Abstract
Mutations in the MET exon 14 RNA splice acceptor and donor sites, which lead to exon skipping, deletion of the juxtamembrane domain containing the Cbl E3-ubiquitin ligase binding site, and decreased turnover of the resultant aberrant MET protein, were previously reported to be oncogenic in preclinical models. We now report responses to the MET inhibitors crizotinib and cabozantinib in four patients with stage IV lung adenocarcinomas harboring mutations leading to MET exon 14 skipping, highlighting a new therapeutic strategy for the 4% of lung adenocarcinoma patients whose tumors harbor this previously underappreciated genetic alteration.
Keywords: MET, exon skipping, lung cancer
Introduction
Lung cancer is the leading cause of cancer death in both men and women.(1) Great therapeutic strides have been made for those with the most common subtype of lung cancer- lung adenocarcinoma- in which an oncogenic driver and target for therapy can now be identified in the majority of patients.(2) One proto-oncogene, the mesenchymal epithelial transition factor (MET) tyrosine kinase, has been the focus of therapeutic studies in lung cancer for a number of years. MET, along with its ligand hepatocyte growth factor (HGF), plays a role in cell proliferation, apoptosis, and motility/invasion.(3, 4) Gain of function alterations in MET are varied, and include gene amplification, protein overexpression, and mutations in the juxtamembrane and semaphorin domains.(5) The overall incidence of MET mutations varies, occurring in 3% of squamous cell lung cancers(6) and 8% of lung adenocarcinomas.(7) MET gene amplification occurs in about 4% of lung adenocarcinomas and 1% of squamous cell lung cancers.(6, 7) Clinical trials of MET-directed therapies have taken two approaches: monoclonal antibody therapy directed against the receptor or HGF ligand; and tyrosine kinase inhibition. In a recent phase 3 trial of stage IV non-small cell lung cancer (NSCLC) patients whose tumors demonstrated high MET protein expression by immunohistochemistry (IHC), patients were randomized to receive second-line erlotinib +/− onartuzumab, an inhibitory anti-MET monoclonal antibody. This study showed no benefit to the addition of onartuzumab to erlotinib over erlotinib alone.(8) In contrast, early reports do suggest that the tyrosine kinase inhibitor crizotinib has activity in patients with MET-amplified NSCLCs.(9)
In 2003 and 2005, Ma et al. reported a series of novel MET exon 14 splicing variants, two in small cell lung cancer tumors involving a 2 base-pair insertion in a splice acceptor site 5’ of exon 14 and one in a NSCLC tumor involving an in-frame skip of exon 14.(10, 11) In 2006, Kong-Beltran et al. identified another series of somatic intronic mutations in lung cancer cell lines and patient samples immediately flanking exon 14, which encodes the juxtamembrane domain and Y1003 residue that serves as the binding site for Cbl, the E3-ubiquitin ligase that controls MET turnover.(12) RT-PCR confirmed exon 14 skipping in each case. Co-precipitation of Cbl and MET was lost in the presence of these variants. MET exon 14 skipping also led to a decrease in MET ubiquitination and delayed receptor downregulation after stimulation with HGF. Downstream ligand-dependent signaling through MAPK was also prolonged in cells transfected with a MET exon 14 splice variant. A xenograft model of Rat1a fibroblasts stably transfected with a MET exon 14 splice variant exhibited tumors whose growth rates were significantly higher than those with wild-type MET. Finally, H596 lung adenocarcinoma cells, which harbor a MET exon 14 splice variant, exhibited MET signaling down-regulation and a decrease in cell viability following treatment with onartuzumab. The scope of MET exon 14 splice variants in lung adenocarcinomas has since been defined by a number of other groups, including The Cancer Genome Atlas.(7, 13, 14)
Based on these data, we prospectively identified a series of 8 patients with MET exon 14 splice site alterations and treated 4 of them with one of the small molecule MET inhibitors, crizotinib or cabozantinib.
Results
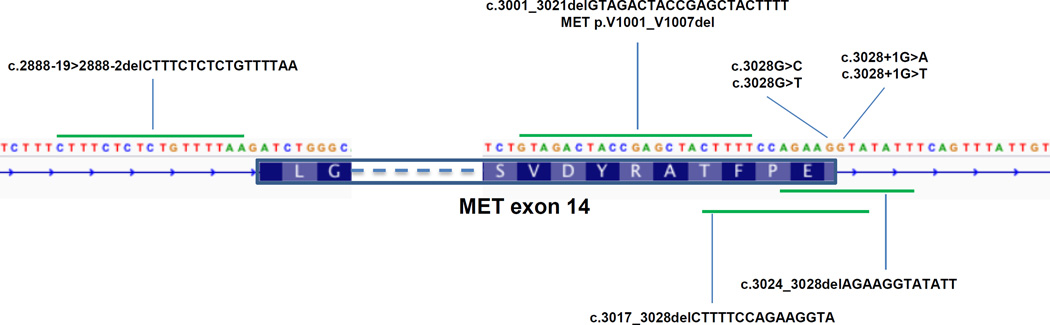
Table 1 summarizes the clinicopathological data for the 8 patients with MET exon 14 splice site alterations. There were no ROS1, RET, or ALK fusion events detected by MSK-IMPACT. The mutations we detected flanking MET exon 14 or deleting Y1003 are shown pictorially in Figure 1. nanoString confirmed MET exon 14 skipping in all 5 patients who had leftover tumor material for this analysis (Supplementary Figures 1 and 2). The specific case reports for four patients treated with a MET inhibitor follow below.
Table 1.
Clinical, pathologic, and molecular characteristics of patients with stage IV lung adenocarcinomas harboring MET exon 14 splice site mutations
| Patient ID |
Age | Sex | Smoking status (pack years) |
MET exon 14 variant (mutant allele frequency) |
Histology | Prior therapies |
MET therapy |
RECIST response |
PFS (months) |
OS (months) |
Somatic mutations |
Copy number alterations |
MET fold change |
MET IHC (H-score) |
MET mRNA exon 14 skip (Nanostring) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | F | F (6) | MET c.2888- 19>2888- 2delCTTTCTCTCTGTTTTAA exon 14 splicing variant (0.51) |
poorly differentiated adenocarcinoma |
MPDL3280A | none | NA | NA | 9.2+ | TP53 C135F, ASXL1 V1332F, ATRX E1159X, FAT1 M4205I, MET S204fs |
MYC amp, RPS6KA4 amp, MEN1 amp, RPS6KB2 amp |
not amplified |
300 | Yes |
| 2 | 80 | F | N | MET c.3028G>C exon 14 splicing variant (0.94) |
moderately differentiated adenocarcinoma |
docetaxel; pemetrexed |
cabozantinib (3rd line) |
SD (0%), CR (PERCIST response) |
5.1+ | 55.1+ | RB1 intragenic deletion, DICER1 Q1776X, EPHA5 R853Q, KLF4 M1I, MLL I1929M, MTOR D537N, TERT G225E |
FUBP1 amp, GSK3B amp, SDHA amp, TERT amp, MET amp, KDM5A amp, RAD52 amp, MDM2 amp, BCL2L1 amp, NKX3- 1 del, ETV6 del |
6 | 300 | Yes |
| 3 | 79 | F | N | MET c.3028+1G>A exon 14 splicing variant (0.16) |
adenocarcinoma with squamous and spindle cell components |
unknown | none | NA | NA | 5.3 | NF1 L2755fs | none | not amplified |
300 | Yes |
| 4a (primary lung tumor) |
80 | M | F (20) | MET c.3024_3028del AGAAGGTATATT exon 14 splicing variant (0.38) |
adenocarcinoma | carboplatin + pemetrexed + bevacizumab ; abraxane |
crizotinib (3rd line) |
PR (−30%) | 3.6 | 22.2 | TP53 exon8 splice variant, FAT1 R782fs, FBXW7 G539V, MLL E1678K, NF1 E572fs, NTKR2 I191T |
YES1 amp | not amplified |
300 | Yes |
| 4b (liver metastasis) |
80 | M | F (20) | MET c.3024_3028del AGAAGGTATATT exon 14 splicing variant (0.57) |
adenocarcinoma | carboplatin + pemetrexed + bevacizumab ; abraxane |
crizotinib (3rd line) |
PD (+133%) | 0 | 22.2 | TP53 exon 8 splice variant, FAT1 R782fs, FBXW7 G539V, MLL E1678K, NF1 E572fs, TERT G674S |
CDKN2B del, CDKN2A del |
not amplified |
300 | Yes |
| 5 | 65 | M | C (20) | MET p.V1001_F1007 del (c.3001_3021d elGTAGACTACC GAGCTACTTTT) (0.44) |
poorly differentiated adenocarcinoma |
cisplatin + pemetrexed + bevacizumab ; gemcitabine |
crizotinib (3rd line) |
PR (−31%) | 4.6+ | 17.9+ | TP53 R248P, RB1 intragenic deletion, BRCA1 E648Q, MYC E137D, NF1 exon56 splice variant, NDS1 E1902K, PDGFRB R397W |
TERT gain, MET amp, MYC amp, NKX2-1 amp |
3.8 | NA | NA |
| 6 | 63 | M | F (20) | MET c.3028+1G>T exon 14 splicing variant (0.67) |
adenocarcinoma | cisplatin + pemetrexed |
none | NA | NA | 15.8+ | PIK3CA R108L, TET2 I1871fs |
none | not amplified |
NA | NA |
| 7 | 90 | F | N | MET c.3028G>T exon 14 splicing variant |
adenocarcinoma | pemetrexed; gemcitabine |
crizotinib (3rd line) |
PR (−47%) | 3.1+ | 73.3+ | none | CDK4 amp, MDM2 amp |
not amplified |
300 | NA |
| 8 | 86 | M | N | MET c.3017_3028del CTTTTCCAGAA GGTA exon 14 splicing variant |
adenocarcinoma | pemetrexed; gemcitabine |
none | NA | NA | 12.0+ | none | none | not amplified |
NA | Yes |
Figure 1.
Diagram of MET exon 14 alterations in relation to the 5’ and 3’ splice sites.
Patient 2
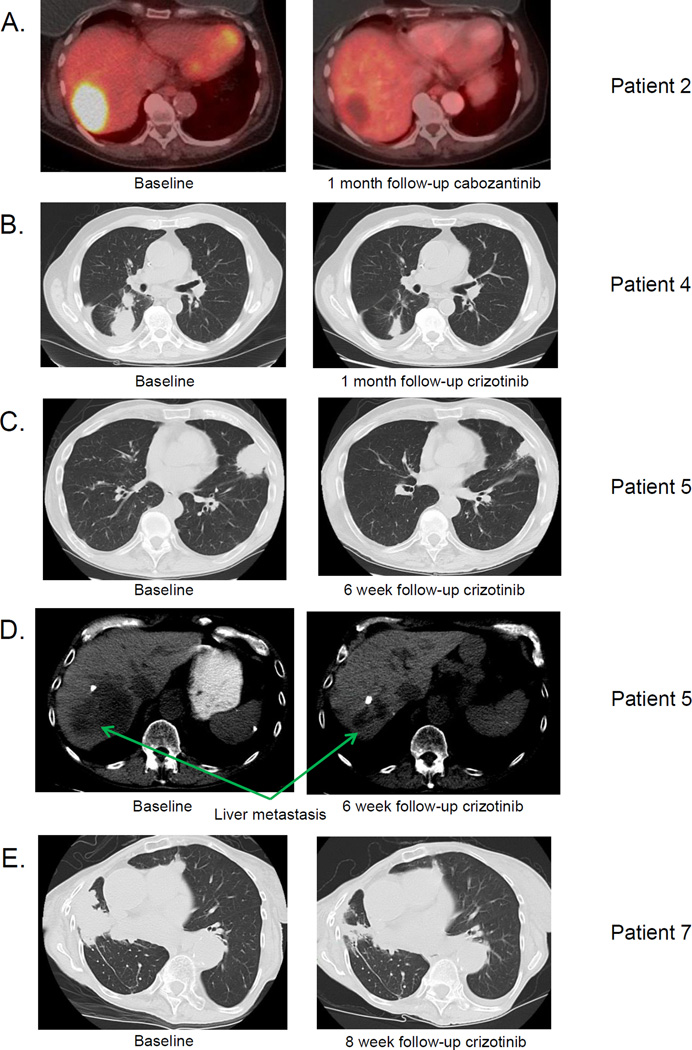
Patient 2 is an 80 year-old woman and a never smoker who was diagnosed with a pT1aN0M0 stage IA lung adenocarcinoma in 2008. In 2010, the patient developed recurrence in a precarinal lymph node and received weekly docetaxel for 2 months. This was poorly tolerated. Her therapy was changed to pemetrexed which she received from September 2011 until February 2012. Following disease progression, she underwent radiation therapy to the mediastinum totaling 66Gy. She did well for just over 2 years when she developed a liver metastasis in June 2014. Her liver tumor was biopsied and sequenced revealing lung adenocarcinoma positive for a MET c.3028G>C exon14 splice site mutation and MET amplification. MET IHC showed high MET expression, with an H-score of 300. The patient provided written informed consent for participation in a phase 2 clinical trial of cabozantinib, a tyrosine kinase inhibitor with activity against MET (IRB #12-097; NCT01639508). She began treatment with cabozantinib at a dose of 60mg oral daily in September 2014. Baseline PET imaging showed a 5cm liver metastasis with SUVmax 10.4. Follow-up imaging 4 weeks later showed complete resolution of FDG-uptake in the liver lesion, meeting the definition of a PERCIST complete response (Figure 2A). The patient remains on therapy with a dose reduction, having experienced grade 2 fatigue as her most severe adverse event to date.
Figure 2.
A. Baseline and 4 week PET scan from Patient 2 (MET c.3028G>C exon 14 splice variant) following treatment with cabozantinib. Imaging shows complete PET response by PERCIST criteria in the patient’s liver metastasis.
B. Baseline and 4 week CT scan from Patient 4 (MET c.3024_3028delAGAAGGTATATT exon 14 splice variant) following treatment with crizotinib. Imaging shows a response in the patient’s lung tumor.
C. Baseline and 6 week CT scan from Patient 5 (MET c.3028+1G>T exon 14 splice variant) following treatment with crizotinib. Imaging shows a response in the patient’s lung tumor.
D. Baseline and 6 week CT scan from Patient 5 (MET c.3028+1G>T exon 14 splice variant) following treatment with crizotinib. Imaging shows a response in the patient’s liver metastasis.
E. Baseline and 8 week CT scan from Patient 7 (MET c.3028G>T exon 14 splice variant) following treatment with crizotinib. Image shows a response in the patient’s lung tumors.
Patient 4
Patient 4 was a 78 year-old gentleman and former smoker diagnosed with stage IV lung adenocarcinoma in December 2012. Imaging showed metastases to the right pleura, mediastinal lymph nodes, and bone. Initial genotyping of his lung tumor by a mass spectrometry-based multiplex PCR assay (Sequenom MassARRAY™) for common lung cancer oncogene point mutations(15), sizing assays(16) for EGFR and ERBB2 indels, and ALK fluorescence in-situ hybridization test was negative. He received treatment with carboplatin + pemetrexed + bevacizumab followed by maintenance pemetrexed + bevacizumab from January 2013 to February 2014 with a partial response. CT imaging in March 2014 showed disease progression with a new 3cm right lung tumor and growth of an existing right lower lobe lung tumor. A biopsy of his new lung tumor was performed to obtain additional material for sequencing, revealing a MET c.3024_3028delAGAAGGTATATT exon 14 splice site mutation. MET IHC showed strong MET expression (H-score=300). Second-line albumin-bound paclitaxel was given every other week in the interim, with imaging after 2 cycles showing disease progression in his lung tumors as well as new liver metastases. The patient began treatment with crizotinib 250mg oral twice daily in June 2014, provided off-label with insurance approval. Imaging after 4 and 8 weeks of therapy showed a response in his lung tumors, meeting RECIST partial response criteria (−30%) (Figure 2B). Unfortunately, his liver metastases grew despite efficacy in the lung. Because of a past history of resected early stage sarcomas, a biopsy of a liver metastasis was performed to confirm histology. This showed a poorly differentiated adenocarcinoma morphologically similar to his lung primary. The tumor contained the same MET exon 14 splice site mutation and showed strong MET expression (H-score 300). Additional changes were present, however, including CDKN2A deletion (fold-change −2.3) and CDKN2B deletion (fold change −2.3).
The patient signed written informed consent to participate in a clinical trial of the MET inhibitor cabozantinib (IRB #12-097, NCT01639508) and ceased taking crizotinib as part of the washout period for the study. Unfortunately, prior to starting the clinical trial, he developed multilobar pneumonia and died despite treatment with broad-spectrum antibiotics.
Patient 5
Patient 5 is a 65 year-old gentleman and a former smoker who was diagnosed with stage IV lung adenocarcinoma in July 2013 following work-up for an episode of chest pain. Imaging showed widespread metastatic disease in the liver and bones, including the skull, sternum, spine, and bilateral hips. Outside molecular studies showed no evidence of alterations in EGFR and ALK. He was treated with first-line cisplatin + pemetrexed + bevacizumab followed by maintenance pemetrexed + bevacizumab from September 2013 to July 2014 with a partial response. CT imaging in July 2014 showed increase in his lung tumor and a new liver metastasis, prompting a biopsy of his lung tumor for more comprehensive genotyping. This demonstrated adenocarcinoma morphologically similar to his initial biopsy and a MET c.3028+1G>T exon 14 splice site mutation. He was treated with second-line gemcitabine for two months, with CT imaging showing further disease progression in keeping with his worsening dyspnea and bone pain. He began crizotinib 250mg oral twice daily in September 2014, provided off-label with insurance approval. Two weeks after starting treatment, the patient noted substantial improvement in his dyspnea and bone pain. CT imaging 6 weeks after initiation of crizotinib demonstrated a partial response to therapy (−31%) (Figure 2C and 2D). The patient remains on treatment without side effects.
Patient 7
Patient 7 is a 90 year-old woman and never-smoker who was diagnosed with recurrent metastatic lung adenocarcinoma to lymph node and lung in February 2009. She was treated with single-agent pemetrexed from April 2009 until June 2010 and single-agent gemcitabine from July 2010 until January 2014. A CT scan in January 2014 showed evidence of slight progression in multiple lung nodules in the setting of cumulative fatigue from chemotherapy. A treatment holiday was instituted and the patient observed with serial imaging. CT scan from July and September 2014 showed further progression. Based on this, the patient underwent a new biopsy of her lung tumor in September 2014 for sequencing. MSK-IMPACT showed a single somatic mutation, a MET c.3028G>T exon 14 splicing variant, and two copy number alterations-CDK4 and MDM2 amplification. Treatment with off-label crizotinib at a dose of 250mg oral twice daily was instituted in November 2014 with subsequent imaging in January 2015 showing a partial response to therapy (−47%) (Figure 2E). The patient remains on treatment without side effects.
Discussion
These data are, to our knowledge, the first to show that patients with lung adenocarcinomas harboring MET exon 14 splice site mutations can respond to MET-directed targeted therapy. More broadly, they underscore the potential clinical importance of looking beyond the exome for cancer-specific mutations that affect RNA processing and differential exon use. While MET exon 14 splice site mutations occur with highest frequency in lung adenocarcinomas, they have also been identified in small cell lung cancer(10), glioblastoma multiforme (1%)(17) and squamous cell head and neck cancers (1%).(18) In addition to specific splice site mutations, larger scale changes in pre-mRNA processing caused by recurrent mutations in U2AF1, which encodes an auxiliary factor that is required for U2 snRNP identification of 3’ splice sites, have been recently characterized and can lead to significantly different splicing programs involving many, many gene products.(19) Mutations in U2AF1 occur with appreciable frequency in leukemias, lung adenocarcinomas(7), pancreatic cancers, and endometrial cancers.(18) We believe this report may represent the first clinical validation of a new class of actionable driver events with potential relevance to patients across cancer types. Unlike most splice site mutations which result in loss of the reading frame and protein truncation, these splice site mutations inducing MET exon 14 skipping and are activating and targetable.
Although somatic splice site mutations in MET have been previously reported, the absence of broadly applied comprehensive clinical sequencing platforms has limited our ability to routinely detect these mutations. Somatic splice site mutations flanking exon 14 occur in 4% of lung adenocarcinomas based on recently published TCGA data and our own series.(7) This frequency is comparable to that of ALK rearrangements, for which crizotinib is FDA-approved, and encompasses about 7,000 new patients per year in the United States alone. It is important to note that Ma et al. also identified exon 14 splice site mutations in small cell lung cancer tumors, highlighting another thoracic malignancy in which patients may derive benefit from MET inhibitors.
Cabozantinib and crizotinib have low nanomolar specificity for a number of tyrosine kinases. The in vitro kinase inhibition profile for cabozantinib includes VEGFR2 (IC50 = 0.035 nM), MET (IC50 = 1.3 nM), RET (IC50 = 5.2nM), TIE2 (IC50 = 14.3nM), AXL (IC50 = 7nM), FLT3 (IC50 = 11.3nM), ROS1 (IC50 = 10.9nM) and KIT (IC50 = 4.6nM).(20, 21) The kinase inhibition profile for crizotinib includes MET (IC50 = 11nM), ALK (IC50 = 24nM), and ROS1 (IC50 = 2.1nM).(21, 22) Mutational profiling of the tumors from Patients 4 and 7 did not reveal any other clear-cut somatic alterations that might contribute to the response to crizotinib. On the other hand, the tumors from Patients 2 and 5 demonstrated both MET exon 14 splice site mutations and MET amplification. Given data showing in vitro responses to the MET inhibitor SU11274 in a MET amplified NSCLC cell line(11) and responses to crizotinib in lung adenocarcinoma patients whose tumors bear high degrees of MET amplification(9), the co-occurrence of both events may reflect the degree to which Patient 2’s and 5’s tumors are dependent on MET signaling, and both may have contributed to drug sensitivity. An alternative explanation is that some or all of the responses to crizotinib seen in those patients with high MET amplified tumors reported by Camidge et al. were driven by undiagnosed MET exon 14 alterations. Indeed, lack of stratification for MET exon 14 alterations may also explain the discrepant survival outcomes reported in the phase 2 and phase 3 trials of erlotinib +/− onartuzumab in NSCLC patients.(8, 23) Finally, it is possible that the tumors are dependent on other receptor tyrosine kinases in ways not captured by DNA sequencing, although this has not been the case for most other validated targets in NSCLC. As a whole, our data, in which 4/4 patients had radiographic responses to MET inhibitors and 2/4 patients had tumors that did not harbor concomitant MET amplification, suggest that the responses seen in these patients are in fact driven by MET exon 14 skip events.
As for the significance of the 3 alterations that differed between the primary lung tumor and liver metastasis from Patient 4, two- the NTRK2 and TERT mutations- fall in domains that do not currently have attributed functions. CDKN2A/B deletion leading to loss of the G1/S checkpoint in the liver metastasis could very well have caused primary resistance to crizotinib. Functional studies will be needed to address this, as well as other potential causes of resistance.
In conclusion, our data support prospective identification of MET exon 14 splice site mutations in patients with lung adenocarcinomas. Patients with these splice site mutations should ideally be treated in the context of a clinical trial of a MET inhibitor. For those patients who do not have access to a clinical trial and for whom standard therapy does not exist, use of off-label crizotinib should be considered.
Methods
Patients at MSK with stage IV lung adenocarcinomas harboring MET exon 14 splice site mutations (N=7) or a mutation deleting Y1003 (N=1) were identified through a clinical assay based on hybrid capture and next-generation sequencing of 341 oncogenes and tumor suppressors termed MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets).(24) Prospective testing began in February 2014 with data cut-off for this study in December 2014. One hundred seventy eight lung adenocarcinoma patients were screened in total (MET exon 14 mutation frequency = 4%). Somatic mutations were called using matched germline DNA. This study was performed in accordance with the Declaration of Helsinki and was approved by the MSK IRB/Privacy Board through a Waiver of Authorization for the study of existing data.
MET immunohistochemistry (IHC) was performed on archival FFPE tissue using a rabbit monoclonal antibody (Ventana clone SP44) in a CLIA laboratory. Membranous reactivity for MET was assessed using an H-score according to the following formula: H-Score = % cells staining (0–100%) × intensity (range from 1 to 3), where an H-score of 0 corresponded to no staining and a score of 300 to maximum staining intensity in the entire tumor (Supplementary Figure 3).
Confirmation of the MET exon 14 skip was performed using the nCounter Analysis System (nanoString Technologies, Seattle, WA), a fluorescence-based platform for multiplexed digital mRNA profiling without amplification or generation of cDNA.(25) A custom code set measuring the expression of 13 cancer-related genes, including MET, 16 different combinations of fusion genes, and 8 house-keeping genes was used in our experiment. Detailed sequence information for the MET gene target regions is provided in Supplementary Table 1. There was sufficient archived tumor tissue from 5 of 8 patients for mRNA confirmation of exon 14 skipping. NCI-H596 cells (ATCC© HTB-178™) were tested as a positive control and 24 MET wild-type patient cases were tested as negative controls. H596 cells were obtained December 2014, passaged for fewer than 6 months, and were not reauthenticated.
Radiographic response to MET inhibition was performed by a single radiologist (MSG) using RECIST 1.1 and PERCIST criteria.
Supplementary Material
Statement of Significance.
Oncogenic mutations in the MET exon 14 splice sites that cause exon 14 skipping occur in 4% of lung adenocarcinomas. We report responses to the MET inhibitors crizotinib and cabozantinib in patients with lung adenocarcinomas harboring MET exon 14 splice site mutations, identifying a new potential therapeutic target in this disease.
Acknowledgments
AD (consulting for Exelixis).
Footnotes
Conflicts of interest: There are no other conflicts of interest.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadiq AA, Salgia R. MET As a Possible Target for Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2013;31:1089–1096. doi: 10.1200/JCO.2012.43.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treatment Reviews. 2013;39:793–801. doi: 10.1016/j.ctrv.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaswamy S, Kanteti R, Duke-Cohan JS, Loganathan S, Liu W, Ma PC, et al. Ethnic Differences and Functional Analysis of MET Mutations in Lung Cancer. Clinical Cancer Research. 2009;15:5714–5723. doi: 10.1158/1078-0432.CCR-09-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spigel D, Edelman M, O'Byrne K, Paz-Ares L, Shames D, Yu W, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol. 2014;32 doi: 10.1200/JCO.2016.69.2160. abstr 8000. [DOI] [PubMed] [Google Scholar]
- 9.Camidge D, Ou S-H, Shapiro G, Otterson G, Villacruz L, Villalona-Calero M, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32 abstr 8001. [Google Scholar]
- 10.Ma PC, Kijima T, Maulik G, Fox EA, Sattler M, Griffin JD, et al. c-MET Mutational Analysis in Small Cell Lung Cancer: Novel Juxtamembrane Domain Mutations Regulating Cytoskeletal Functions. Cancer research. 2003;63:6272–6281. [PubMed] [Google Scholar]
- 11.Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional Expression and Mutations of c-Met and Its Therapeutic Inhibition with SU11274 and Small Interfering RNA in Non–Small Cell Lung Cancer. Cancer research. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 12.Kong-Beltran M, Seshagiri S, Zha J, Zhu W, Bhawe K, Mendoza N, et al. Somatic Mutations Lead to an Oncogenic Deletion of Met in Lung Cancer. Cancer Research. 2006;66:283–289. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 13.Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by Gene Amplification or by Splice Mutations Deleting the Juxtamembrane Domain in Primary Resected Lung Cancers. Journal of Thoracic Oncology. 2009;4:5–11. doi: 10.1097/JTO.0b013e3181913e0e. 0.1097/JTO.0b013e3181913e0e. [DOI] [PubMed] [Google Scholar]
- 14.Seo J-S, Ju YS, Lee W-C, Shin J-Y, Lee JK, Bleazard T, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Research. 2012;22:2109–2119. doi: 10.1101/gr.145144.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, Clinicopathologic Associations, and Molecular Spectrum of ERBB2 (HER2) Tyrosine Kinase Mutations in Lung Adenocarcinomas. Clinical Cancer Research. 2012;18:4910–4918. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, et al. EGFR Exon 20 Insertion Mutations in Lung Adenocarcinomas: Prevalence, Molecular Heterogeneity, and Clinicopathologic Characteristics. Molecular Cancer Therapeutics. 2013;12:220–229. doi: 10.1158/1535-7163.MCT-12-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci Signal. 2013;6 doi: 10.1126/scisignal.2004088. pl1-pl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilagan JO, Ramakrishnan A, Hayes B, Murphy ME, Zebari AS, Bradley P, et al. U2AF1 mutations alter splice site recognition in hematological malignancies. Genome Research. 2014;25:14–26. doi: 10.1101/gr.181016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a Novel MET and VEGFR2 Inhibitor, Simultaneously Suppresses Metastasis, Angiogenesis, and Tumor Growth. Molecular Cancer Therapeutics. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 21.Katayama R, Kobayashi Y, Friboulet L, Lockerman EL, Koike S, Shaw AT, et al. Cabozantinib Overcomes Crizotinib Resistance in ROS1 Fusion–Positive Cancer. Clinical Cancer Research. 2015;21:166–174. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An Orally Available Small-Molecule Inhibitor of c-Met, PF-2341066, Exhibits Cytoreductive Antitumor Efficacy through Antiproliferative and Antiangiogenic Mechanisms. Cancer Research. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 23.Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH, Blumenschein GR, et al. Randomized Phase II Trial of Onartuzumab in Combination With Erlotinib in Patients With Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2013;31:4105–4114. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng D, Mitchell T, Zehir A, Shah R, Benayed R, Syed A, et al. MSK-IMPACT: A hybridization capture-based next generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2014 doi: 10.1016/j.jmoldx.2014.12.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotech. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.