Abstract
A potential cause of neurodegenerative diseases, including Parkinson’s disease (PD), is protein misfolding and aggregation that in turn leads to neurotoxicity. Targeting Hsp90 is an attractive strategy to halt neurodegenerative diseases, and benzoquinone ansamycin (BQA) Hsp90 inhibitors such as geldanamycin (GA) and 17-(allylamino)-17-demethoxygeldanamycin have been shown to be beneficial in mutant A53T α-synuclein PD models. However, current BQA inhibitors result in off-target toxicities via redox cycling and/or arylation of nucleophiles at the C19 position. We developed novel 19-substituted BQA (19BQA) as a means to prevent arylation. In this study, our data demonstrated that 19-phenyl-GA, a lead 19BQA in the GA series, was redox stable and exhibited little toxicity relative to its parent quinone GA in human dopaminergic SH-SY5Y cells as examined by oxygen consumption, trypan blue, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), and apoptosis assays. Meanwhile, 19-phenyl-GA retained the ability to induce autophagy and potentially protective heat shock proteins (HSPs) such as Hsp70 and Hsp27. We found that transduction of A53T, but not wild type (WT) α-synuclein, induced toxicity in SH-SY5Y cells. 19-Phenyl-GA decreased oligomer formation and toxicity of A53T α-synuclein in transduced cells. Mechanistic studies indicated that mammalian target of rapamycin (mTOR)/p70 ribosomal S6 kinase signaling was activated by A53T but not WT α-synuclein, and 19-phenyl-GA decreased mTOR activation that may be associated with A53T α-synuclein toxicity. In summary, our results indicate that 19BQAs such as 19-phenyl-GA may provide a means to modulate protein-handling systems including HSPs and autophagy, thereby reducing the aggregation and toxicity of proteins such as mutant A53T α-synuclein.
Introduction
Hsp90 is a crucial molecular chaperone that functions to correctly fold client proteins, thereby preventing them from degradation by the ubiquitin-proteasome system (UPS) (Neckers, 2002). More than 200 client proteins of Hsp90 have been identified, including a range of prosurvival proteins (also known as oncoproteins) such as Raf-1, Akt, p53, and cdk4 (Kamal and Burrows, 2004), as well as neural proteins, including polyglutamine-expanded mutant androgen receptor (Tokui et al., 2009) and leucine-rich repeat kinase 2 (Hurtado-Lorenzo and Anand, 2008).
One of the major causes of neurodegenerative diseases, including Parkinson’s disease (PD), is the accumulation of misfolded protein aggregates that in turn leads to toxicity and loss of neurons (Olzmann et al., 2008; Olanow and McNaught, 2011). In the brain, α-synuclein (α-Syn) is highly abundant and plays a key role in the modulation of synaptic activity. α-Syn is also the main component of Lewy bodies and Lewy neurites in the PD brain, and mutations in the gene encoding α-Syn (SNCA gene) have been implicated in the early onset of familial forms of PD (Lashuel et al., 2013). A53T α-Syn is one of the mutant forms of α-Syn that has been intensively studied. Despite accumulating evidence for mutant A53T α-Syn in the pathogenesis of PD, its role has not been fully elucidated. However, it is believed that the toxicity of A53T α-Syn is associated with formation of prefibrillar α-Syn oligomers (Kalia et al., 2013).
Recent studies suggest that inhibition of Hsp90 could be beneficial for neurodegenerative diseases (McLean et al., 2004; Waza et al., 2005; Tokui et al., 2009; Baldo et al., 2012). Pharmacologic inhibition of Hsp90 has been shown to be useful in neurodegenerative disease models (Gallo, 2006; Luo et al., 2010). There are at least three potential protective mechanisms associated with inhibition of Hsp90: 1) Hsp90 inhibition leads to activation of heat shock factor 1, which further induces a range of small heat shock proteins (HSPs; including Hsp70 and Hsp27) that assist in appropriate folding of misfolded proteins (Dimant et al., 2012); 2) inhibition of Hsp90 leads to the degradation of mutant neuronal client proteins such as polyglutamine-expanded mutant androgen receptor and leucine-rich repeat kinase 2, which reduces the load of toxic Hsp90 client proteins (Waza et al., 2005; Tokui et al., 2009; Taipale et al., 2010; Baldo et al., 2012); and 3) Hsp90 inhibitors including 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) have been shown to induce protective autophagy that led to degradation of mutant and misfolded protein aggregates (Riedel et al., 2010). The benzoquinone ansamycin (BQA) Hsp90 inhibitors geldanamycin (GA), 17-AAG, and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) have been shown to induce a robust compensatory heat shock response and amelioration of protein aggregation and toxicity. Specifically with respect to PD, either GA or 17-AAG can inhibit α-synuclein aggregation and toxicity in cell, fly, and yeast models (Waza et al., 2005; Tokui et al., 2009; Putcha et al., 2010; Baldo et al., 2012).
However, current BQA inhibitors were relatively hepatotoxic in animal models, and their off-target toxicities were mediated via redox cycling and arylation of nucleophiles at the C19 position of the benzoquinone ring (Kamal and Burrows, 2004; Cysyk et al., 2006; Guo et al., 2008). We developed novel 19-substituted BQAs (19BQAs) as a means to prevent arylation, and we have validated this approach by using model thiols (Kitson et al., 2013; Chang et al., 2014). This study was designed to evaluate the use of novel 19BQAs as potential therapeutic agents for PD. We established a transient A53T α-Syn overexpression model and used 19-phenyl-geldanamycin (19-Ph-GA) as proof of principle that 19BQAs 1) had reduced toxicity relative to GA in SH-SY5Y cells and 2) were able to induce compensatory changes in protein handling to ameliorate A53T α-Syn oligomer formation and toxicity.
Materials and Methods
Materials and Antibodies.
17-AAG and GA were purchased from LC Laboratories (Woburn, MA); 19-Ph-GA, 19-methyl-GA, 19-(4-phenyl-phenyl)-GA, 19-(4-morpholinyl-phenyl)-GA, 19-(4-methoxy-phenyl)-GA, 19-dimethylamino-GA, and 19-hydroxymethyl-GA were synthesized as described previously (Kitson et al., 2013; Kitson and Moody, 2013). NADH, 4′,6-diamidino-2-phenylindole, chloroquine diphosphate, ATP disodium salt, digitonin, phenylmethylsulfonyl fluoride, bovine serum albumin, and mouse monoclonal anti–β-actin (A5441) antibody were obtained from Sigma-Aldrich (St. Louis, MO). Dithiothreitol was purchased from Fisher Scientific (Pittsburgh, PA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was obtained from Research Products International (Mount Prospect, IL). Mouse anti–α-synuclein (610786) antibody was from BD Biosciences (San Jose, CA). Mouse monoclonal anti-Hsp27 (ADI-SPA-800), anti-Hsp70 (ADI-SPA-810) antibodies, and rabbit polyclonal anti-p62 (BML-PW9860) antibody were purchased from Enzo (Farmingdale, NY). Fluorescein isothiocyanate (FITC)/annexin V (640906) and annexin V binding buffer (422201) were purchased from BioLegend (San Diego, CA). The proteasome substrate Suc-Leu-Leu-Val-Tyr-AMC was purchased from Bachem (Torrance, CA). Rabbit polyclonal anti-LC3 (NB100-2331 and NB600-1384) antibody was from Novus (Littleton, CO). Rabbit polyclonal anti–phospho-mTOR (mammalian target of rapamycin) (Ser2448, #2971), anti-mTOR (#2972), anti–phospho-p70S6K (p70 ribosomal S6 kinase) (Thr389, #9205), anti-p70S6K (#9202), anti–phospho-eIF2α (eukaryotic translation initiation factor 2α) (Ser51, #9721), and anti-eIF2α (#9722) antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Cell Culture and Treatment.
SH-SY5Y cells were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle’s medium/F-12 medium (Cellgro, Manassas, VA) containing 10% (v/v) fetal bovine serum, 2 mM glutamine (American Type Culture Collection), 100 units/ml penicillin, and 100 μg/ml streptomycin (Cellgro) in a humidified atmosphere of 5% CO2 at 37°C.
Adenoviral Transient Transduction.
Adenoviruses expressing A53T mutated α-Syn, wild-type (WT) α-Syn, or green fluorescent protein were constructed and produced in two-step AdEasy systems (Agilent Technologies, Santa Clara, CA) (Zhou et al., 2002). Adenoviruses were mixed with culture medium and incubated with cells at a concentration of 100–400 plaque-forming units/cell as described previously (Zhou et al., 2002, 2011).
MTT Growth Inhibition Assay.
Drug-induced toxicity was determined using the MTT assay. SH-SY5Y cells were seeded at 5000 cells per well in 96-well plates or 40,000 cells per well in 48-well plates (in triplicate). After 24 hours, the cells were incubated with adenoviruses expressing either A53T mutated α-synuclein (A53T α-Syn) or WT α-Syn in complete medium in 48-well plates. One day after adenovirus transduction, cells were exposed to 19BQAs at varying concentrations for 48 hours, after which the medium was removed and replaced with complete medium containing MTT (0.4 mg/ml). The MTT containing medium was removed after 2-hour incubation, and the MTT formazan product was extracted from cells with 200 µl of dimethylsulfoxide. The optical density of the extract was determined at 550 nm with a microplate reader. IC50 values were defined as the concentration of drugs that resulted in a 50% reduction in cell density compared with dimethylsulfoxide-treated controls.
Trypan Blue Exclusion Assay.
Cells were seeded at 0.5 × 106 cells into 60 × 15–mm tissue culture dishes in 3 ml of complete medium and allowed to grow for 24 hours. Following treatment with the indicated compound for 24 hours, cell pellets containing both floating and attached cells were collected and then resuspended in 1 ml of phosphate-buffered saline (PBS). To assess cell viability, the cell suspension (10 μl) was stained with 10 μl of trypan blue dye (0.4% w/v in PBS). The number of viable cells was determined using a Life Technologies (Carlsbad, CA) Countess automated cell counter immediately after staining.
Apoptosis Assay.
Apoptosis was determined by flow cytometry using FITC-conjugated anti–annexin V and propidium iodide (PI) as previously described (Xiong et al., 2013). After the indicated treatments, cell pellets including both the floating and attached cells were collected and resuspended in 500 μl of annexin V binding buffer and incubated with anti–annexin V antibody (2 μl) and PI (0.7 μl of 100 μg/ml stock) for 10 minutes at 37°C in the dark. Samples were kept on ice and then analyzed using a BD Biosciences (San Jose, CA) FACSCalibur flow cytometer. The fluorescence was measured at 530 nm (FL1, FITC) and PI at above 600 nm (FL2), and the data were acquired and analyzed using Cellquest software (Becton Dickinson, Mountain View, CA).
Proteasomal Activity Assay.
Proteasomal activity was determined by measuring the fluorescence of cleaved proteasome substrate Suc-Leu-Leu-Val-Tyr-AMC (chymotrypsin-like activity) as described previously (Kisselev and Goldberg, 2005; Xiong et al., 2013, 2014). In brief, after the indicated treatments, the medium was removed and the cells were washed with PBS (3 ml) three times, and then lysed with proteasomal activity assay buffer and centrifuged at 10,000g for 14 minutes at 4°C. The supernatants were collected and protein concentrations were determined using the method of Lowry (Lowry et al., 1951). To assay proteasome activity, cell lysate (20 µg) was added to 100 µl of proteasome activity assay buffer [50 mM Tris-HCl (pH 7.5), 250 mM sucrose, 1 mM dithiothreitol, 5 mM MgCl2, 2 mM ATP, 0.5 mM EDTA, and 0.025% (w/v) digitonin] followed by the addition of 100 μM proteasome substrate Suc-Leu-Leu-Val-Tyr-AMC. After incubation at 37°C for 30 minutes, 40-µl samples were transferred to a 96-well plate, and the fluorescence of liberated 7-amino-4-methylcoumarin (Excitation: 380 nm; Emission: 460 nm) was measured in duplicate samples.
Immunoblot Analysis.
Cells were seeded at 400,000 cells per well in a six-well plate. Following the indicated treatments, cells were washed with PBS, and then attached cells were collected and lysed with radioimmunoprecipitation assay buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% (w/v) SDS, 1% (v/v) NP40, and 0.5% (w/v) sodium deoxycholate] supplemented with protease inhibitors (Complete, Mini Protease Inhibitor Cocktail; Roche Diagnostics, Indianapolis, IN), phenylmethylsulfonyl fluoride (2 mM), and phosphatase inhibitors (Sigma-Aldrich). Cells were sonicated on ice and then centrifuged at 10,000g for 14 minutes at 4°C. The supernatants were collected, and protein concentrations were measured using the method of Lowry (Lowry et al., 1951). Proteins were diluted in 2× Laemmli SDS sample buffer and heated to 70°C for 5 minutes, and then separated on a 12% SDS-PAGE precast minigel (Bio-Rad Laboratories, Hercules, CA). A 7.5% SDS-PAGE precast minigel was used to separate the high molecular weight oligomers. Proteins were transferred to polyvinylidene fluoride membranes in 25 mM Tris and 192 mM glycine containing 20% (v/v) methanol at 4°C. Membranes were incubated in blocking buffer [10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.2% (v/v) Tween 20 (TBST) containing 5% (w/v) nonfat dry milk] for 1 hour, and then incubated with primary antibodies overnight at 4°C. Membranes were washed every 10 minutes in TBST for 1 h and then probed with horseradish peroxidase–conjugated goat anti-rabbit or horseradish peroxidase–conjugated goat anti-mouse IgG (1:5000; Jackson Immunoresearch Laboratories, West Grove, PA) for 30 minutes at room temperature. Membranes were washed three times in TBST and protein bands were visualized using enhanced chemiluminescence (Thermo Scientific, Rockford, IL). Photoshop CS6 (Adobe, San Jose, CA) was used to quantitate immunoblots from three independent experiments.
Oxygen Consumption Assay.
The ability of 19BQAs to undergo redox cycling in SH-SY5Y cells was determined using an airtight Clark electrode (Yellow Springs Instrument Company, Yellow Springs, OH). For these studies, 5 million cells were suspended in 3 ml of complete cell culture medium at 37°C in the presence or absence of compounds, and the rate of oxygen consumption was measured over 10 minutes. Dissolved oxygen concentrations were adjusted for altitude (5280 feet) and temperature (37°C).
Statistical Analysis.
All experiments were repeated at least three times, and the values were expressed as means ± S.D. The statistical significance was determined using Prism 6.0 software (GraphPad Software, San Diego, CA). One-way analysis of variance followed by Tukey’s or Dunnett’s multiple comparison test was used.
Results
Decreased Toxicity of 19BQAs Relative to Their Parent Quinone GA in SH-SY5Y Cells.
The synthesis of 19BQAs (Fig. 1) was described previously (Kitson et al., 2013). We first examined the potential toxicity of 19BQAs in human dopaminergic SH-SY5Y (5Y) cells using a short-term MTT test as an initial screen. As shown in Fig. 2A, GA induced a significant loss in cell viability at a low concentration (IC50 287 nM), compared with 19-Ph-GA (IC50 > 10 µM). In addition, we found that 19BQAs were also less toxic than 17-DMAG (IC50 6.8 µM). Overall, using this initial screen, these results indicated that 19BQAs induced substantially decreased toxicity relative to GA and 17-DMAG.
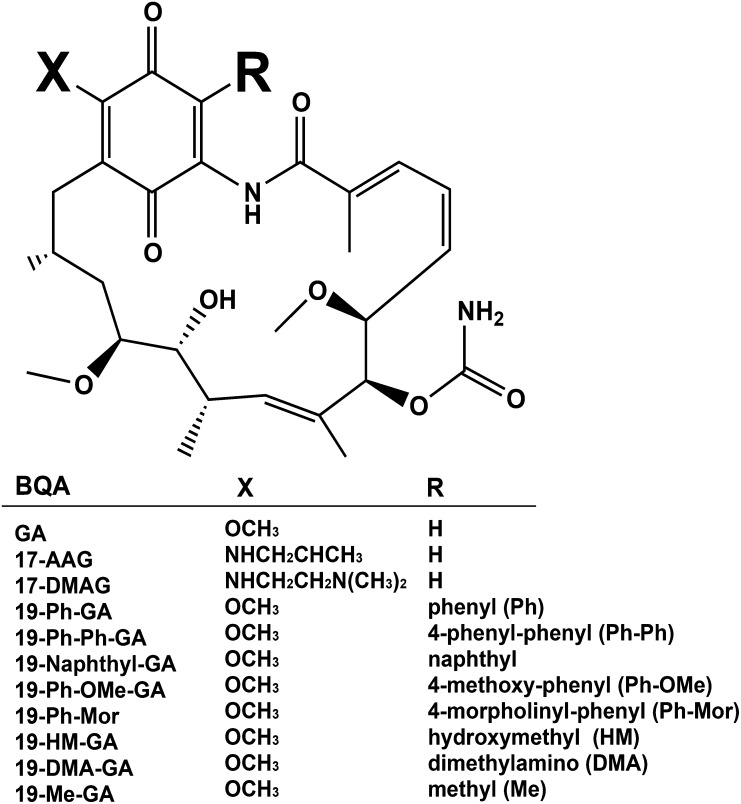
Fig. 1.
Chemical structures of BQAs. Substitutions at the 19-position (R) include phenyl (Ph), 4-phenyl-phenyl (Ph-Ph), naphthyl, 4-methoxy-phenyl (Ph-OMe), 4-morpholinyl-phenyl (Ph-Mor), hydroxymethyl (HM), dimethylamino (DMA), and methyl (Me).
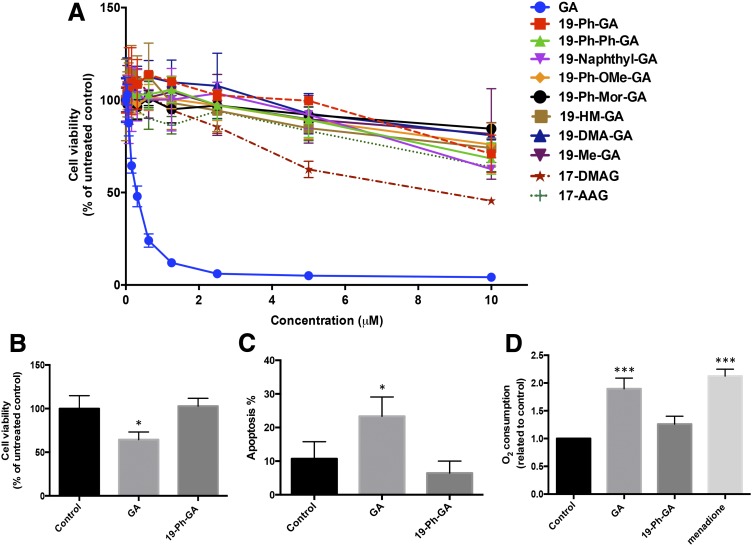
Fig. 2.
19BQAs exerted decreased toxicity compared with their parent quinone GA. (A) Growth inhibition induced by 19BQAs in 5Y cells. Growth inhibition was measured using the MTT assay. Cells were treated with BQAs for 4 hours, then allowed to grow for an additional 72 hours. (B) Cells were treated with GA and 19-Ph-GA at 0.25 μM for 24 hours and harvested for determination of cell number using a trypan blue exclusion assay. (C) Apoptosis was measured using annexin V/PI cell staining in combination with flow cytometry in cells after exposure to the equivalent concentration (5 μM) of GA or 19-Ph-GA for 16 hours. (D) Oxygen consumption was measured in cells in the absence (basal) or presence of the indicated compounds. Measurements were made over 10 minutes using a Clark electrode at 37°C. Menadione was included as a redox-cycling quinone (positive control). Results are expressed as the mean ± S.D., n = 3. *P < 0.05, ***P < 0.001 is considered significantly different from basal by one-way analysis of variance using Dunnett’s multiple comparison test. 19-DMA-GA, 19-dimethylamino-GA; 19-HM-GA, 19-hydroxymethyl-GA; 19-Me-GA, 19-methyl-GA; 19-Ph-Mor-GA, 19-(4-morpholinyl-phenyl)-GA; 19-Ph-OMe-GA, 19-(4-methoxy-phenyl)-GA; 19-Ph-Ph-GA, 19-(4-phenyl-phenyl)-GA.
The relatively low toxicity of 19-Ph-GA was then confirmed using the trypan blue exclusion assay and flow cytometry using annexin V and PI double staining for apoptosis. As shown in Fig. 2B, no significant toxicity could be detected following treatment with 19-Ph-GA (250 nM) for 24 hours, whereas exposure to GA at the equivalent concentration resulted in over 30% of cell death and significant morphologic changes in 5Y cells (Supplemental Fig. 1). Significant induction of apoptosis (Fig. 2C) was also observed after 24-hour treatment with a higher concentration (5 μM) of GA, whereas exposure to 19-Ph-GA (5 μM) did not increase the number of apoptotic cells.
19-Substitution prevents the ability of BQAs to induce toxicity via arylation of cellular nucleophiles (Chang et al., 2014). An additional concern with quinone-based drugs is their ability to undergo redox cycling and generate reactive oxygen species, which in turn results in off-target toxicities. To determine whether 19BQAs undergo appreciable redox cycling, we measured the rates of oxygen consumption in 5Y cells in the absence and presence of GA or 19-Ph-GA. Results from these studies demonstrated that exposure to 19-Ph-GA at a relatively high concentration (10 μM) did not significantly increase the rate of oxygen consumption in contrast to exposure to GA, which resulted in 2-fold increases in the rates of oxygen consumption (Fig. 2D). These results demonstrated that 19BQAs such as 19-Ph-GA were less redox active and had significantly decreased toxicity relative to GA in 5Y cells.
19BQAs Are Potent Inducers of Heat Shock Proteins.
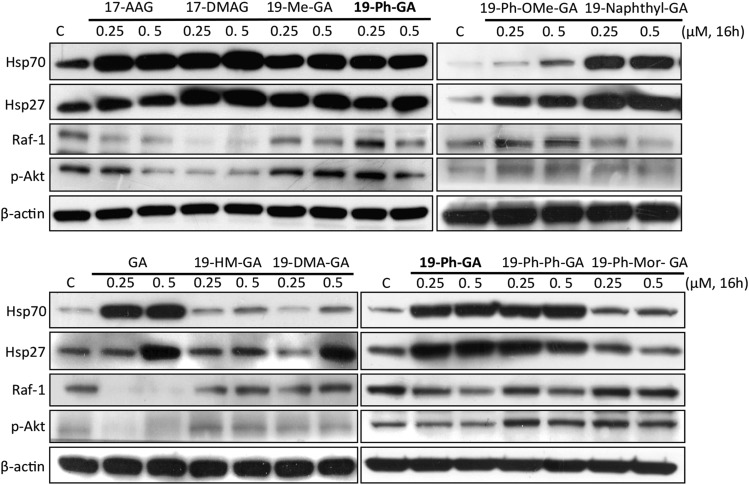
Previously, we demonstrated that 19BQAs inhibit Hsp90 ATPase activity and induce cytotoxicity in human cancer cells (Chang et al., 2014). We therefore performed the experiments to examine the efficacy of 19BQAs as Hsp90 inhibitors in dopaminergic 5Y cells. Screening was carried out at nanomolar concentrations (250–500 nM) for 16 hours, and results from these experiments (Fig. 3) clearly show that many 19BQAs, including 19-Ph-GA, are potent inducers of HSPs. At these low concentrations of 19-Ph-GA that were examined, significant degradation of prosurvival kinases (phosphorylated Akt, Raf-1) did not occur, but treatment with higher concentrations (5–10 μM) of 19-Ph-GA resulted in apparent degradation of phosphorylated Akt and Raf-1 (data not shown). These results also demonstrate that many 19-substituted geldanamycin analogs, although not all, are comparable or superior to the clinically tested Hsp90 inhibitor 17-AAG in their ability to induce protective HSPs in 5Y cells. In subsequent studies, we focused on 19-Ph-GA to demonstrate proof of principle.
Fig. 3.
Treatment with 19BQAs induced HSPs in 5Y cells. Immunoblot analysis of biomarkers of Hsp90 inhibition following treatment with BQAs at the indicated concentrations for 16 hours. Note that many 19BQAs induced potent expression of Hsp70 and Hsp27. 19-DMA-GA, 19-dimethylamino-GA; 19-HM-GA, 19-hydroxymethyl-GA; 19-Me-GA, 19-methyl-GA; 19-Ph-Mor-GA, 19-(4-morpholinyl-phenyl)-GA; p-Akt, phosphorylated Akt; 19-Ph-OMe-GA, 19-(4-methoxy-phenyl)-GA; 19-Ph-Ph-GA, 19-(4-phenyl-phenyl)-GA.
Overexpression of A53T α-Syn Resulted in Protein Handling Changes and Toxicity.
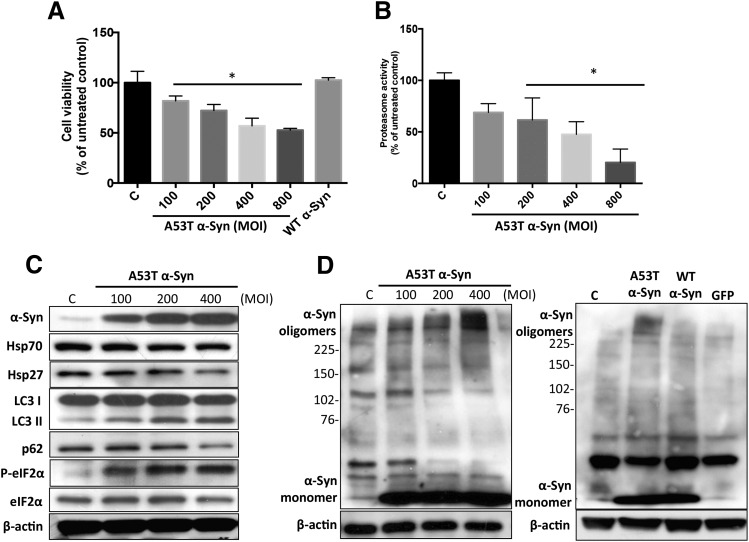
We used adenoviral transduction of 5Y cells with mutant A53T α-Syn as a cellular model. Interestingly, the overexpression of A53T α-Syn perturbed several key protein handling systems that have been previously shown to be important for determining the fate of misfolded or aggregated proteins (Dimant et al., 2012; Ebrahimi-Fakhari et al., 2012a; Xiong et al., 2013). As shown in Fig. 4, increased expression of A53T, but not WT, α-Syn led to significant dose-dependent growth inhibition of 5Y cells (Fig. 4A), and these data correlated with decreased proteasome activity (Fig. 4B), suggesting that impaired proteasome function may be associated with A53T α-Syn toxicity as previously reported in PC12 cells (Stefanis et al., 2001). In addition to the UPS, the autophagy-lysosome pathway, molecular chaperones, and the endoplasmic reticulum (ER) stress response/unfolded protein response all work in concert to maintain intracellular protein homeostasis (Olanow and McNaught, 2011; Xiong et al., 2013, 2014). In these studies, we found that overexpression of A53T α-Syn induced the ER stress response and macroautophagy (hereafter referred to as autophagy) in 5Y cells, as indicated by the sustained increase in the ER stress marker phosphorylated eIF2α (p-eIF2α) and the autophagy marker LC3 II (Fig. 4C). To further examine the effect of A53T α-Syn expression on autophagy, we treated 5Y cells (previously transduced with A53T α-Syn for 24 hours) with chloroquine (CQ) for an additional 48 hours. CQ is a lysosome inhibitor and suppresses the fusion of autophagosomes with lysosomes and blocks autophagic degradation. The levels of LC3 II further increased after CQ treatment, excluding the possibility that A53T α-Syn–stimulated autophagic flux was due to the blockade of autophagic degradation (Supplemental Fig. 2A). In addition, we also observed enhanced degradation of the autophagy substrate p62 (Fig. 4C) following transduction with A53T α-Syn. These results demonstrated that autophagy was activated by increased A53T α-Syn expression in 5Y cells. Surprisingly, expression of the protective chaperones Hsp70 and Hsp27 was slightly reduced after elevated expression of A53T α-Syn in 5Y cells (Fig. 4C). It is possible that these changes were induced as secondary responses to A53T α-Syn toxicity. Additionally, we were able to detect increases in the formation of high molecular weight α-Syn oligomers in 5Y cells transduced with mutant A53T, but not WT α-Syn (Fig. 4D). These bands were not ubiquitinated forms of A53T α-Syn as indicated by a lack of reactivity with an antiubiquitin antibody (data not shown). Since the transduction with WT α-Syn did not result in significant toxicity (Fig. 4A), these data are consistent with previous literature (Conway et al., 2000; Kalia et al., 2013), suggesting a link between A53T α-Syn oligomer formation and toxicity in 5Y cells. It is also noteworthy that treatment with the lysosome inhibitor CQ resulted in a greater level of α-Syn oligomers in 5Y cells, indicating that autophagy may be an important protective mechanism for α-Syn oligomer degradation (Supplemental Fig. 2B).
Fig. 4.
Overexpression of human mutant A53T α-Syn induced protein handling changes and toxicity in 5Y cells. (A) One day after seeding, cells were transduced with mutant A53T α-Syn (MOI from 100 to 800 plaque-forming units/cell) and WT α-Syn (MOI 400 plaque-forming units/cell) for 72 hours. Cell viability was then determined by the MTT assay. (B) Overexpression of A53T α-Syn inhibited intracellular 20/26S proteasomal activity (chymotrypsin-like active site) in a dose-dependent manner. (C) Hsp70 and Hsp27 protein levels and induction of the ER stress response and autophagy following transduction with A53T α-Syn. (D) MOI-dependent increase in higher molecular weight α-Syn oligomers was detected 72 hours after transduction with A53T α-Syn, but not WT α-Syn, and green fluorescent protein (GFP) adenovirus vector control (MOI 400 plaque-forming units/cell). β-Actin was included as a loading control. A representative blot from three separate experiments is shown for each figure. Values in (A) and (B) are presented as the mean ± S.D. (n = 3); *P < 0.05 is considered significant to control group (one-way analysis of variance using Dunnett’s multiple comparison test). P-eIF2α, phosphorylated eIF2α; MOI, multiplicity of infection.
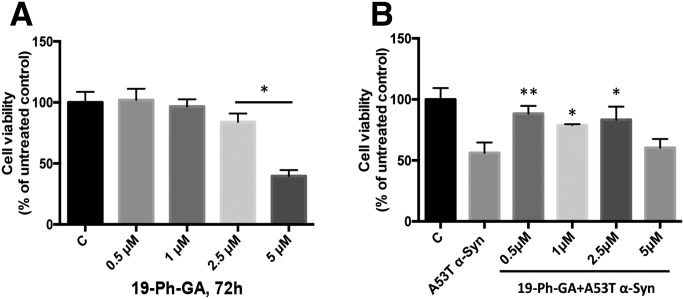
19-Ph-GA Alleviated A53T α-Syn Induced Toxicity.
Continuous exposure (72 hours) of 5Y cells to 19-Ph-GA at a concentration of 2.5 μM resulted in a small but significant loss of cell viability, whereas treatment with higher concentrations showed increased toxicity. Lower concentrations (0.5–1 μM) of 19-Ph-GA were nontoxic (Fig. 5A). Importantly, we found that 19-Ph-GA was able to significantly alleviate A53T α-Syn–induced toxicity. As shown in Fig. 5B, after 48-hour treatment with nontoxic concentrations of 19-Ph-GA (0.5–1 μM), the viability of A53T α-Syn–overexpressing cells was increased over 20% and was not significantly different from untreated cells. We have tested pretreatment and cotreatment with 19-Ph-GA, relative to A53T α-Syn transduction, but neither was as effective as post-treatment in terms of improving cell viability (data not shown). In addition, after 48-hour post-treatment with either 17-AAG or 17-DMAG at the equivalent concentrations (0.5–1 μM), significant cell death was observed, and there was no beneficial effect on A53T α-Syn toxicity (Supplemental Fig. 3). These results demonstrated that exposure to low concentrations of 19-Ph-GA were beneficial and ameliorated A53T α-Syn–induced toxicity in 5Y cells.
Fig. 5.
19-Ph-GA significantly attenuated A53T α-Syn–induced toxicity in 5Y cells. (A) Treatment with low concentrations of 19-Ph-GA (0.5–1 μM) for 72 hours was not cytotoxic to 5Y cells. (B) After 24-hour incubation with adenovirus expressing mutant A53T α-Syn (400 plaque-forming units/cell), cells were exposed to the indicated concentrations of 19-Ph-GA for 48 hours, after which cell viability was determined by the MTT assay. Triplicate treatments in 48-well plates were used. Results represent one experiment typical of n = 5. Values are presented as the mean ± S.D. (n = 3); *P < 0.05, **P < 0.01 is considered significant compared with control in (A) and relative to the A53T α-Syn group in (B), respectively, by one-way analysis of variance using Tukey’s multiple comparison test.
19-Ph-GA Activated Heat Shock Protein Response and Autophagy That May Contribute to the Decreased Oligomer Formation and Toxicity of A53T α-Syn.
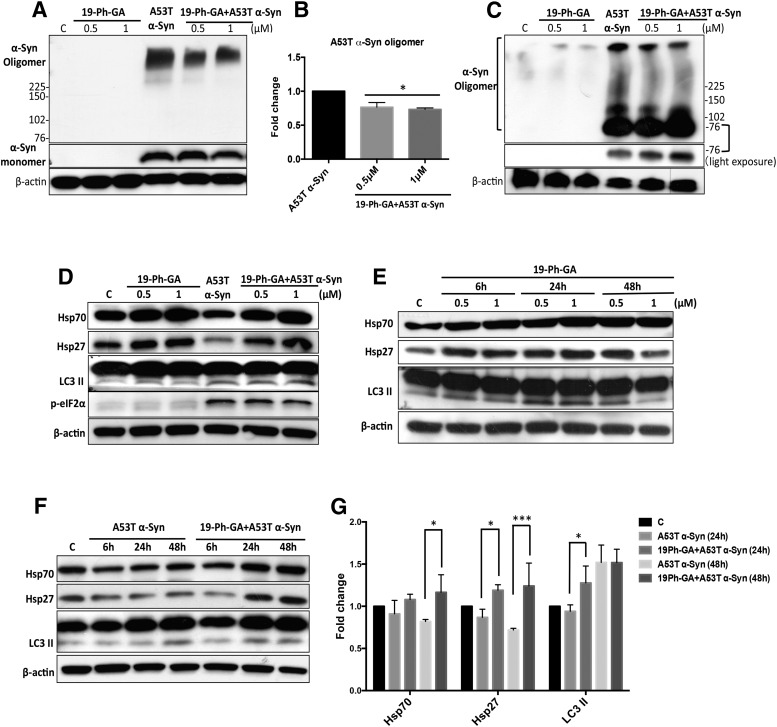
To further examine potential mechanisms underlying 19-Ph-GA–mediated protective effects, we characterized biochemical changes modulated by 19-Ph-GA, particularly with respect to protein handling systems. As shown in Fig. 6, A and B, 24 hours after transduction with A53T α-Syn, exposure of 5Y cells to 19-Ph-GA for 48 hours resulted in a significant decrease in higher molecular weight α-Syn (HW α-Syn) oligomers, whereas α-Syn monomers were not significantly perturbed. We also blotted α-Syn oligomers using nondenaturing native PAGE. Interestingly, in these studies, treatment with 19-Ph-GA also led to a decrease in native HW α-Syn oligomers as well as some redistribution of HW α-Syn oligomers to smaller oligomers (∼76 kD) (Fig. 6C). 19-Ph-GA might mediate its protective effects via induction of Hsp70 and Hsp27 to refold mutant A53T α-Syn and prevent higher molecular weight oligomer formation in addition to stimulation of autophagy causing oligomer degradation. However, by the end of 48-hour treatment, we only observed the concomitant induction of Hsp70 and Hsp27 but not induction of LC3 II (Fig. 6D). To further examine potential alterations of HSP response and autophagic flux induced by 19-Ph-GA, we conducted a time-course study and found that 19-Ph-GA was able to induce sustained expression of Hsp70 and Hsp27 in 5Y cells while it stimulated a temporal autophagic flux, particularly during the early phase (up to 24 hours) of drug treatment (Fig. 6E). Since 19-Ph-GA did not modulate the ER stress response as indicated by the expression of phosphorylated eIF2α (Fig. 6D) and had little effect on A53T α-Syn–induced proteasomal inhibition (Supplemental Fig. 4), the temporal autophagy activated by 19-Ph-GA might be particularly important as an alternative pathway for α-Syn oligomer degradation. Indeed, we found that 19-Ph-GA not only could significantly restore the expression of Hsp70 and Hsp27 in A53T α-Syn–overexpressing cells after 48 hours post-treatment, but also significantly stimulated an early induction of autophagy at 24 hours (Fig. 6, F and G). Taken together, our results suggest that the ability of 19-Ph-GA to both induce HSP expression as well as activate autophagy should be considered as potential mechanisms for decreasing A53T α-Syn oligomer formation and toxicity in 5Y cells.
Fig. 6.
19-Ph-GA significantly reduced A53T α-Syn oligomer formation through induction of heat shock protein responses and autophagy. (A and B) α-Syn oligomers (denatured) were significantly reduced after 48-hour post-treatment of 5Y cells with 19-Ph-GA. (B) Fold changes of α-Syn oligomers in (A) after the drug treatment are normalized to respective controls and estimated by densitometry. (C) Exposure of 5Y cells to 19-Ph-GA resulted in decreased levels of native high molecular weight α-Syn oligomers as well as an increase in the smaller (76 kDa) oligomer species. (D) After 48-hour post-treatment with 19-Ph-GA, Hsp70 and Hsp27 protein levels were elevated 2- to 3-fold, but biomarkers of autophagy and ER stress were not significantly perturbed. (E) Time-course study showed that 19-Ph-GA was able to upregulate both HSPs and LC3 II expression in 5Y cells. Note that exposure to 19-Ph-GA for 24 hours induced the maximum increase in LC3 II, suggesting 19-Ph-GA stimulated a temporal autophagic flux in 5Y cells. (F and G) Significant induction of HSPs and LC3 II could be detected in A53T α-Syn–overexpressing cells after post-treatment with 19-Ph-GA (0.5 μM) for 24 hours, suggesting that HSP response and autophagic flux were stimulated by 19-Ph-GA at relatively early time points. β-Actin was included as a loading control. A representative blot from three separate experiments is shown for each figure. (G) Fold changes of Hsp70, Hsp27, and LC3 II in (F) after the drug treatment at the indicated times are normalized to respective controls and estimated by densitometry. Values in (B) and (G) are presented as the mean ± S.D. (n = 3); *P < 0.05 considered significant compared with the A53T α-Syn group by one-way analysis of variance using Tukey’s multiple comparison test. p-eIF2α, phosphorylated eIF2α.
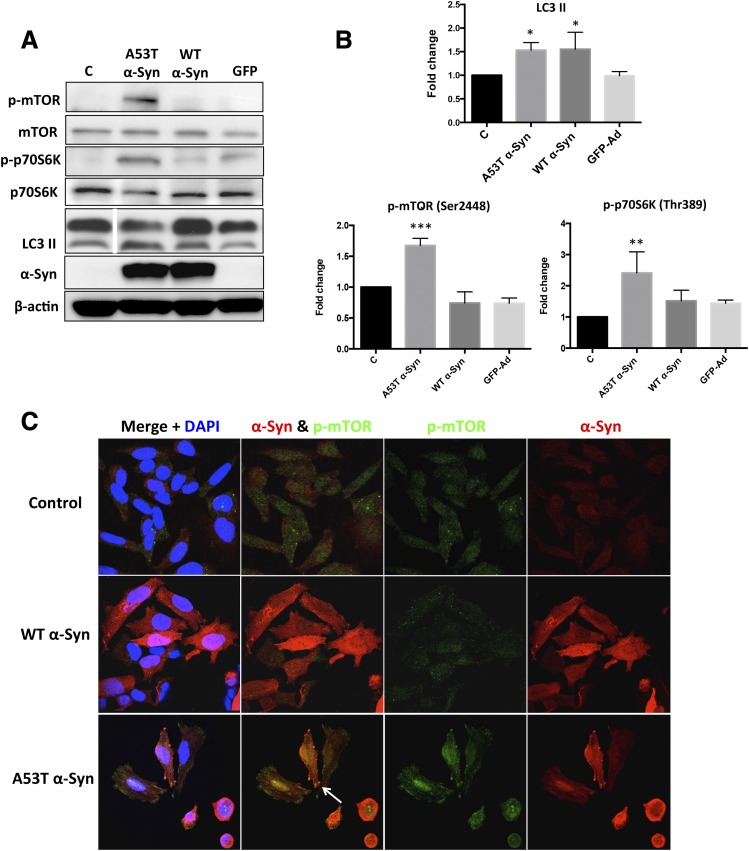
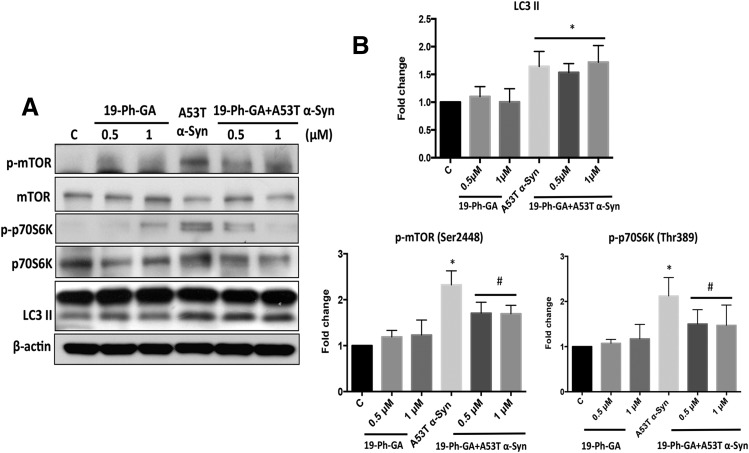
19-Ph-GA Significantly Downregulated mTOR/p70S6K Signaling in A53T α-Syn–Overexpressing Cells.
mTOR (mTORC1) regulates many signaling proteins involved in various cellular processes, including cell growth, protein synthesis, and autophagy (Maiese et al., 2013). Since mTOR has been reported to be a client protein of Hsp90 (Moulick et al., 2011), we hypothesized that 19-Ph-GA might modulate mTOR activity in 5Y cells overexpressing A53T α-Syn. In contrast to a recent study (Jiang et al., 2013), we observed a sustained increase in the levels of LC3 II after overexpression of either A53T or WT α-Syn in 5Y cells, whereas the concomitant induction of phosphorylated mTOR (p-mTOR) and phosphorylated p70S6K (p-p70S6K) was only detected in A53T α-Syn–overexpressing cells (Fig. 7). Moreover, post-treatment with 19-Ph-GA for 48 hours significantly attenuated the levels of p-mTOR and p-p70S6K in A53T α-Syn–overexpressing cells but had little effect on the amount of LC3 II (Fig. 8). It is noteworthy that increased A53T α-Syn expression activated mTOR/p70S6K signaling in both a time- and dose-dependent manner (Supplemental Fig. 5), suggesting a potential role of mTOR/p70S6K signaling in A53T α-Syn–induced toxicity.
Fig. 7.
mTOR/p70S6K signaling was activated by overexpression of A53T but not WT α-Syn. (A) Overexpression of A53T, but not WT for 72h, α-Syn significantly increased the levels of phosphorylated mTOR and p70S6K in 5Y cells. An increased amount of LC3 II was observed in both A53T and WT α-Syn–overexpressing cells, suggesting that autophagy was not directly mediated via the mTOR/p70S6K signaling. The white dividing line in the LC3 II blot indicates omission of three lanes on the gel; all lanes shown were from the same gel. (B) Fold changes of LC3 II, p-mTOR, and p-p70S6K levels in (A) are normalized to respective controls and estimated by densitometry. These values are presented as the mean ± S.D. (n = 3); *P < 0.05 is considered significant relative to the control group using Dunnett’s multiple comparison test. (C) Increased expression of p-mTOR was observed in A53T, but not WT, α-syn–overexpressing cells. After transduction with adenovirus expressing A53T α-Syn or WT α-Syn at MOI 400 plaque-forming units/cell for 72 hours, 5Y cells were fixed and immunostained for α-Syn (red), p-mTOR (green), and nuclei (DAPI, blue) and then examined by confocal microscopy. Note that the colocalization of p-mTOR and α-Syn (white arrow), suggesting an interaction between p-mTOR and A53T α-Syn, may exist. Ad, adenovirus; GFP, green fluorescent protein; DAPI, 4′,6-diamidino-2-phenylindole; MOI, multiplicity of infection.
Fig. 8.
19-Ph-GA decreased mTOR activation that was associated with A53T α-Syn–induced toxicity. (A) Post-treatment with 19-Ph-GA for 48 hours significantly blocked the activation of p-mTOR and p-p70S6K induced by A53T α-Syn. (B) Fold changes of LC3 II, p-mTOR, and p-p70S6K levels in (A) are normalized to respective controls and estimated by densitometry. These values are presented as the mean ± S.D. (n = 3); *P < 0.05 is considered significant relative to the control group; #P < 0.05 is considered significant compared with the A53T α-Syn group by one-way analysis of variance using Tukey’s multiple comparison test.
Discussion
In this study, we demonstrated that 19-Ph-GA, a novel BQA Hsp90 inhibitor, induced significantly less toxicity relative to its parent quinone GA while still retaining the ability to induce HSPs in human dopaminergic 5Y cells. To evaluate the potential therapeutic effects of 19-Ph-GA in PD, we established an A53T α-Syn cell model using transient adenoviral transduction of human mutant A53T α-Syn in 5Y cells. Our results demonstrated that 19-Ph-GA could effectively decrease the formation of high molecular mass α-Syn oligomers and toxicity of A53T α-Syn. 19-Ph-GA triggered a sustainable increase in expression of HSP and the temporal induction of autophagy, both of which may play a role in the protective effects observed with 19-Ph-GA. Intriguingly, we found that mTOR/p70S6K signaling was activated by overexpression of A53T, but not WT, α-Syn, and 19-Ph-GA decreased mTOR activation. It is possible that this effect may also contribute to the protective mechanism of 19-Ph-GA against A53T α-Syn–induced toxicity, but further investigation is required.
The mechanisms of α-Syn degradation have not been fully elucidated, but protein handling systems have been shown to play a role in modulating the levels of intracellular α-Syn via multiple pathways: the 20/26S proteasome and/or autophagy mediates degradation of α-Syn to alleviate intracellular protein load (Sarkar et al., 2007; Ebrahimi-Fakhari et al., 2011), and α-Syn could be chaperoned by heat shock proteins, including Hsp70 and Hsp27, to limit the formation of toxic oligomers (Auluck et al., 2002; Zourlidou et al., 2004; Kilpatrick et al., 2013). Interestingly, overexpression of α-Syn, particularly its pathogenic form A53T α-Syn, has been shown to impair functions of the UPS or chaperone-mediated autophagy and lead to further accumulation of α-Syn (Ebrahimi-Fakhari et al., 2012b). In this study, we found that increased levels of A53T α-Syn resulted in a significant loss of cell viability, and that was associated with the formation of α-Syn oligomers as well as alterations in protein handling systems, including decreased protein levels of Hsp27 and Hsp70 and decreased proteasomal activity. Overexpression of WT α-Syn did not induce oligomer formation and toxicity in 5Y cells as observed with A53T α-Syn overexpression, suggesting that formation of oligomers was important for triggering the toxicity of α-Syn.
Inhibiting Hsp90 leads to compensatory induction of HSPs and has been proposed as an effective strategy for the therapy of neurodegenerative diseases (Luo et al., 2010; Blair et al., 2014). In fact, because of their ability to induce Hsp70, several Hsp90 inhibitors have recently been tested in a rat α-Syn PD model (Putcha et al., 2010; McFarland et al., 2014). However, the failure of these candidate molecules in ameliorating dopaminergic neurodegeneration indicated that the compensatory upregulation of Hsp70 by itself may be insufficient to protect against α-Syn toxicity (Zourlidou et al., 2004; Shimshek et al., 2010). The BQA class of Hsp90 inhibitors, including GA and 17-AAG, has been found to be effective in inducing both multiple HSPs and autophagy (Finn et al., 2005; Riedel et al., 2010; Watanabe et al., 2010). However, the protective effects of BQAs are accompanied by off-target toxicities, limiting their use in neurodegenerative diseases. Therefore, we examined the ability of novel 19-substituted BQA analogs to alleviate toxicity induced by α-Syn overexpression. 19-Substituted BQA analogs were synthesized to prevent direct conjugation with thiols at the 19 position (Kitson et al., 2013), and studies have demonstrated that 19-substitutions resulted in less toxicity when compared with their unsubstituted parent BQAs (Chang et al., 2014). Our data demonstrated that 19-Ph-GA was able to induce HSP expression and autophagy and exerted significant protective effects when tested in an A53T α-Syn cell model. After treatment with 19-Ph-GA, we observed a significant reduction in HW α-Syn oligomers as well as a redistribution of HW α-Syn oligomers to smaller oligomers. These effects on HW α-Syn oligomers following treatment with 19-Ph-GA may be the result of induction of Hsp70, Hsp27, and/or autophagy.
mTOR (TORC1) plays a key role in many cellular processes, including cell growth, proliferation, metabolism, and protein synthesis (Laplante and Sabatini, 2012). Its activity can be monitored by following the phosphorylation of substrates such as p70S6K at Thr389 (Yip et al., 2010). The inhibition of mTOR typically results in induction of autophagy and has been proposed as an attractive strategy for the therapy of misfolded protein diseases (Bové et al., 2011). However, in some cases, mTOR inhibition does not necessarily result in autophagy, but instead autophagy may be positively regulated by mTOR (Zeng and Kinsella, 2008) or may even be mTOR-independent (Sarkar, 2013). Our results demonstrated that overexpression of either A53T or WT α-Syn in 5Y cells could induce autophagy, whereas the activation of mTOR was only detected in A53T α-Syn–overexpressing cells. In addition, post-treatment with 19-Ph-GA for 48 hours significantly blocked the activation of mTOR/p70S6K signaling, whereas it had no significant effect on LC3 II levels in A53T α-Syn–overexpressing cells. These results suggest that mTOR activation is not necessary for synuclein-induced autophagy, and a number of previous studies have demonstrated overexpression of pathogenic forms of α-Syn could activate compensatory autophagy in response to either impaired chaperone-mediated autophagy or UPS activity (Stefanis et al., 2001; Cuervo et al., 2004; Xilouri et al., 2009).
The activation of mTOR/p70S6K signaling may be important for A53T α-Syn–induced toxicity in 5Y cells, although the mechanisms remain unclear. It is possible that mTOR activated downstream p70S6K to upregulate protein synthesis, including the synthesis of aberrant proteins such as A53T α-Syn, and resulted in proteolytic stress and toxicity as previously reported in a cell model of Huntington’s disease (King et al., 2008). Recently, mTOR has also been found to drive the phosphorylation status of transcriptional factor EB, preventing it from translocating to the nucleus and activating lysosome biogenesis (Decressac et al., 2013). Since lysosomes are critical to the process of autophagy, overexpression of A53T α-Syn might activate mTOR to inhibit lysosome biogenesis and lead to incomplete autophagic degradation of α-Syn oligomers and toxicity in 5Y cells. Our data also demonstrated that 19-Ph-GA was very effective in decreasing the levels of p-mTOR and p-p70S6K in A53T α-Syn–overexpressing cells. Under stress or disease conditions, mTOR/p70S6K signaling may be overactivated, and since mTOR has been reported as an Hsp90 client protein (Moulick et al., 2011), Hsp90 inhibitors such as 19-Ph-GA may be particularly effective.
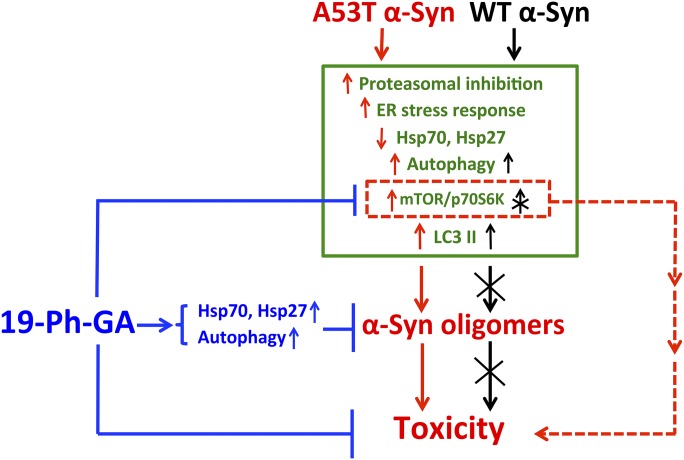
In summary, we demonstrated that 19-Ph-GA, an analog of GA, showed decreased toxicity when compared with GA while maintaining the ability to induce a robust HSP response as well as modulating the activation of autophagy in 5Y cells. The multiple protective effects of 19-Ph-GA rendered 5Y cells more resistant to mutant A53T α-Syn–induced adverse effects (Fig. 9). The data provide proof of principle that compounds which stimulate the HSP response and autophagy, such as 19-Ph-GA, may be worthy of further translational studies for the therapy of protein folding diseases, including PD.
Fig. 9.
Protective effects of 19-Ph-GA on A53T α-Syn induced protein handling changes and toxicity in 5Y cells. Overexpression of A53T α-Syn (labeled in red) resulted in α-Syn oligomer formation and toxicity via perturbing major protein handling systems, including increased proteasomal inhibition, ER stress response, and decreased levels of HSPs. Autophagy could be activated by overexpression of either A53T or WT α-Syn (labeled in black), excluding the possibility that autophagic cell death was induced specifically by A53T α-Syn overexpression. mTOR/p70S6K signaling was not directly involved in A53T α-Syn–induced autophagy but was positively related to the toxicity of A53T α-Syn. 19-Ph-GA (labeled in blue) activated Hsp70, Hsp27, and autophagy and ameliorated A53T α-Syn–induced mTOR activation. Collectively, these mechanisms may contribute to protection against A53T α-Syn oligomer formation and toxicity.
Supplementary Material
Abbreviations
- 17-AAG
17-(allylamino)-17-demethoxygeldanamycin
- AMC
7-amino-4-methylcoumarin
- BQA
benzoquinone ansamycin
- 19BQA
19-substituted benzoquinone ansamycin
- CQ
chloroquine
- 17-DMAG
17-(dimethylaminoethylamino)-17-demethoxygeldanamycin
- eIF2α
eukaryotic translation initiation factor 2α
- FITC
fluorescein isothiocyanate
- GA
geldanamycin
- HSP
heat shock protein
- HW α-Syn
higher molecular weight α-Syn
- mTOR
mammalian target of rapamycin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- 19-Ph-GA
19-phenyl-geldanamycin
- PI
propidium iodide
- p-mTOR
phosphorylated mTOR
- p-p70S6K
phosphorylated p70S6K
- p70S6K
p70 ribosomal S6 kinase
- α-Syn
α-synuclein
- UPS
ubiquitin-proteasome system
- WT
wild type
- 5Y
SH-SY5Y cells
Authorship Contributions
Participated in research design: Xiong, Siegel, Ross.
Conducted experiments: Xiong.
Contributed new reagents or analytic tools: Zhou, Freed, Kitson, Moody.
Performed data analysis: Xiong, Siegel, Ross.
Wrote or contributed to the writing of the manuscript: Xiong, Siegel, Ross.
Footnotes
This work was supported by the National Institutes of Health [Grants R01ES018943 and CA51210 (to D.R.)] and the Parkinson’s Disease Society UK (to C.J.M.).
D.S., R.R.A.K., C.J.M., D.R., and the University of Colorado have a patent interest in 19-substituted benzoquinone ansamycins.
Preliminary data from this work were presented in part at the following meeting: Xiong R, Zhou W, Siegel D, Kitson RAR, Freed CR, Moddy CJ, Ross D (2015) A novel Hsp90 inhibitor activates compensatory heat shock protein responses and autophagy and alleviates mutant A53T alpha synuclein toxicity. Society of Toxicology Annual Meeting; 2015 March 22–26; San Diego, CA.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Auluck PK, Chan HYE, Trojanowski JQ, Lee VM-Y, Bonini NM. (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 295:865–868. [DOI] [PubMed] [Google Scholar]
- Baldo B, Weiss A, Parker CN, Bibel M, Paganetti P, Kaupmann K. (2012) A screen for enhancers of clearance identifies huntingtin as a heat shock protein 90 (Hsp90) client protein. J Biol Chem 287:1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair LJ, Sabbagh JJ, Dickey CA. (2014) Targeting Hsp90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin Ther Targets 18:1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bové J, Martínez-Vicente M, Vila M. (2011) Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 12:437–452. [DOI] [PubMed] [Google Scholar]
- Chang C-H, Drechsel DA, Kitson RRA, Siegel D, You Q, Backos DS, Ju C, Moody CJ, Ross D. (2014) 19-substituted benzoquinone ansamycin heat shock protein-90 inhibitors: biological activity and decreased off-target toxicity. Mol Pharmacol 85:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr (2000) Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann N Y Acad Sci 920:42–45. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295. [DOI] [PubMed] [Google Scholar]
- Cysyk RL, Parker RJ, Barchi JJ, Jr, Steeg PS, Hartman NR, Strong JM. (2006) Reaction of geldanamycin and C17-substituted analogues with glutathione: product identifications and pharmacological implications. Chem Res Toxicol 19:376–381. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A. (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci USA 110:E1817–E1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimant H, Ebrahimi-Fakhari D, McLean PJ. (2012) Molecular chaperones and co-chaperones in Parkinson disease. Neuroscientist 18:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. (2011) Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci 31:14508–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, McLean PJ, Unni VK. (2012a) Alpha-synuclein’s degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy 8:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Wahlster L, McLean PJ. (2012b) Protein degradation pathways in Parkinson’s disease: curse or blessing. Acta Neuropathol 124:153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PF, Mesires NT, Vine M, Dice JF. (2005) Effects of small molecules on chaperone-mediated autophagy. Autophagy 1:141–145. [DOI] [PubMed] [Google Scholar]
- Gallo KA. (2006) Targeting HSP90 to halt neurodegeneration. Chem Biol 13:115–116. [DOI] [PubMed] [Google Scholar]
- Guo W, Reigan P, Siegel D, Ross D. (2008) Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: relevance for toxicity and mechanism of action. Drug Metab Dispos 36:2050–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Anand VS. (2008) Heat shock protein 90 modulates LRRK2 stability: potential implications for Parkinson’s disease treatment. J Neurosci 28:6757–6759 Soc Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T-F, Zhang Y-J, Zhou H-Y, Wang H-M, Tian L-P, Liu J, Ding J-Q, Chen S-D. (2013) Curcumin Ameliorates the Neurodegenerative Pathology in A53T α-synuclein Cell Model of Parkinson's Disease Through the Downregulation of mTOR/p70S6K Signaling and the Recovery of Macroautophagy. J Neuroimmune Pharmacol 8:356–369. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. (2013) α-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol 73:155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Burrows FJ. (2004) Hsp90 inhibitors as selective anticancer drugs. Discov Med 4:277–280. [PubMed] [Google Scholar]
- Kilpatrick K, Novoa JA, Hancock T, Guerriero CJ, Wipf P, Brodsky JL, Segatori L. (2013) Chemical induction of Hsp70 reduces α-synuclein aggregation in neuroglioma cells. ACS Chem Biol 8:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Hands S, Hafiz F, Mizushima N, Tolkovsky AM, Wyttenbach A. (2008) Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol Pharmacol 73:1052–1063. [DOI] [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL. (2005) Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol 398:364–378. [DOI] [PubMed] [Google Scholar]
- Kitson RRA, Chang C-H, Xiong R, Williams HEL, Davis AL, Lewis W, Dehn DL, Siegel D, Roe SM, Prodromou C, et al. (2013) Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90. Nat Chem 5:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson RRA, Moody CJ. (2013) An improved route to 19-substituted geldanamycins as novel Hsp90 inhibitors--potential therapeutics in cancer and neurodegeneration. Chem Commun (Camb) 49:8441–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. (2012) mTOR signaling in growth control and disease. Cell 149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- Luo W, Sun W, Taldone T, Rodina A, Chiosis G. (2010) Heat shock protein 90 in neurodegenerative diseases. Mol Neurodegener 5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Wang S. (2013) mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med 19:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Dimant H, Kibuuka L, Ebrahimi-Fakhari D, Desjardins CA, Danzer KM, Danzer M, Fan Z, Schwarzschild MA, Hirst W, et al. (2014) Chronic treatment with novel small molecule Hsp90 inhibitors rescues striatal dopamine levels but not α-synuclein-induced neuronal cell loss. PLoS One 9:e86048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Klucken J, Shin Y, Hyman BT. (2004) Geldanamycin induces Hsp70 and prevents alpha-synuclein aggregation and toxicity in vitro. Biochem Biophys Res Commun 321:665–669. [DOI] [PubMed] [Google Scholar]
- Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, et al. (2011) Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol 7:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. (2002) Heat shock protein 90 inhibition by 17-allylamino-17- demethoxygeldanamycin: a novel therapeutic approach for treating hormone-refractory prostate cancer. Clin Cancer Res 8:962–966. [PubMed] [Google Scholar]
- Olanow CW, McNaught K. (2011) Parkinson’s disease, proteins, and prions: milestones. Mov Disord 26:1056–1071. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Li L, Chin LS. (2008) Aggresome formation and neurodegenerative diseases: therapeutic implications. Curr Med Chem 15:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha P, Danzer KM, Kranich LR, Scott A, Silinski M, Mabbett S, Hicks CD, Veal JM, Steed PM, Hyman BT, et al. (2010) Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity. J Pharmacol Exp Ther 332:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel M, Goldbaum O, Schwarz L, Schmitt S, Richter-Landsberg C. (2010) 17-AAG induces cytoplasmic α-synuclein aggregate clearance by induction of autophagy. PLoS One 5:e8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S. (2013) Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41:1103–1130. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282:5641–5652. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Mueller M, Wiessner C, Schweizer T, van der Putten PH. (2010) The HSP70 molecular chaperone is not beneficial in a mouse model of α-synucleinopathy. PLoS One 5:e10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. (2001) Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci 21:9549–9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528. [DOI] [PubMed] [Google Scholar]
- Tokui K, Adachi H, Waza M, Katsuno M, Minamiyama M, Doi H, Tanaka K, Hamazaki J, Murata S, Tanaka F, et al. (2009) 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse. Hum Mol Genet 18:898–910. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nagase K, Chosa M, Tobinai K. (2010) Schwann cell autophagy induced by SAHA, 17-AAG, or clonazepam can reduce bortezomib-induced peripheral neuropathy. Br J Cancer 103:1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. (2005) 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 11:1088–1095. [DOI] [PubMed] [Google Scholar]
- Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. (2009) Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One 4:e5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R, Siegel D, Ross D. (2013) The activation sequence of cellular protein handling systems after proteasomal inhibition in dopaminergic cells. Chem Biol Interact 204:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R, Siegel D, Ross D. (2014) Quinone-induced protein handling changes: implications for major protein handling systems in quinone-mediated toxicity. Toxicol Appl Pharmacol 280:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. (2010) Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell 38:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Kinsella TJ. (2008) Mammalian target of rapamycin and S6 kinase 1 positively regulate 6-thioguanine-induced autophagy. Cancer Res 68:2384–2390. [DOI] [PubMed] [Google Scholar]
- Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR. (2011) Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem 286:14941–14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Schaack J, Zawada WM, Freed CR. (2002) Overexpression of human alpha-synuclein causes dopamine neuron death in primary human mesencephalic culture. Brain Res 926:42–50. [DOI] [PubMed] [Google Scholar]
- Zourlidou A, Payne Smith MD, Latchman DS. (2004) HSP27 but not HSP70 has a potent protective effect against α-synuclein-induced cell death in mammalian neuronal cells. J Neurochem 88:1439–1448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.