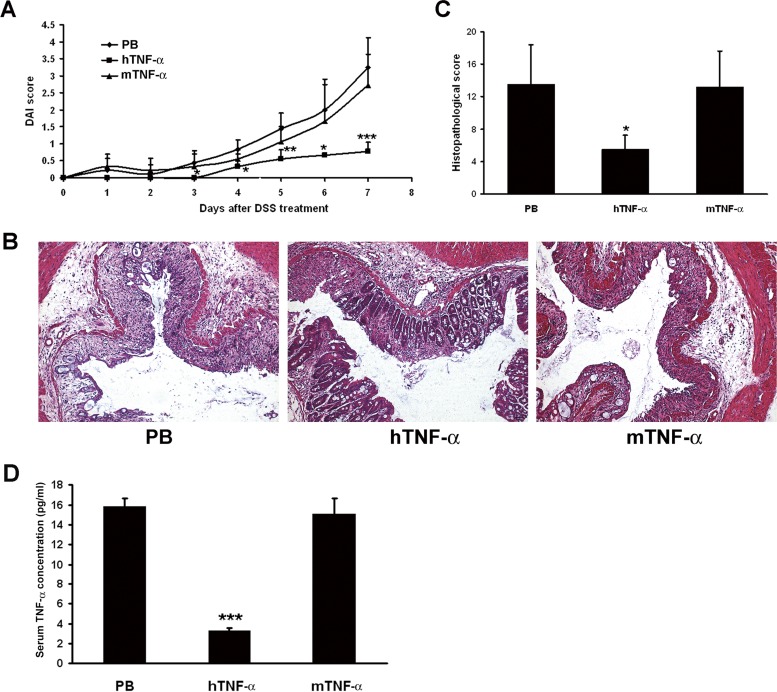
We first tested the protective effect of TNF-α vaccine in an acute colitis model. One week after the last immunization, mice were fed 2.5% DSS for 7 days to induce acute colitis. mTNF-α-vaccinated mice or control mice showed body weight loss, diarrhea, and bleeding in stool, resulting in a sharp increase in the DAI score. In contrast, hTNF-α-vaccinated mice exhibited a significantly reduced DAI score from day 4 to day 7 (P < 0.05) (Fig. 3A).
FIG 3.
Protective effect of TNF-α vaccine in acute colitis model. One week after the last immunization, mice were fed 2.5% DSS for 7 days to induce acute colitis. (A) The DAI score was recorded every 2 days. Data represent mean DAI scores ± SD for n = 6 mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with the results from the PB control group). (B) Photomicrographs of representative H&E-stained colon sections. The colon section photographs are representative of 6 mice for each group (magnification, ×100). (C) Histological scoring was performed by two experienced pathologists in a blind fashion. Data represent mean histological scores ± SD for n = 6 mice/group. *, P < 0.05 (compared with the results from the PB control group). (D) The effect of hTNF-α vaccination on serum TNF-α concentrations. Data represent mean serum TNF-α concentrations ± SD for n = 3 mice/group. ***, P < 0.001 (compared with the results from the PB control group).
A well-established histopathological event, including infiltration of inflammatory cells, ulceration, and crypt damage, was detected by H&E staining of colonic tissue sections from mTNF-α-vaccinated mice or control mice. In contrast, the colons from hTNF-α-vaccinated mice were relatively normal, exhibiting only mild evidence of infiltration of inflammatory cells and mucosal injury (Fig. 3B). Furthermore, colonic tissue sections from hTNF-α-vaccinated mice also showed a significantly lower histological score than colonic tissue sections from mTNF-α-vaccinated or control mice (P = 0.006; Fig. 3C).
We further studied the effect of hTNF-α vaccination on serum TNF-α concentrations at the end of the acute-colitis experiment. As shown in Fig. 3D, TNF-α concentrations of serum from hTNF-α-vaccinated mice were significantly reduced (P < 0.001).
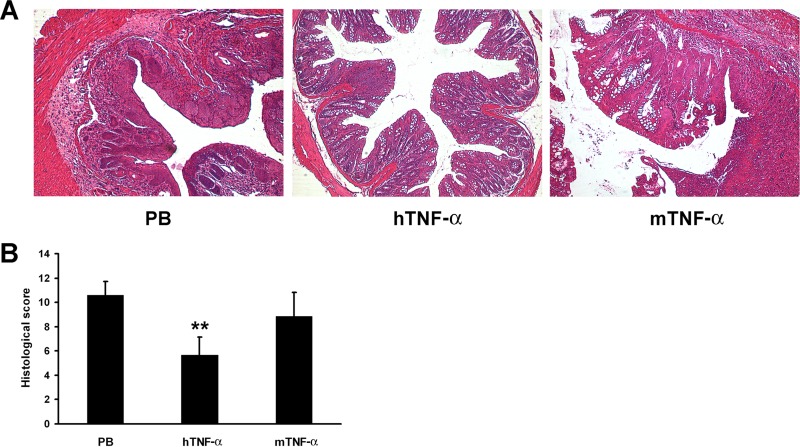
IBD is characterized by chronic relapsing inflammatory disorders of the gastrointestinal tract, so we further tested the protective effect of TNF-α vaccine in a chronic colitis model. Similarly to the results seen in the acute colitis model, hTNF-α vaccination protected mice from crypt loss, erosions, and inflammatory cell infiltrations as well from as the DSS-induced increase in the histological score (Fig. 4).
FIG 4.
Protective effect of TNF-α vaccine in chronic colitis model. One week after the last immunization, mice were fed by three cycles of administration of 2% DSS in drinking water for 5 days, alternating with 5-day periods of recovery. (A) Photomicrographs of representative H&E-stained colon sections. The colon section photographs are representative of 6 mice for each group (magnification, ×100). (B) Histological scoring was performed by two experienced pathologists in a blind fashion. Data represent mean histological scores ± SD for n = 6 mice/group. **, P < 0.01 (compared with the results from the PB control group).
Moreover, no change of weight loss, ruffling of fur, behavior, or feeding was recorded during TNF-α vaccination. And no pathological changes in the liver, kidneys, lungs, spleen, or heart were observed (see Fig. S1 in the supplemental material).
Self-specific Abs developed in hTNF-α-vaccinated mice are neutralizing in vitro.
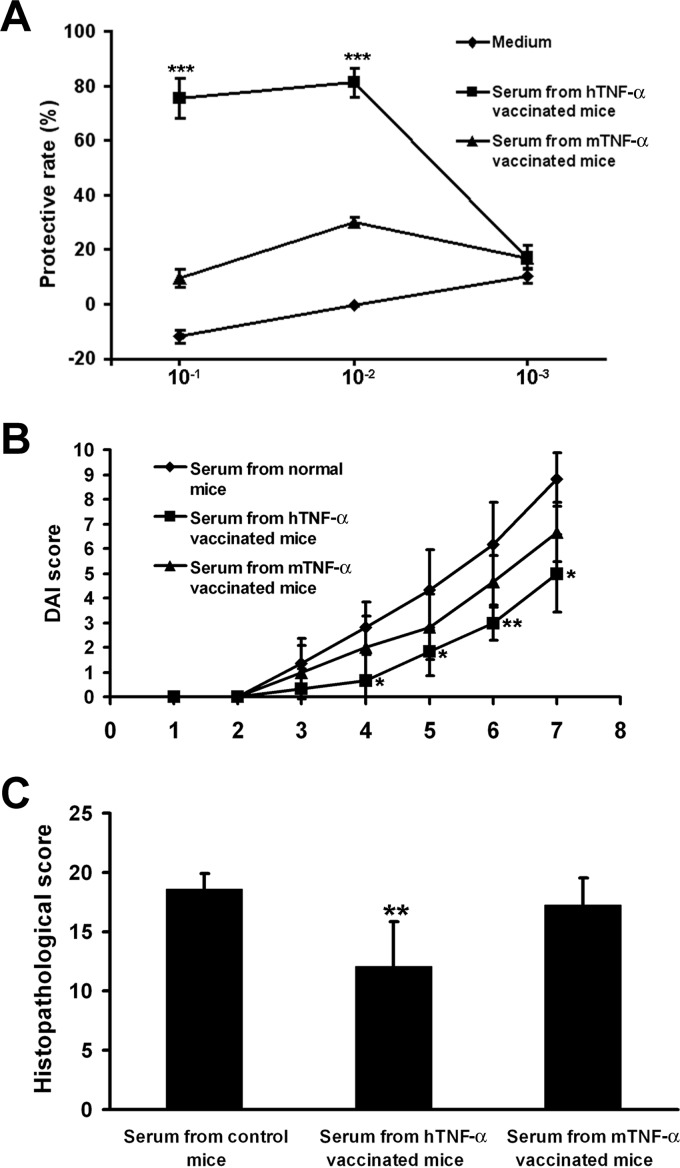
To evaluate the ability of the Abs to neutralize TNF-α in vitro, L929 cells were treated with mTNF-α and serially diluted sera. As shown in Fig. 5A, serum at a high dilution from hTNF-α-vaccinated mice abolished the cytotoxic activity of mTNF-α on L929 cells, whereas there were no protective effects of sera from mTNF-α-vaccinated or control mice.
FIG 5.
Neutralizing effects of TNF-α-specific antibodies. (A) L929 cells were treated with mTNF-α and serially diluted sera, and protective rates were calculated. Data represent mean protective rates ± SD for n = 3 mice/group. ***, P < 0.001 (compared with the results from the PB control group). (B and C) Mice were treated with DSS to induce acute colitis and administered sera from hTNF-α- or mTNF-α-vaccinated mice. Mice were sacrificed on day 7 after DSS treatment, and colons were collected for histological examination. (B) Data represent mean DAI scores ± SD for n = 6 mice/group. *, P < 0.05; **, P < 0.01 (compared with the results from the PB control group). (C) Data represent mean histological scores ± SD for n = 6 mice/group. **, P < 0.01 (compared with the results from the PB control group).
Furthermore, a serum adoptive-transfer experiment was performed in this study. Mice were treated with DSS to induce acute colitis and administered sera from hTNF-α- or mTNF-α-vaccinated mice. Mice were sacrificed on day 7 after DSS treatment, and colons were collected for histological examination. Serum from mTNF-α-vaccinated mice showed no protective effects on colitis, whereas serum from hTNF-α-vaccinated mice significantly reduced the DAI and histological damage scores (Fig. 5B and C).
TNF-α-specific antiserum fails to induce T lymphocyte apoptosis.
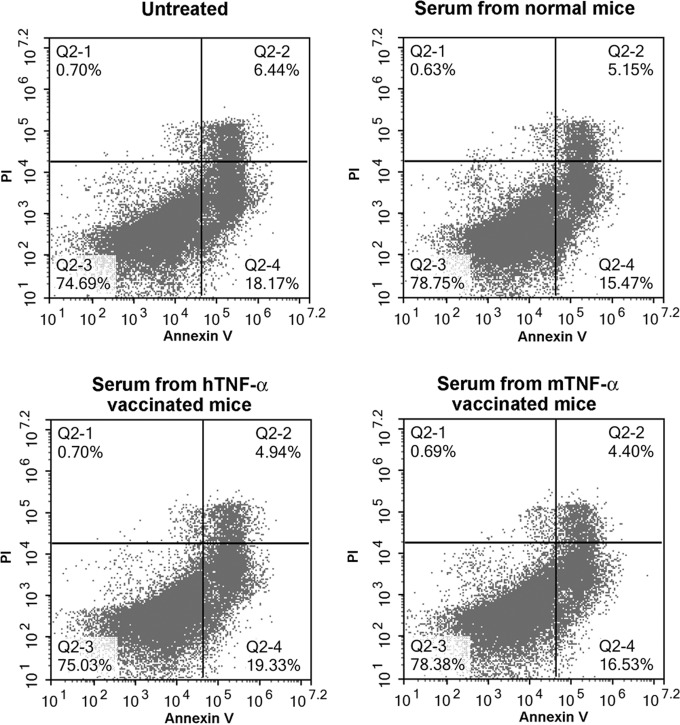
It was reported that, in addition to exhibiting a neutralizing effect, infliximab, a chimeric anti-TNF-α monoclonal antibody, could induce apoptosis in activated T lymphocytes (20, 21). We further investigated the effects of antiserum on T lymphocyte apoptosis. As shown in Fig. 6, no obvious increase of T lymphocyte apoptosis was detected after treatment with serum from hTNF-α-vaccinated mice.
FIG 6.
The effect of TNF-α-specific antiserum on activated T lymphocyte apoptosis. Activated T lymphocytes were treated with antiserum at a dilution of 1:100 for 24 h, and then cells were doubly stained with annexin V and propidium iodide (PI) to detect apoptosis. Data shown are representative of the results of 3 independent experiments.
DISCUSSION
Recently, the introduction of TNF-α inhibitors highlighted a pivotal role in the pathogenesis of IBD (22). Levels of TNF-α, which is mainly produced by activated macrophages, monocytes, eosinophils, and T cells, are increased in the mucosa and serum of IBD patients (23). TNF-α is a pleiotropic proinflammatory cytokine that binds to its receptors TNFR1 and TNFR2 followed by the intracellular activation of nuclear factor-κB (NF-κB) (7), resulting in the activation of immune cells, increased angiogenesis, the induction of Paneth cell death, the production of matrix metalloproteinases (MMPs) by myofibroblasts, and the direct damage of intestinal epithelial cells (24–27). These studies indicated that inhibition of TNF-α by monoclonal antibodies or soluble receptors is among the effective approaches for IBD treatment (28). However, because of the immunogenicity of the xenogeneic TNF-α inhibitors, ADAs can be triggered after repeated administration. Previous studies have investigated formation of ADAs and its influence on infliximab concentrations and clinical outcomes (29, 30). ADAs might reduce the efficiency of the drug by competing with the endogenous ligands and/or by forming immune complexes, which accelerates the clearance of the drug from the circulation (31).
In this study, we investigated the efficiency of a protein vaccine against TNF-α in IBD treatment by eliciting the production of neutralizing antibodies. This strategy allows the production of polyclonal autologous anti-TNF-α antibodies, potentially bypassing the risk of formation of ADAs. However, it is difficult to elicit immune responses to self-antigens because of immune tolerance (32). Recently, a heterocomplex vaccine, called human TNF-α kinoid (TNF-K), consisting of a biologically inactive but immunogenic hTNF-α protein conjugated to a carrier protein, keyhole limpet hemocyanin (KLH), was developed by Neovacs (Paris, France). This compound is capable of inducing the production of neutralizing anti-hTNF-α antibodies and avoiding the risk of induction of ADAs. The results of a phase I/phase II clinical trial of TNF-K showed good efficiency and safety (33, 34). However, the KLH carrier protein cannot be reproduced synthetically and can be purified only from the keyhole limpet Megathura crenulata, which may limit future clinical usage.
Our previous studies explored the feasibility of immunotherapy for treatment of tumors with xenogeneic homologous molecules as a vaccine against those on autologous cells in a cross-reaction between the xenogeneic homologous molecules and self-molecules (11–14). In this study, we tested this strategy in mice IBD models. Our results showed that xenogeneic TNF-α protein vaccination, rather than homologous vaccination, could elicit a neutralizing antibody response. Although the homology level of the soluble forms of TNF-α protein in human and mice was 79%, the differences between the two amino acid sequences were sufficient to overcome immune tolerance in mice after xenogeneic vaccination.
Moreover, the xenogeneic TNF-α protein vaccine could protect mice from acute and chronic colitis induced by DSS. One possible explanation for this protective effect is the production of TNF-α-specific neutralizing antibody, which absorbs the biological activity of mTNF-α, as shown by neutralization assay and serum transfer experiment results. However, it was reported that, in addition to its neutralizing ability, infliximab could induce apoptosis in activated T lymphocytes (20, 21). We also tested the effect of antiserum on lymphocyte apoptosis. As shown in Fig. 6, TNF-α-specific antiserum failed to induce T lymphocyte apoptosis, suggesting that the neutralizing ability of TNF-α-specific antibody may derive from mechanisms of the xenogeneic TNF-α protein vaccine.
Furthermore, potential adverse effects were tested in this study. Characteristics of weight loss, ruffling of fur, behavior, or feeding were not changed in xenogeneic TNF-α protein-vaccinated mice, and no obvious pathological changes were observed. In summary, use of the xenogeneic TNF-α protein vaccine may be a potent therapeutic strategy for treatment of IBD.
ACKNOWLEDGMENT
This work was supported by the National High-Tech Research and Development Program of China (863 Plan) (no. 2014AA020706).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00331-15.
REFERENCES
- 1.Peng XD, Wu XH, Chen LJ, Wang ZL, Hu XH, Song LF, He CM, Luo YF, Chen ZZ, Jin K, Lin HG, Li XL, Wang YS, Wei YQ. 2010. Inhibition of phosphoinositide 3-kinase ameliorates dextran sodium sulfate-induced colitis in mice. J Pharmacol Exp Ther 332:46–56. doi: 10.1124/jpet.109.153494. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan F, Bernstein CN. 2009. The evolving epidemiology of inflammatory bowel disease. Curr Opin Gastroenterol 25:301–305. doi: 10.1097/MOG.0b013e32832b12ef. [DOI] [PubMed] [Google Scholar]
- 3.Burisch J, Munkholm P. 2013. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol 29:357–362. doi: 10.1097/MOG.0b013e32836229fb. [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg B, Hommes D. 2007. Biological therapies in inflammatory bowel disease: top-down or bottom-up? Curr Opin Gastroenterol 23:395–399. doi: 10.1097/MOG.0b013e32815b601b. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen OH, Ainsworth MA. 2013. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med 369:754–762. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JR. 2008. TNF-mediated inflammatory disease. J Pathol 214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 7.Neurath MF. 2014. Cytokines in inflammatory bowel disease. Nat Rev Immunol 14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 8.de Bie CI, Escher JC, de Ridder L. 2012. Antitumor necrosis factor treatment for pediatric inflammatory bowel disease. Inflamm Bowel Dis 18:985–1002. doi: 10.1002/ibd.21871. [DOI] [PubMed] [Google Scholar]
- 9.van Schouwenburg PA, Rispens T, Wolbink GJ. 2013. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 9:164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 10.Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, Van der Woude J, Baert F, Eliakim R, Katsanos K, Brynskov J, Steinwurz F, Danese S, Vermeire S, Teillaud JL, Lemann M, Chowers Y. 2010. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 4:355–366. doi: 10.1016/j.crohns.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, Xiao F, He QM, Shu JM, Xie XJ, Mao YQ, Lei S, Luo F, Zhou LQ, Liu CE, Zhou H, Jiang Y, Peng F, Yuan LP, Li Q, Wu Y, Liu JY. 2000. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med 6:1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 12.Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, Shu JM, Lu CJ, Niu T, Kang B, Mao YQ, Liu F, Wen YJ, Lei S, Luo F, Zhou LQ, Peng F, Jiang Y, Liu JY, Zhou H, Wang QR, He QM, Xiao F, Lou YY, Xie XJ, Li Q, Wu Y, Ding ZY, Hu B, Hu M, Zhang W. 2001. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci U S A 98:11545–11550. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Wen YJ, Ding ZY, Fu CH, Wu Y, Liu JY, Li Q, He QM, Zhao X, Jiang Y, Li J, Deng HX, Kang B, Mao YQ, Wei YQ. 2006. Immunotherapy of tumors with protein vaccine based on chicken homologous Tie-2. Clin Cancer Res 12:1813–1819. doi: 10.1158/1078-0432.CCR-05-1990. [DOI] [PubMed] [Google Scholar]
- 14.Liu JY, Wei YQ, Yang L, Zhao X, Tian L, Hou JM, Niu T, Liu F, Jiang Y, Hu B, Wu Y, Su JM, Lou YY, He QM, Wen YJ, Yang JL, Kan B, Mao YQ, Luo F, Peng F. 2003. Immunotherapy of tumors with vaccine based on quail homologous vascular endothelial growth factor receptor-2. Blood 102:1815–1823. doi: 10.1182/blood-2002-12-3772. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Xie N, He W, Liu R, Lei Y, Chen Y, Tang H, Liu B, Huang C, Wei Y. 2013. An integrated proteomics and bioinformatics analyses of hepatitis B virus X interacting proteins and identification of a novel interactor apoA-I. J Proteomics 84:92–105. doi: 10.1016/j.jprot.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Tong A, Wu L, Lin Q, Lau QC, Zhao X, Li J, Chen P, Chen L, Tang H, Huang C, Wei YQ. 2008. Proteomic analysis of cellular protein alterations using a hepatitis B virus-producing cellular model. Proteomics 8:2012–2023. doi: 10.1002/pmic.200700849. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto H, Fukudome K, Kohara J, Nakatake H, Kimoto M. 2007. Preparation of recombinant murine tumor necrosis factor-alpha in Escherichia coli: a rapid method to remove tags from fusion proteins by thrombin-cleavage and ion-exchange chromatography. Protein Expr Purif 56:138–144. doi: 10.1016/j.pep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69:238–249. [PubMed] [Google Scholar]
- 19.Dieleman LA, Elson CO, Tennyson GS, Beagley KW. 1996. Kinetics of cytokine expression during healing of acute colitis in mice. Am J Physiol 271:G130–G136. [DOI] [PubMed] [Google Scholar]
- 20.Ringheanu M, Daum F, Markowitz J, Levine J, Katz S, Lin X, Silver J. 2004. Effects of infliximab on apoptosis and reverse signaling of monocytes from healthy individuals and patients with Crohn's disease. Inflamm Bowel Dis 10:801–810. doi: 10.1097/00054725-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. 2003. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology 124:1774–1785. doi: 10.1016/S0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]
- 22.Papadakis KA, Targan SR. 2000. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 23.Ahluwalia JP. 2012. Immunotherapy in inflammatory bowel disease. Med Clin North Am 96:525–544, x. doi: 10.1016/j.mcna.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, Hildner K, Hoffman A, Kiesslich R, Rink AD, Rau TT, Rose-John S, Kessler H, Schmidt J, Neurath MF. 2011. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14(+) macrophages. Gastroenterology 141:2026–2038. doi: 10.1053/j.gastro.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Rutella S, Fiorino G, Vetrano S, Correale C, Spinelli A, Pagano N, Arena V, Maggiano N, Repici A, Malesci A, Danese S. 2011. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn's disease. Am J Gastroenterol 106:762–770. doi: 10.1038/ajg.2011.48. [DOI] [PubMed] [Google Scholar]
- 26.Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. 2011. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijer MJ, Mieremet-Ooms MA, van Duijn W, van der Zon AM, Hanemaaijer R, Verheijen JH, van Hogezand RA, Lamers CB, Verspaget HW. 2007. Effect of the anti-tumor necrosis factor-alpha antibody infliximab on the ex vivo mucosal matrix metalloproteinase-proteolytic phenotype in inflammatory bowel disease. Inflamm Bowel Dis 13:200–210. doi: 10.1002/ibd.20051. [DOI] [PubMed] [Google Scholar]
- 28.D'Haens G, Daperno M. 2006. Advances in biologic therapy for ulcerative colitis and Crohn's disease. Curr Gastroenterol Rep 8:506–512. doi: 10.1007/s11894-006-0041-5. [DOI] [PubMed] [Google Scholar]
- 29.Steenholdt C, Al-khalaf M, Brynskov J, Bendtzen K, Thomsen OO, Ainsworth MA. 2012. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm Bowel Dis 18:2209–2217. doi: 10.1002/ibd.22910. [DOI] [PubMed] [Google Scholar]
- 30.Afif W, Loftus EV Jr, Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ. 2010. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 105:1133–1139. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcês S, Demengeot J, Benito-Garcia E. 2013. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 72:1947–1955. doi: 10.1136/annrheumdis-2012-202220. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Liu J, Wu Y, Xiao F, Wang Y, Wang R, Yang H, Wang G, Yang J, Deng H, Li J, Wen Y, Wei Y. 2008. Immunotherapy of hepatocellular carcinoma with a vaccine based on xenogeneic homologous alpha fetoprotein in mice. Biochem Biophys Res Commun 376:10–14. doi: 10.1016/j.bbrc.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 33.Le Buanec H, Delavallee L, Bessis N, Paturance S, Bizzini B, Gallo R, Zagury D, Boissier MC. 2006. TNFalpha kinoid vaccination-induced neutralizing antibodies to TNFalpha protect mice from autologous TNFalpha-driven chronic and acute inflammation. Proc Natl Acad Sci U S A 103:19442–19447. doi: 10.1073/pnas.0604827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semerano L, Biton J, Delavallee L, Duvallet E, Assier E, Bessis N, Bernier E, Dhellin O, Grouard-Vogel G, Boissier MC. 2013. Protection from articular damage by passive or active anti-tumour necrosis factor (TNF)-alpha immunotherapy in human TNF-alpha transgenic mice depends on anti-TNF-alpha antibody levels. Clin Exp Immunol 172:54–62. doi: 10.1111/cei.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]