Abstract
Alkylation of potassium selenosulfate with allylic halides gives Se-allyl seleno Bunte salts. On reaction with thiols at room temperature these afford mixed dialkyl selenosulfides, which undergo 2,3-sigmatropic rearrangement with loss of selenium, either spontaneously or with assistance by triphenylphosphine, thereby providing mixed dialkyl sulfides and a new permanent chemical ligation method. The process is illustrated through the lipidation of cysteine containing tripeptides and by the allylation of 1-thioglucose tetraacetate.
The development of methods for the chemical ligation of small molecules to peptides and proteins, for conjoining two proteins, and for the attachment of oligosaccharides to peptides and proteins is a current frontier in chemical biology.1 One established ligation method involves the formation of mixed disulfide linkages,2 various modifications of which have recently been applied to the synthesis of glycopeptides and proteins.3 The disulfide ligation is especially attractive in conjunction with native peptide ligation, however, the ease of disulfide exchange renders the method impermanent.4 We report preliminary results on the development of a new method of chemical ligation based on the facile formation of mixed selenosulfides, which are then rendered permanent by a sigmatropic rearrangement with loss of selenium.
Allylic disulfides are in equilibrium with allylic thiosulfoxides, by virtue of a 2,3-sigmatropic rearrangement.5 The equilibrium very strongly favors the allylic disulfide and is only revealed when the reaction is driven in the forward direction, either by a further rearrangement, or by the addition of a thiophilic reagent, typically a phosphine. At 60 °C in benzene rate constants for the rearrangement with transfer of sulfur to triphenyl phosphine were found to range from 0.7 – 190 × 10−4 s−1 depending on the substituent pattern.5a Allylic diselenides on the other hand undergo the analogous rearrangement at room temperature in a matter of hours.6,7
We hypothesized that Se-allyl selenosulfides 2 would undergo a similar 2,3-sigmatropic rearrangement at room temperature and that the weakness of the Se-S bond in the selenosulfoxide product 3 would result in the facile loss of selenium, thereby driving the reaction even in the absence of a phosphine. We further hypothesized that the requisite Se-allylic selenosulfides could be readily accessed at room temperature from simple Se-allyl seleno Bunte salts 1, as was known for Se-alkyl seleno Bunte salts,8 and that the combination of these two processes would provide a convenient new chemical ligation process (Scheme 1).
Scheme 1.
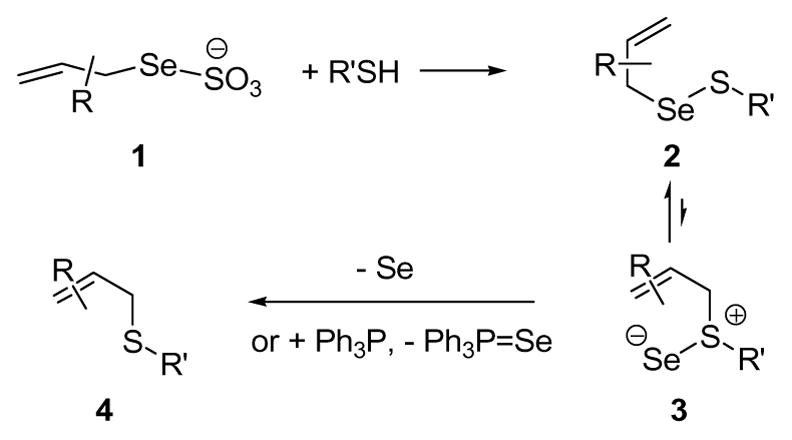
Chemical Ligation via Se-Allyl Selenosulfides
Reaction of K2SO3 with Se powder and, then, allyl bromide gave potassium Se-allyl selenosulfate 5 as an orange solid.8b,9 Reaction of this Se-Bunte salt with primary aliphatic thiols in methanol at room temperature resulted in transfer of the allyl group to sulfur, with loss of selenium (Scheme 1), establishing the validity of the initial hypothesis.
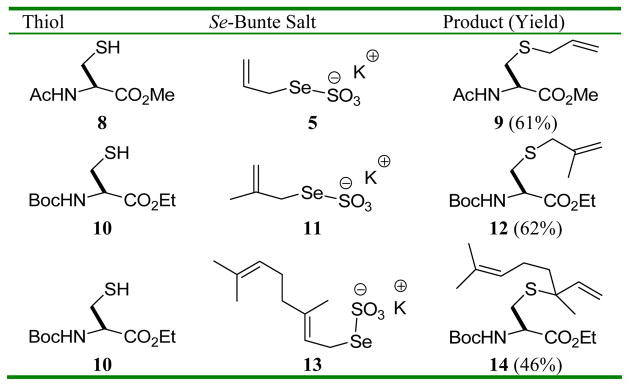
We next examined the attachment of allyl and substituted allyl groups to cysteine derivatives (Table 1). These reactions were conducted in two stages, with formation of the selenosulfide in methanol, and, following filtration on silica gel, triphenylphosphine-promoted deselenylative rearrangement in CDCl3, all at room temperature. The 2,3-sigmatropic nature of the rearrangement step is unmasked with the formation of the S-linalyl cysteine 14 starting from the Se-geranyl Se-Bunte salt 13.10
Table 1.
: With Se Bunte salt 13, the first step was conducted in a methanol/CH2Cl2 mixture.
: Compound 14 was formed as an approximately 1:1 unassigned mixture of diastereomers.
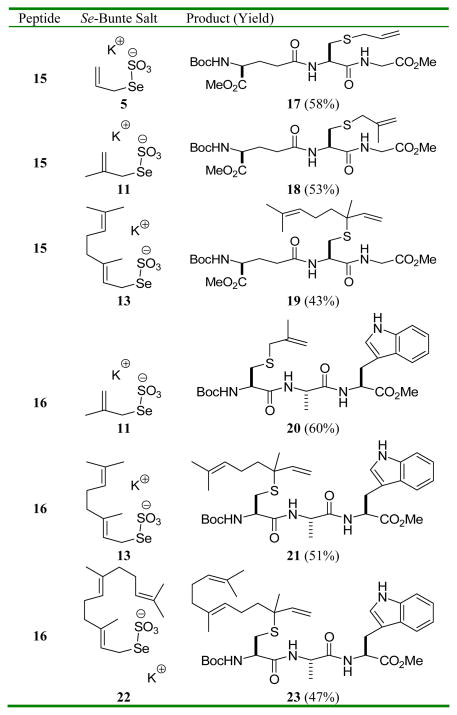
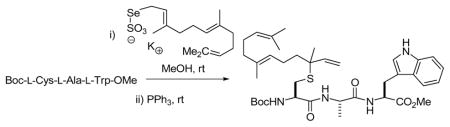
Functionalization of small cysteine-containing peptides was explored with Boc-(α-OMe)-γ-L-Glu-L-Cys-Gly-OMe, 15, and with Boc-L-Cys-L-Ala-L-Trp-OMe 16 using a selection of allylic Se-Bunte salts (Table 2). These reactions were conducted in methanol at room temperature with filtration of the excess Bunte salt before addition of the phosphine. It is noteworthy that this new cysteine functionalization method is compatible with the indole in tryptophan, and enables the incorporation of the geranyl and farnesyl chains as the transposed linalyl and nerolidyl groups. We have also demonstrated application to a carbohydrate-based thiol 24 (Scheme 3). This rearrangement, which proceeded spontaneously over a period of several days at room temperature, or in a matter of hours on addition of triphenyl phosphine, took place with complete retention of the anomeric stereochemistry.
Table 2.
Application to Cysteine Containing Tripeptidesa
: Compounds 19, 21, and 23 were formed as an approximately 1:1 unassigned mixture of diastereomers.
Scheme 3.
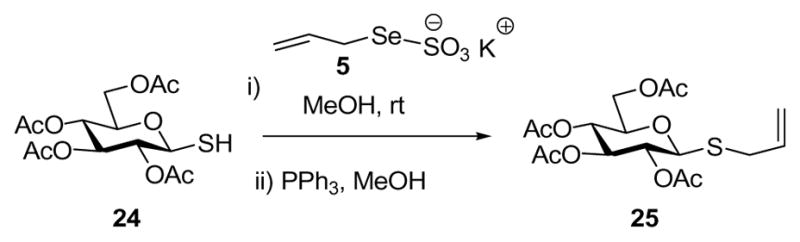
Allylation of an Anomeric Thiol
In summary, we describe a new, permanent chemical ligation method. The method has potential for the attachment of lipids to protein-based thiols, an area of considerable current interest.11 Applications in combinatorial chemistry and for the attachment of a wide variety of groups, including polyethylene glycol12 and fluorous chains,13 and oligosaccharide units to thiols3,14 of biological interest, are currently under development.
Supplementary Material
Scheme 2.
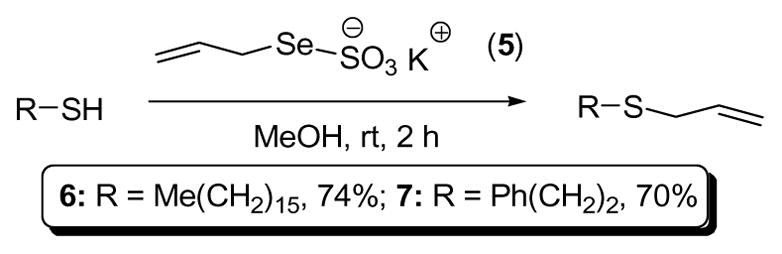
Allylation of Simple Thiols
Acknowledgments
We thank the NIH (AI 56575) for partial support of this work and Dr. Franck Brebion for the preparation of peptide 16.
Footnotes
Supporting Information Available: Full experimental details and copies of spectra of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Kochendoerfer GG, Kent SBH. Curr Op Chem Biol. 1999;3:665. doi: 10.1016/s1367-5931(99)00024-1. [DOI] [PubMed] [Google Scholar]; b) Dawson PE, Kent SBH. Ann Rev Biochem. 2000;69:923. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]; c) Köhn M, Breinbauer R. Angew Chem Int Ed. 2004;43:3106. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]; d) van Swieten PF, Leeuwenburgh MF, Kessler BM, Overkleeft HS. Org Biomol Chem. 2005;3:20. doi: 10.1039/b412558d. [DOI] [PubMed] [Google Scholar]; e) Peri F, Nicotra F. Chem Comm. 2004:623. doi: 10.1039/b308907j. [DOI] [PubMed] [Google Scholar]; f) Breinbauer R, Köhn M. ChemBioChem. 2003;4:1147. doi: 10.1002/cbic.200300705. [DOI] [PubMed] [Google Scholar]; g) Kubler-Kielb J, Pozsgay V. J Org Chem. 2005;70:6987. doi: 10.1021/jo050934b. [DOI] [PubMed] [Google Scholar]; h) Langenhan JM, Thorson JS. Curr Org Synth. 2005;2:59. [Google Scholar]; i) He Y, Hinklin RJ, Chang J, Kiessling L. Org Lett. 2004;6:4479. doi: 10.1021/ol048271s. [DOI] [PubMed] [Google Scholar]; j) Grandjean C, Boutonnier A, Guerreiro C, Fournier JM, Mulard LA. J Org Chem. 2005;70:7123. doi: 10.1021/jo0505472. [DOI] [PubMed] [Google Scholar]; k) Lin H, Walsh CT. J Am Chem Soc. 2004;126:13998. doi: 10.1021/ja045147v. [DOI] [PubMed] [Google Scholar]; l) Liu H, Wang L, Brock A, Wong CH, Schultz PG. J Am Chem Soc. 2003;125:1702. doi: 10.1021/ja029433n. [DOI] [PubMed] [Google Scholar]; m) Manetsch R, Krasinski A, Radic Z, Raushel J, Taylor P, Sharpless KB, Kolb HC. J Am Chem Soc. 2004;126:12809. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]; n) Krasinski A, Radic Z, Manetsch R, Raushel J, Taylor P, Sharpless KB, Kolb HC. J Am Chem Soc. 2005;127:6686. doi: 10.1021/ja043031t. [DOI] [PubMed] [Google Scholar]; o) Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; p) Davis BG. Chem Rev. 2002;102:579. doi: 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]; q) Shin Y, Winans KA, Backes BJ, Kent SBH, Ellman JA, Bertozzi CR. J Am Chem Soc. 1999;121:11684. [Google Scholar]; r) Macmillan D, Bertozzi CR. Angew Chem Int Ed. 2004;43:1355. doi: 10.1002/anie.200352673. [DOI] [PubMed] [Google Scholar]; s) MacMillan DWC, Bill RM, Sage KA, Fern D, Flitsch SL. Chem Biol. 2001;8:133. doi: 10.1016/s1074-5521(00)90065-6. [DOI] [PubMed] [Google Scholar]
- 2.a) Lundblad RL. Chemical Reagents for Protein Modification. 3. CRC Press; Boca Raton: 2005. [Google Scholar]; b) Kenyon GL, Bruice TW. Meth Enzym. 1977;47:407. doi: 10.1016/0076-6879(77)47042-3. [DOI] [PubMed] [Google Scholar]; c) Wynn R, Richards FM. Meth Enzym. 1995;251:351. doi: 10.1016/0076-6879(95)51138-5. [DOI] [PubMed] [Google Scholar]; d) Faulstich H, Heintz D. Meth Enzym. 1995;251:357. doi: 10.1016/0076-6879(95)51139-3. [DOI] [PubMed] [Google Scholar]
- 3.a) Gamblin DP, Garnier P, van Kasteren S, Oldham NJ, Fairbanks AJ, Davis BG. Angew Chem Int Ed. 2004;43:828. doi: 10.1002/anie.200352975. [DOI] [PubMed] [Google Scholar]; b) Gamblin DP, Garnier SJ, Ward NJ, Oldham AJ, Fairbanks AJ, Davis BG. Org Biomol Chem. 2003;1:3642. doi: 10.1039/b306990g. [DOI] [PubMed] [Google Scholar]; c) Macindoe WM, van Oijen AH, Boons GJ. Chem Commun. 1998:847. [Google Scholar]
- 4.As illustrated by applications in dynamic combinatorial libraries. Otto S, Furlan RLE, Sanders JKM. J Am Chem Soc. 2000;122:12063.Otto S, Furlan RLE, Sanders JKM. Science. 2002;297:590. doi: 10.1126/science.1072361.Brisig B, Sanders JKM, Otto S. Angew Chem Int Ed. 2003;42:1270. doi: 10.1002/anie.200390326.Leclaire J, Vial L, Otto S, Sanders JKM. Chem Commun. 2005:1959. doi: 10.1039/b500638d.
- 5.a) Höfle G, Baldwin JE. J Am Chem Soc. 1971;93:6307. doi: 10.1021/ja00738a055. [DOI] [PubMed] [Google Scholar]; b) Baechler RD, Hummel JP, Mislow K. J Am Chem Soc. 1973;95:4442. [Google Scholar]; c) Block E, Iyer R, Grisoni S, Saha C, Belman S, Lossing FP. J Am Chem Soc. 1988;110:7813. [Google Scholar]; d) Kutney GW, Turnbull K. Chem Rev. 1982;82:333. [Google Scholar]
- 6.Sharpless KB, Lauer KB. J Org Chem. 1972;37:3973. [Google Scholar]
- 7.Synthesis of selenosulfides by reaction of selenols with disulfides was discounted due to the complex synthesis and instability of allylic selenols. Riague EH, Guillemin JC. Organometallics. 2002;21:68.
- 8.a) Patarasakulchai N, Southwell-Keely PT. Phosphorus and Sulfur. 1985;22:277. [Google Scholar]; b) Klayman DL, Günther WHH, editors. Organic Selenium Compounds: their Chemistry and Biology. Wiley Interscience; New York: 1973. [Google Scholar]
- 9.a) Price TS, Jones LM. J Chem Soc. 1909;95:1729. [Google Scholar]; b) Günther WHH, Mautner HG. J Med Chem. 1964;7:229. doi: 10.1021/jm00332a023. [DOI] [PubMed] [Google Scholar]
- 10.Attempted acceleration of the rearrangement with more nucleophilic phosphines resulted in a changed mechanism: nucleophilic attack on the selenosulfide with loss of the sigmatropic rearrangement component. Presumably, this occurs analogously to loss of sulfur from disulfides with hexaalkylphosphoramines. Hexamethylphosphoramine also caused racemization of the cysteine moiety as evidenced by incorporation of deuterium at the α-position when the reaction was conducted in CD3OD. Harpp DN, Gleason JG. J Am Chem Soc. 1971;93:2437.
- 11.a) Schelhaas M, Nägele E, Kuder N, Bader B, Kuhlmann J, Wittinghofer A, Waldmann H. Chem Eur J. 1999;5:1239. [Google Scholar]; b) Schelhaas M, Glomsda S, Hänsler M, Jakubke HD, Waldmann H. Angew Chem Int Ed. 1996;35:106. [Google Scholar]; c) Brown MJ, Milano PD, Lever DC, Epstein WW, Poulter CD. J Am Chem Soc. 1991;113:3176. [Google Scholar]; d) Yang CC, Marlowe CK, Kania R. J Am Chem Soc. 1991;113:3177. [Google Scholar]; e) Pachamuthu K, Zhu X, Schmidt RR. J Org Chem. 2005;70:3720. doi: 10.1021/jo0482357. [DOI] [PubMed] [Google Scholar]
- 12.a) Veronese FM, Pasut G. Drug Disc Today. 2005;10:1451. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]; b) Doherty DH, Rosendahl MS, Smith DJ, Hughes JM, Chlipala EA, Cox GN. Bioconj Chem. 2005;16:1291. doi: 10.1021/bc050172r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Manjula BN, Tsai A, Upadhya R, Perumalsamy K, Smith PK, Malavalli A, Vanderegriff K, Winslow RM, Intaglietta M, Prabhakaran M, Friedman JM, Acharya AS. Bioconj Chem. 2003;14:464. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 13.Brittain SM, Ficarro SB, Brock A, Peters EC. Nature Biotechnology. 2005;23:463. doi: 10.1038/nbt1076. [DOI] [PubMed] [Google Scholar]
- 14.a) Ohnishi Y, Ichikawa M, Ichikawa Y. Biorg Med Chem Lett. 2000;10:1289. doi: 10.1016/s0960-894x(00)00223-7. [DOI] [PubMed] [Google Scholar]; b) Jobron L, Hummel G. Org Lett. 2000;2:2265. doi: 10.1021/ol006019o. [DOI] [PubMed] [Google Scholar]; c) Cohen SB, Halcomb RL. Org Lett. 2001;3:405. doi: 10.1021/ol006908b. [DOI] [PubMed] [Google Scholar]; d) Knapp S, Myers DS. J Org Chem. 2002;67:2995. doi: 10.1021/jo0110909. [DOI] [PubMed] [Google Scholar]; e) Zhu XM, Pachamuthu K, Schmidt RR. Org Lett. 2004;6:1083–1085. doi: 10.1021/ol036186z. [DOI] [PubMed] [Google Scholar]; f) Zhu XM, Schmidt RR. Chem Eur J. 2004;10:875. doi: 10.1002/chem.200305163. [DOI] [PubMed] [Google Scholar]; g) Thayer DA, Yu HN, Galan MC, Wong CH. Angew Chem Int Ed. 2005;44:4596. doi: 10.1002/anie.200500090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.