Abstract
The National Institute for Occupational Safety and Health (NIOSH) has conducted an occupational exposure assessment study of manganese (Mn) in welding fume of construction workers rebuilding tanks, piping, and process equipment at two oil refineries. The objective of this study was to evaluate exposures to different Mn fractions using a sequential extraction procedure. Seventy-two worker-days were monitored for either total or respirable Mn during stick welding and associated activities both within and outside of confined spaces. The samples were analyzed using an experimental method to separate different Mn fractions by valence states based on selective chemical solubility. The full-shift total particulate Mn time-weighted average (TWA) breathing zone concentrations ranged from 0.013 – 29 for soluble Mn in a mild ammonium acetate solution; from 0.26 – 250 for Mn0,2+ in acetic acid; from non-detectable (ND) – 350 for Mn3+,4+ in hydroxylamine-hydrochloride; and from ND – 39 micrograms per cubic meter (μg/m3) for insoluble Mn fractions in hydrochloric and nitric acid. The summation of all Mn fractions in total particulate TWA ranged from 0.52 to 470 μg/m3. The range of respirable particulate Mn TWA concentrations were from 0.20 – 28 for soluble Mn; from 1.4 – 270 for Mn0,2+; from 0.49 – 150 for Mn3+,4+; from ND – 100 for insoluble Mn; and from 2.0 – 490 μg/m3 for Mn (sum of fractions). For all jobs combined, total particulate TWA GM concentrations of the Mn(sum) were 99 (GSD=3.35) and 8.7 (GSD=3.54) μg/m3 for workers inside and outside of confined spaces; respirable Mn also showed much higher levels for welders within confined spaces. Regardless of particle size and confined space work status, Mn0,2+ fraction was the most abundant followed by Mn3+,4+ fraction, typically >50% and ~30-40% of Mn(sum), respectively. Eighteen welders’ exposures exceeded the ACGIH Threshold Limit Values for total Mn (100 μg/m3) and 25 exceeded the recently adopted respirable Mn TLV (20 μg/m3). This study shows that a welding fume exposure control and management program is warranted, especially for welding jobs in confined spaces.
Keywords: welding, manganese fractionation, stick welding, shielded metal arc welding (SMAW), construction, petroleum refineries
INTRODUCTION
NIOSH has identified important research needs for workers exposed to welding fume. The principal objective of the Industrywide Studies Branch welding study is to evaluate workers’ exposures to Mn in welding fume in multiple industries where appreciable levels of Mn occur, often associated with mild carbon steel and stainless steel welding. The specific aim is to identify the forms of Mn in welding emissions collected in field settings by applying extraction methods to measure different valence states based on solubility of Mn compounds, utilizing industrial hygiene air sampling and analytical methods.
The focus of this manuscript is to report on site evaluations of welding tasks associated with construction projects needed to refurbish tanks, process vessels and piping systems at two oil refineries. Three different construction projects were monitored in 2010: an asphalt tank refurbishing job; a boiler house and sewer piping construction job; and a refinery “turn-around” project where the facility was shut down, processing equipment and vessels were disassembled, inspected, and rebuilt, if necessary. The vast majority of the hot work for these construction jobs used shielded metal arc welding (SMAW) on carbon steel. A few welders were also exposed to lesser amounts of grinding dust as well as to fumes from gas metal arc welding (GMAW) from gouging and torch cutting on carbon steel, and from stick welding on stainless steel.
Currently, occupational evaluation criteria for Mn are based on chemical measurement of elemental, inorganic Mn without further characterization of the Mn compound fractions. However, Mn in welding fume binds with many other elements(1), and Mn can exist in six valence states.(2) Although Mn neurotoxicity has been reported for many years, the mechanism is not fully understood.(3) Archibald and Tyree(4) proposed that Mn can produce varying toxicities contingent upon oxidation state. Oberdoster and Cherian(5) reported that they believed that Mn3+ and Mn4+ were the most toxic. However, the World Health Organization(6) report that “little is known about the relative toxicity of different Mn compounds.”
An in vitro study conducted by Chen et al.(7) examined the effect of Mn oxidation state on some mitochondrial (Fe-S) containing enzymes. Their results suggest that Mn3+ species appear more cytotoxic than Mn 2+ compounds, possibly due to higher oxidative reactivity. The rate of (saturable) Mn2+ transport through the blood-brain barrier is also believed to be an important determinant of Mn neurotoxicity.(8) Transferrin-mediated transfer is another proposed pathway into cellular tissues; Mn is in the trivalent oxidation state when conjugated with transferrin.(9) The olfactory nerve route, which directly transports Mn from the nasal cavities to the olfactory bulb, is also a factor for Mn deposition.(10,11)
Frequently, symptomatic workers have presented with Mn accumulation in the brain in area(s) normally associated with divalent Mn concentrations which may impact transport regulation.(3,12) The chemical and biological solubility of Mn compounds is dependent on valence state but this is not well (and easily) characterized with conventional analytical methods.(13) Roels et al.(14) noted that despite similar mean exposure concentration (0.94 vs. 0.95 mg/m3) to total elemental Mn dust, the mean levels of Mn in blood and urine of battery workers exposed only to MnO2 were substantially lower than for workers exposed to mixed salts and oxides; they proposed that this may be due, in part, to different bioavailability of the absorbed Mn oxides and salts. More research is needed to determine critical information pertaining to welding exposures and the risk of developing neurological effects. It is unclear how Mn is absorbed in various chemical forms and valence states, how much is bioavailable, and how it is distributed in humans.(15) The paucity of occupational exposure studies regarding Mn valence states served as the impetus of the present study to evaluate welders’ exposures because the valence state of Mn in welding aerosols may affect the transport of Mn across cellular membranes, influence brain deposition, and may have implications for Mn cytotoxicity and neurotoxicity.(7,16-17)
New methods for measuring Mn fractions were explored in this research study by applying the sequential extraction procedures reported by Thomassen et al.(18) and Ellingsen et al.(19) for Mn fractionation; in those studies workers were monitored for dust and fume exposures from raw materials, intermediate materials and finished products in the Mn alloy industry in Norway. The Mn compounds one could expect with this method in each extraction step include: i) water soluble Mn (in neutral 0.01M ammonium acetate – MnF2, MnCl2); ii) Mn0,2+ (in 25% acetic acid – Mn metal, MnO, Mn2+ part of Mn3 O4); iii) Mn3+,4+ (in 0.5% hydroxylamine-hydrochloride in 25% acetic acid – Mn3+ part of Mn3O4, Mn2O3, MnO2); and iv) insoluble Mn (in HCl-HNO3-HF acids – SiMn).(18-19) At the Norway Mn alloy plants, none of the different areas monitored were characterized by a single Mn fraction, and Mn0,2+ was the most abundant oxidation state for both inhalable and respirable aerosols regardless of the production and maintenance departments. Thomassen et al. concluded that it is feasible to fractionate Mn compounds in Mn smelters. The sequential leaching, inductively coupled argon plasma–atomic emission spectroscopy (ICP-AES ) procedure was critiqued with Mn in welding fume by Berlinger et al.(20) using fixed area sampling with Higgens-Dewell cyclones. X-ray diffraction results did not fully confirm the ICP-AES data but, even with this limitation, some data for different Mn compounds in complex welding fume matrices may be useful for assessing bioaccessibility.
BACKGROUND
Welding
Welding is an indispensable manufacturing activity in the US and throughout the world which exposes workers in a multitude of industries. In excess of 300,000 US workers are defined as welders, brazers and solderers(21), most of which are employed in the manufacturing, construction, energy, and transportation sectors. This number does not include workers in other job titles who may also perform welding as part of their job tasks.
Approximately 100 different welding, thermal cutting, and other allied processes exist.(22) The majority of welding operations are performed on low-alloy or carbon steels but stainless steel may account for up to 5% of welding. Shielded metal arc welding, commonly called ‘stick’ welding, on mild carbon steel is one of the most prevalent processes. Shielded metal arc welding uses an electrical power supply to produce an arc by grounding the work piece with a wire and clamp to the electric supply unit and then striking it with a welding rod, which is attached to an electrode cable of the welding power source to form a closed circuit. The welding rods have a coating over the metal rod that provides a flux shielding over the molten metal pool to minimize oxidation of the weld by the atmosphere and produce a strong joint between the metal parts.
Welding fume is generated from the melting of the base metal, electrode rods or filler wire, with the majority of the fume being emitted from the consumable rod or wire.(23) As a result of the high process temperatures, welding tasks expose workers to gases such as carbon monoxide, ozone, and nitrogen oxides as well as aerosol emissions composed of metals, metal oxides, silicates and fluorides.(24-26) Occupational studies have reported a number of work-related adverse health effects in welders, such as lung disease and possibly neurological toxicity. Epidemiologic studies and case reports of welders have shown an excessive incidence of acute and chronic respiratory diseases.(27) In a criteria document, NIOSH concluded that exposure to welding fumes should be considered as a potential occupational lung carcinogen.(25)
The effect of welding fumes on one's health varies depending on the duration and intensity of the exposure and the specific emissions involved. The air contaminant content of welding fumes depends on the composition of the welding electrode or filler wire and base metal; the welding process and operating parameters; the shielding gas; coatings or contaminants on the surface of base metal; use of anti-spatter treatment; and effectiveness of ventilation. Mild carbon steels are distinguished by relatively low carbon content; these types of steel consist mainly of iron, carbon, and manganese, but may also contain other elements. Stainless steels contain higher levels of toxic metals in the metal alloy, such as nickel and chromium, which are not typically present in mild carbon steels.
The particle size of welding fume aerosol is variable but the primary particle nodule is typically in nanometer sizes (i.e., < 0.1 micrometer), which will form larger aggregates and agglomerates, most of which are produced in sub-micron particle sizes.(28-31) The size of the welding aerosol will determine the location of particle deposition within the naso-pharnyx and respiratory system; even the larger welding particle clusters are typically of respirable sizes, much of which deposits in the gas exchange regions of the lungs.
Manganese
There are many important industrial uses for manganese (Mn); it is used in the steel and metal alloy industries; in ceramic and glass products; in rubber and wood preservatives; and in dry-cell alkaline batteries.(32) Manganese is a common ingredient of many steels, welding rods, and filler wires to impart strength, hardness, and ductility to the metal.(22)
The principal health effects of excessive occupational Mn exposure are primarily neurological and respiratory effects including irritation, metal fume fever, pneumonitis, and chronic bronchitis.(32-33) Most notably, occupational exposure to excessive Mn concentrations can cause a Parkinsonism (i.e., manganism), a neurological syndrome with well-recognized characteristics, most of which are movement disorders. These may include neurological signs and symptoms such as poor hand-eye coordination, slow movement and disturbed gait, increased tremor, reduced response speed, mood disturbance, and possibly memory and intellectual loss.(14,34-38) Manganism is a progressive occupational disease which may develop gradually over time, but it is often unrecognized until the worker is irreversibly affected.(39)
Manganism has been reported in workers who had high Mn exposures in ore mining and refining, ferroalloy production, and the dry cell battery industries.(32) Yet the relationship between chronic low level Mn exposure, including those associated with welding, and manganism is unclear because the initial signs and symptoms may be subtle, sub-clinical neurobehavioral effects (i.e., mild abnormalities that may or may not be recognized as a medical problem). Some recent studies of welders have shown neurological and neurobehavioral effects from low level Mn exposures (< 200 μg/m3) including short term memory loss, mood swings, altered reaction times and eye-hand coordination deficits.(40-41) However, the use of neurobehavioral tests for epidemiological and risk assessment studies is not universally accepted.(42-44) Santamaria et al.(15) concluded that, for welders, interpretations of abnormal neurobehavioral tests are subjective and abnormalities found in asymptomatic workers do not necessarily progress to clinical disease.
Occupational Exposure Criteria
General occupational exposure limits (OELs) for total welding metal fume mixtures have not been established by NIOSH, OSHA nor by the American Conference of Governmental Industrial Hygienists (ACGIH). However, OELs have been set for individual welding fume components (e.g., iron, chromium, nickel, manganese, etc.), as full-shift time-weighted average (TWA) concentrations. The OELs available for Mn are inconsistent and the bases for establishing these criteria are based on different potential acute or chronic health effects. Currently, the NIOSH REL for Mn is an 8-hr TWA of 1000 μg/m3, with a Short Term Exposure Limit (STEL) of 3000 μg/m3 over 15 minutes; these are based on central nervous system effects and pneumonitis, respectively.(45) The OSHA PEL for Mn is a ceiling limit of 5000 μg/m3 for protection against eye and respiratory irritation.(46) The ACGIH TLV for inorganic Mn is 100 μg/m3, measured as an 8-hr TWA for both Mn-dust and Mn-fume compounds.(47) The TLV for Mn was established to reduce adverse pulmonary symptoms (e.g., coughing, shortness of breath, acute bronchitis); central nervous system effects (e.g., preclinical psychomotor abnormalities); and decreased male fertility.(32,48-49) In 2012, ACGIH adopted a notice of intended change, which modified the TLV for inorganic Mn from total to inhalable particle size and includes a respirable Mn TLV of 20 μg/m3 measured by an 8-hr TWA. For welding fume exposures, the sampling of total Mn using closed-face filter cassettes approximates the inhalable levels because of the very small particle size distribution of the fume emissions. All of these OELs for Mn are not specific to Mn in welding fume but are applicable to all sources of Mn exposure.
METHODS
Seventy-two worker-days were monitored from several welding areas throughout two petroleum refineries, both within and outside of confined spaces, up on scaffolds or equipment platforms with or without partial enclosures, in trenches, and in open air environments at ground level. Full-shift breathing zone (BZ) exposure concentrations were measured on each welder either for total or respirable airborne welding fume over 8 – 12 hour work shifts. Personal air sampling pumps were used at nominal flow rates of 2.5 liters per minute (lpm) for total particulate or 2.0 lpm for respirable particulate aerosols.
Total particulate samples were collected using 25-millimeter (mm) diameter, 0.8 μm pore size, mixed cellulose ester (MCE) sample filters in closed-face cassettes. The filter cassettes were attached on workers’ lapels close to their neck such that the filter position was inside of their welding helmets when closed in accordance with the ISO welding standard.(50) Respirable particulate Mn samples were collected on 37-mm, 0.8 μm pore size, MCE filters which were placed in a SKC Parallel Particle Impactor®, a respirable particle size selecting device reported to more closely approximate the alveolar particle deposition curve than cyclone samplers. The respirable samples were collected outside of the welding helmets since the dimensions of the impactor precluded placement within the helmet.
The cassette and impactor filters were transferred to 50-mL polypropylene centrifuge tubes with 25-mL filter cup inserts equipped with 0.2-μm polyvinylidene fluoride membranes and prepared using a triplicate sequential extraction procedure: i) 10-mL of 0.01 molar (M) ammonium acetate at room temperature for 90 minutes (extracts soluble Mn); ii) 10-mL of 25% acetic acid, heated at 75°C for 90 minutes (extracts Mn0 and Mn2+ valence state compounds); and iii) 10-mL of 0.5% hydroxylamine, hydrochloride in 25% acetic acid, heated at 75°C for 90 minutes (extracts Mn3+ and Mn4+ valence state compounds).
The NIOSH extraction method modified the Thomassen et al.(18) procedures by eliminating the fourth extraction step due to its use of hydrofluoric acid, a very hazardous chemical. For the turn-around reconstruction project, a fourth extraction step was added to measure “insoluble” Mn remaining after the first three extractions using the same digestion procedure as published in NMAM 7303.(51) This was important so that the summation of the Mn fractions would be more directly comparable to published OELs and legacy data, and for inclusion in the NIOSH Manual of Analytical Methods. The fourth extraction procedure measures the remaining insoluble Mn using three additional steps: i) 2.5-mL of 12.1 M hydrochloric acid, heated at 95°C for 15 minutes and cooled; ii) 2.5-mL of 15.6 M nitric acid added to the extract, heated at 95°C for 15 minutes and cooled; and iii) dilution to 25-mL with deionized water.
Between each extraction step, the samples were spun in a centrifuge; the extract was collected; and the filter cup was transferred to a clean 50-mL polypropylene centrifuge tube. The sample extracts were analyzed by ICP-AES using instrument parameters described in NMAM 7303. The LODs were 0.003 μg/filter, 0.2 μg/filter, and 0.3 μg/filter, and 0.2 μg/filter for soluble Mn; Mn0 and Mn2+; Mn3+ and Mn4+; and insoluble Mn fractions, respectively.
Various exposure groups stratified by particle size, construction projects as well as by working in confined space (at least for part of the day) versus an outdoor environment were compared using simple descriptive statistics. Assumptions of normality were better met by taking logarithms of the TWA exposure data for both groups of welders working inside and outside of confined spaces. All statistical analyses were performed using SAS Software (version 9.2, SAS Institute Inc., Cary, NC). Data were summarized by reporting the minimum, maximum, geometric mean (GM) and geometric standard deviation (GSD). In the case of values below the LOD, the maximum likelihood(52) GM and GSD were calculated by analyzing the log of the values in the PROC LIFEREG procedure of SAS (9.3). In addition, the percent of the each Mn fraction to the sum was calculated for each TWA measurement. These percentages were also summarized by reporting minimum, maximum, geometric mean (GM) and geometric standard deviation (GSD), using the maximum likelihood estimate in the case of values below the LOD.
RESULTS
Boiler House and Piping
The welders’ full-shift TWA concentrations for total Mn during boiler house and associated sewer piping construction jobs are presented in Table I and Figure 1. Overall, the Mn data ranged from 0.17 – 3.3 for soluble Mn; 0.26 – 38 for Mn0,2+; and 0.86 – 18 μg/m3 for Mn3+,4+ fractions. The summation of all the total particulate Mn fractions yielded a GM (GSD) for the boiler house workers of 12 μg/m3 (2.83).
TABLE I.
Welders’ TWA Breathing Zone Concentrations of Total Particulate Manganese Fractions Measured During Boiler House, Asphalt Tank, and Turn-Around Construction Jobs.
| TWA Concentration (μg/m3) |
||||||
|---|---|---|---|---|---|---|
| Projects | Parameter | Mn (soluble) | Mn (0,+2) | Mn (+3,+4) | Mn (insoluble) | Mn (sum) |
| Boiler House No Confined Space Jobs | n | 18 | 18 | 18 | n.a.A | 18 |
| Minimum | 0.17 | 0.26 | 0.86 | n.a. | 1.3 | |
| GM (GSD) | 0.79 (2.29) | 5.7 (3.49) | 4.2 (2.74) | n.a. | 12 (2.83) | |
| Maximum | 3.3 | 38 | 18 | n.a. | 57 | |
| Asphalt Tank No Confined Space Jobs | n | 7 | 7 | 7 | n.a. | 7 |
| Minimum | 0.21 | 0.58 | 0.06 | n.a. | 0.85 | |
| GM (GSD) | 1.2 (2.67) | 3.5 (3.67) | 1.7 (5.33) | n.a. | 6.9 (3.53) | |
| Maximum | 3.9 | 25 | 6.8 | n.a. | 34 | |
| Turn-Around No Confined Space Jobs | n | 15 | 15 | 15 | 15 | 15 |
| Minimum | 0.013 | 0.50 | NDB | NDC | 0.52 | |
| GM (GSD) | 0.36 (3.97) | 3.6 (3.82) | 1.6 (5.01) | 0.14 (9.80) | 5.7 (4.31) | |
| Maximum | 2.4 | 30 | 23 | 3.9 | 59 | |
| Asphalt Tank Confined Space Jobs | n | 11 | 9D | 11 | n.a. | 11 |
| Minimum | 1.5 | 0.93 | 2.7 | n.a. | 7.5 | |
| GM (GSD) | 12 (2.41) | 41 (6.31) | 58 (4.49) | n.a. | 110 (3.82) | |
| Maximum | 29 | 250 | 350 | n.a. | 470 | |
| Turn-Around Confined Space Jobs | n | 6 | 6 | 6 | 6 | 6 |
| Minimum | 1.5 | 13 | 3.3 | NDE | 18 | |
| GM (GSD) | 5.9 (2.56) | 50 (2.58) | 18 (3.37) | 4.0 (7.09) | 83 (2.74) | |
| Maximum | 26 | 210 | 52 | 39 | 320 | |
n.a. = not applicable. Insoluble Mn was not analyzed for the first surveys (i.e., Boiler House and Asphalt Tank projects). The Turn-Around survey was conducted 6 months later; during the interim, the method was expanded to include insoluble Mn analysis via NMAM 7303 sample digestion procedures.
ND = non-detectable. Four samples were below the Minimum Detectable Concentration (MDC); censored data for all ND data were used to calculate GM using maximum likelihood statistical method.
Nine samples were below the Minimum Detectable Concentration.
Two samples lost during sample preparation.
One sample was below the Minimum Detectable Concentration.
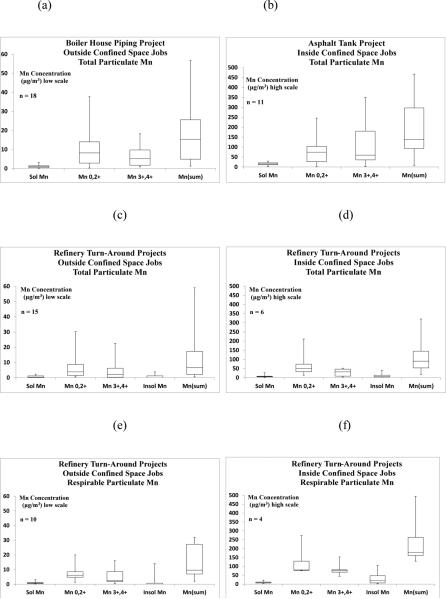
FIGURE 1 (a-f).
Welders’ TWA Breathing Zone Concentrations of Total and Respirable Particulate Manganese Fractions Outside and Inside of Confined Space During Boiler House, Asphalt Tank, and Turn-Around Projects.
All of the full-shift Mn TWA exposure levels for these welders working on this project were well below the current OSHA PEL for Mn (a ceiling limit of 5,000 μg/m3); NIOSH REL (8-10 hour TWA of 1,000 μg/m3); and ACGIH TLV (8-hour TWA of 100 μg/m3). However, many of the boiler house welders were re-assigned away from hot work due to competing construction priorities, resulting in a relatively light production schedule. For those who did weld, often only two to four hours of welding occurred for the entire work shift. Hence, the TWAs for welders would have been higher if their welding activities continued throughout the full work shift.
Asphalt Tank
The worker's full-shift BZ TWA concentrations for total particle size Mn during asphalt tank project are presented in Table I separately for work within and outside of this large confined space and combined as shown in Figure 1. For those workers outside of the tank (e.g., fire watcher, scaffolding set-up, roof cutting, stairway construction), the total particulate Mn(sum) TWAs for outside workers ranged from 0.85 to 34 μg/m3, where the highest exposure occurred when welding stairs on the tank exterior.
As one can expect, higher exposures to Mn usually occurred when working inside rather than outside the tank, as shown in Table I. The total particulate data observed from welders inside of the tank for soluble Mn; Mn0,2+; and Mn3+,4+; and Mn(sum) of the fractions ranged from 1.5 – 29; 0.93 – 250; 2.7 – 350; and 7.5 – 470 μg/m3, respectively. Moreover, the GM (GSD) was 110 μg/m3 (3.82) for welders working within the tank compared to 6.9 μg/m3 (3.53) for those working outside. Some of the exposure levels observed for confined space welding substantially exceeded both the total and respirable Mn ACGIH TLVs.
Respirable particle size full-shift Mn TWA concentrations are summarized for five welders, as provided in Table II. Due to rainy weather, all five welders were inside of the tank on the same day during the monitoring of respirable Mn fractions. The welder assistant was also inside most of the time grinding seams, dry sweeping dust, and performing other tasks. The range of the measured respirable Mn TWA concentrations were from 7.0 – 28; 86 – 200; 18 – 150; and 60 – 380 μg/m3 for soluble Mn, Mn0,2+, Mn3+,4+, and Mn (sum), respectively. The number of welders within confined and enclosed spaces is a prominent exposure determinant because welders are exposed to the fume generated by their own work as well as by the increased background concentration from the other welders. This was visually apparent as the welding emission cloud within the tank was noticeably heavier on this day when five workers, as opposed to two or three, were working in the tank. The highest TWA for total particulate Mn(sum) exposure (470 μg/m3 in Table I) was also, in fact, observed from a sample on this day when it rained.
TABLE II.
Welders’ TWA Breathing Zone Concentrations of Respirable Particulate Manganese Fractions Measured During the Turn-Around and Asphalt Tank Projects.
| TWA Concentration (μg/m3) |
||||||
|---|---|---|---|---|---|---|
| Projects | Parameter | Mn (soluble) | Mn (0,+2) | Mn (+3,+4) | Mn (insoluble) | Mn (sum) |
| Turn-Around No Confined Space Jobs | n | 10 | 10 | 10 | 10 | 10 |
| Minimum | 0.20 | 1.4 | 0.49 | NDA | 2.0 | |
| GM (GSD) | 0.74 (2.27) | 5.9 (2.11) | 3.2 (3.17 ) | 0.11 (14.9 ) | 11 (2.59 ) | |
| Maximum | 3.1 | 20 | 16 | 14 | 32 | |
| Asphalt Tank Confined Space Jobs | n | 5 | 5 | 5 | n.a.B | 5 |
| Minimum | 7.0 | 86 | 18 | n.a. | 60 | |
| GM (GSD) | 16 (1.67) | 110 (1.46) | 94 (1.52) | n.a. | 180 (1.98) | |
| Maximum | 28 | 200 | 150 | n.a. | 380 | |
| Turn-Around Confined Space Jobs | n | 4 | 4 | 4 | 4 | 4 |
| Minimum | 4.1 | 76 | 44 | 1.5 | 130 | |
| GM (GSD) | 8.4 (2.03) | 110 (1.87) | 70 (1.39) | 14 (6.08) | 210 (1.80) | |
| Maximum | 22 | 270 | 95 | 100 | 490 | |
ND = non-detectable. Six samples were below the Minimum Detectable Concentration (MDC); censored data was used for all ND data to calculate GM using maximum likelihood statistical method.
n.a. = not applicable. Insoluble Mn was not analyzed for the Asphalt Tank project). The Turn-Around survey was conducted 6 months later; during the interim, the method was expanded to include insoluble Mn analysis via NMAM 7303 sample digestion procedures.
Turn-Around
The third survey reported in this paper evaluated welders’ exposures during a large ‘turn-around’ project when the refinery was shut down and equipment was disassembled, inspected, and rebuilt, if necessary. The data were combined for five construction contractors’ welders who participated in this monitoring survey. Two of the contractors specialized in process vessel and tank reconstruction, which entailed most of the confined space work.
Overall, the total particulate Mn(sum) TWA concentrations (μg/m3) measured during the turn-around jobs ranged from 0.52 to 320 μg/m3 (see Table I). The wide variability of these data (i.e., over three orders of magnitude) demonstrates the diverse nature of these construction activities due to different work locations, tasks and welding arc times. As seen with the previous construction projects, higher exposures to all Mn fractions occurred when working inside rather than outside of confined spaces, as presented in Table I. Geometric mean exposure concentrations (μg/m3) and (GSD) of total Mn(sum) were 5.7 (4.31) and 83 (2.74), respectively, for outside versus inside confined space welders. The dramatic difference in exposure levels of welders outside of confined space is graphically shown in Figure 1 using an exposure scale one-third of that provided for workers with confined space entry. All of the full-shift Mn TWA exposure levels of workers without confined space entry were below the OELs for Mn established by OSHA and NIOSH. However, three of the highest exposure levels that were measured within confined spaces (120; 150; 320 μg/m3) did exceed the ACGIH TLV for total particulate Mn (100 μg/m3).
Respirable particulate Mn TWA concentrations for fourteen turn-around workers, stratified by confined space work status, are provided in Table II. Respirable Mn(sum) measured during the no confined space turn-around jobs ranged from ranged from 2.0 – 32 μg/m3 with a GM of 11 μg/m3 (GSD = 2.59). Three out of ten respirable Mn measurements collected from jobs outside confined spaces exceeded the ACGIH TLV for respirable Mn of 20 μg/m3 and another sample (19 μg/m3) nearly exceeded it.
For those welders working within confined spaces during the turn-around project (n = 4), the respirable Mn exposure concentrations (μg/m3) provided in Table II were from 4.1 – 22 for soluble Mn; from 76 – 270 for Mn0,2+; from 44 – 95 for Mn3+,4+; from 1.5 – 100 for insoluble Mn; and from 130 – 490 for Mn(sum). All four Mn(sum) concentrations substantially exceeded the ACGIH respirable Mn TLV. In fact, the GM for confined space welders (210 μg/m3; GSD = 1.80) was ten times greater than the respirable Mn TLV. Moreover, as depicted in Table II and Figure 2, each exposure concentration for Mn0,2+ and Mn3+,4+ fractions were also over the TLV by at least double. All of the confined space respirable Mn(sum) levels also exceeded the TLV (100 μg/m3) for total (inhalable) Mn particulate.
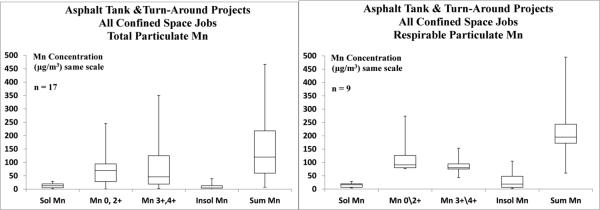
FIGURE 2.
Comparison of Total versus Respirable Particulate TWA Breathing Zone Concentrations of Manganese Fractions for All Confined Space Jobs.
DISCUSSION
Workers’ inhalation exposures presented in this study were affected by variable weather conditions (e.g., rain, drizzle, mist, wind), and welding locations and conditions (e.g., inside confined spaces such as tanks and process vessels; 10-50 ft [3.0-15 m] above ground on a platform, scaffold or lift; ground level in open air; or in trenches 4-6 ft [1.2-1.8 m] deep). Some of the jobs monitored in this survey varied each day, requiring different amounts of set-up and welding arc times. Often welders were re-assigned away from welding tasks due to competing construction priorities, such as erecting scaffolding, disassembly/assembly of process equipment, or awaiting work orders pending inspection decisions. Hence, some of the TWAs for welders would have been higher if their welding activities continued throughout the full work shift. Wide variability between exposure concentrations is typical when measuring welders’ exposures in outdoor and confined space construction environments, as noted with these data, which range over three orders of magnitude.
Analyzing the Mn fractions with the sequential digestion procedure is currently an experimental method. As such, the OELs from NIOSH, OSHA, and ACGIH are not directly relevant to the Mn fractions because the OELs are published for all of the inorganic Mn, measured as elemental Mn. Occupational exposure limits for evaluating the specific Mn fractions have not been established. Summing the component Mn fractions, however, may approximate the full (“total”) elemental Mn concentration, which may be applied to the respective OEL and used for comparison to legacy data. For the samples analyzed with the triplicate sequential extraction method used in the boiler house and asphalt tank projects, this approximation slightly underestimates the true concentration because some of the insoluble Mn compounds not analyzed with the triplicate sequential extraction method could be extracted by the HCl-HNO3 acid/hot plate dissolution of NMAM 7303, the fourth step in the expanded extraction procedures. However, the quantities of insoluble Mn concentrations were substantially lower [GM = 7.9% of Mn(sum-4)] than those observed with the combined Mn0,2+ and Mn3+,4+ [GM = 86% of Mn(sum-4)] analyses as was observed in the full turn-around data set.
The total particulate breathing zone air samples collected during this survey were initially placed inside of the welding helmets in accordance with the International Organization for Standardization.(50) Monitoring within the helmet air space generally would collect lower levels of air contaminants.(53-54) unless the welder was exposed to high concentrations within a confined space.(55) However, due to the difficult nature to maintain the filters inside of welding helmets, particularly when welders frequently remove and reposition their helmets and move sampling filters, it is uncertain if the filters remained within the air space of the helmet when used throughout the entire work shift. Constant surveillance by the industrial hygiene survey team was not possible when multiple welders were simultaneously monitored in disperse locations throughout the two refineries within secured construction zones, including limited access work locations such as elevated platforms, scaffolding and lifts, and inside confined spaces.
It has been shown that the majority of particles associated with welding fume emissions are of respirable sizes, most of which are sub-micrometer.(28-31) Thus, air samples collected in welders’ breathing zones with total particulate size filter cassettes may provide a reasonable estimate of respirable Mn exposure. Figure 4 compares all of the exposure measurements obtained with welders inside the process vessels and tanks, for both total (n = 17) and respirable (n = 9) particulate aerosol, which shows that exposure levels and patterns of the Mn fractions and Mn(sum) appear quite similar, even though respirable samples were collected outside of and total samples were collected within welding helmets. However, after statistical analysis of the total versus respirable Mn from all confined space jobs, the soluble Mn (p = 0.489) and Mn(sum) (p = 0.103) were essentially the same but the Mn0,2+ (p = 0.044) and Mn3+,4+ (p = 0.059) fractions were statistically different. Despite these differences, the finding that Mn(sum) total and respirable Mn medians were the same is consistent with the report by Harris et al.(44) which concluded that total and respirable welding fume concentrations were essentially the same using SMAW within an enclosed space and inorganic, elemental Mn ICP-AES analyses via NMAM 7300.(51) This is due to the inability of NMAM 7300 to distinguish different Mn fractions but the summation of the fractions using the sequential extraction procedure should provide comparable results with those of inorganic, elemental Mn analyses by ICP-AES.
In addition to welding fume, grinding dust particles may be collected in the breathing zone samples as this task is associated with welding to prepare joints and welding beads before welding, as well as to bevel and inspect the joint. Grinding dust produces a larger particle size distribution than welding fume but could still contain respirable particulate. Moreover, given that the welders in this study spent considerably more time welding than grinding, one could expect that their exposures to respirable Mn would be appreciable. Indeed, this was observed with these data sets, as many of the respirable measurements collected during the refinery asphalt tank and turn-around vessel reconstruction jobs were in excess of an order of magnitude above the new respirable Mn TLV, which was adopted a few years after sample collection for this study.
This manuscript presents data regarding Mn fraction distributions for SMAW primarily on mild carbon steel using 7018 (5.4% Mn) and 6010 (1.4%) welding rods with three different construction projects which included open air, partial enclosure and confined space welding. None-the-less, the pattern of median Mn exposures to total particle size Mn showed that the ordinal ranks were consistent for the boiler house, asphalt tank and turn-around projects: Mn0,2+ > Mn3+,4+ > soluble Mn (refer to Figures 1 and 2). For the turn-around project, the median respirable Mn exposures also demonstrated this same ordinal ranking for jobs both outside and inside confined spaces (Figure 3), and the soluble Mn and insoluble Mn levels were very low and nearly identical. The predominant Mn0,2+ oxidation fraction that was observed here is consistent with that found in the Mn alloy plants in Norway.(18-19)
Although the present study cannot distinguish specific Mn compounds from the breathing zone samples, nearly ninety percent of the extracted Mn was measured in the 0,2+ (53%) and 3+,4+ (36%) valence fractions (see Table III). This suggests that SMAW at these construction jobs contained appreciable quantities of Mn compounds with these valence states, possibly MnO, Mn3O4, Mn2O3, or MnO2 (per Thomassen et al., Ellingssen et al.). 18-19) Mixtures of Mn compounds are bound with iron (Fe) and other elements in the complex welding fume matrix. In a chamber study using 7018 welding rods and multiple analytical techniques, Jenkins and Eagar found that SMAW on mild steel produced both a mixed alkali-fluoride phase and Fe-Mn oxide spinel phase that were predominately in the 2+ and 3+ valences; metal cations were approximately 27% Fe, 10% Mn, 10% S, 28% K, and 25% Ca. If present in the fume particle shell, the mixed alkali-fluoride phase probably would be extracted in the soluble Mn fraction.
TABLE III.
Geometric Mean Ratios of Mn Fractions to Mn (sum) for All Projects Combined Stratified by Particle Size.
| Project | Parameter | Total Particulate | Respirable Particulate | Both Total and Respirable Particulate | |||
|---|---|---|---|---|---|---|---|
| n (n < LOD) | GM (GSD) | n (n < LOD) | GM (GSD) | n (n < LOD) | GM (GSD) | ||
| All Projects | Sol Mn:Mn(sum) | 36 (0) | 0.07 (1.70) | 10 (0)B | 0.07 (1.73) | 46 (0) | 0.07 (1.70) |
| Non-Confined Space Jobs | Mn(0,2+):Mn(sum) | 36 (0) | 0.55 (1.36) | 10 (0) | 0.57 (1.30) | 46 (0) | 0.55 (1.35) |
| Mn(3+,4+):Mn(sum) | 36 (4)A | 0.33 (1.51) | 10 (0) | 0.31 (1.43) | 46 (0) | 0.32 (1.49) | |
| All Projects | Sol Mn:Mn(sum) | 17 (0) | 0.09 (1.68) | 9 (0) | 0.07 (1.54) | 26 (0) | 0.08 (1.66) |
| Confined Space Jobs | Mn(0,2+):Mn(sum) | 17 (0) | 0.49 (1.75) | 9 (0) | 0.51 (1.18) | 26 (0) | 0.50 (1.59) |
| Mn(3+,4+):Mn(sum) | 17 (0) | 0.36 (1.77) | 9 (0) | 0.44 (1.39) | 26 (0) | 0.39 (1.66) | |
| All ProjectsC | Sol Mn:Mn(sum) | 53 (4) | 0.08 (1.71) | 19 (0) | 0.07 (1.64) | 72 (4) | 0.07 (1.69) |
| Both Non- and Confined Space Jobs | Mn(0,2+):Mn(sum) | 53 (4) | 0.53 (1.50) | 19 (0) | 0.54 (1.26) | 72 (4) | 0.53 (1.44) |
| Mn(3+,4+):Mn(sum) | 53 (4) | 0.34 (1.61) | 19 (0) | 0.36 (1.47) | 72 (4) | 0.35 (1.57) | |
Four samples were below the Minimum Detectable Concentration (MDC); censored data was used to calculate GM using maximum likelihood method.
Only the Turn-Around project survey collected respirable Mn outside of confined spaces.
The Boiler House project only had non-confined space jobs and total Mn particulate included in these data.
In addition, the percent of the each Mn fraction to the Mn(sum) was calculated for each separate total and respirable particulate TWA measurement. These percentages are summarized in Table V by reporting minimum, maximum, geometric mean (GM), and geometric standard deviation (GSD) for these ratios, combined for all three construction projects, and stratified by confined space status and for the entire data set. For the turn-around project, the fourth Mn fraction was removed for the ratio calculation so that the data was consistent with the other two projects. Again, the same ordinal rank was observed (i.e., Mn0,2+ > Mn3+,4+ > soluble Mn) with Mn0,2+ in excess of 50% in all cases but one; Mn3+,4+ ranged from 31 – 44% and soluble Mn from 7 – 9%. All of the GSDs were 1.77 or less demonstrating relatively low variability.
The bio-accessibility and potential for neurotoxicity of Mn has been proposed to be influenced by several physiochemical parameters including particle size; chemical composition and solubility; and surface area and reactivity.(1, 13) The biological solubility, distribution, accumulation and elimination of this transition metal, determined by its oxidation state,(3,8) underscores the importance of measuring the Mn particle size and fractions based on valence states, to which welders are exposed, as shown in this manuscript.
CONCLUSIONS
The sequential extraction analysis of Mn fractions has demonstrated, with shielded metal arc welding on mild carbon steel, that welders’ are exposed to appreciable levels of respirable Mn in several oxidation states with the vast majority (85-90%) existing in the Mn0,2+ and Mn3+,4+ valence states. Moreover, the prevalence patterns of Mn fractions were quite consistent. Mn0,2+ fractions were observed in the highest quantities and Mn3+,4+ levels were much higher than those for soluble and insoluble Mn. The Mn fractional data discovered in this study may have future research implications regarding the health risk assessment of welders and other workers exposed to Mn compounds.
Although all of the welders’ exposures were measured below the OSHA regulatory PEL and NIOSH REL, there were numerous instances when their exposures exceeded the ACGIH TLV for total Mn and the newly adopted ACGIH TLV for respirable Mn, especially for those welding within confined spaces. Since the signs of manganese toxicity may initially be sub-clinical, and may possibly become irreversible, workplace exposures to Mn and welding fume should be controlled to reduce the risk of chronic disease. Moreover, in the 1988 NIOSH criteria document addressing welding fume, NIOSH concluded that welders were potentially at risk for developing acute and chronic respiratory effects, possibly including lung cancer; as such, NIOSH recommended in that document that exposures to the chemical agents associated with welding be minimized. Moreover, given the very low criteria of the recently adopted TLVs by ACGIH, the construction industry will be challenged to reduce welders’ exposures below these recommended levels, particularly for respirable Mn when welding within confined spaces.
Acknowledgments
This study was partially funded by an interagency agreement between the National Toxicology Program (NTP), National Institute of Environmental Health Sciences (NIEHS) and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC) [NIEHS/NIOSH Interagency Agreement No. Y1-E5-9018-02]. The authors wish to acknowledge the company, contractor management, and union staff who provided valuable input for the success of this endeavor, and the workers who provided their voluntary consent prior to their participation in this exposure monitoring study. The authors would also like to acknowledge Kevin Dunn MS and Kenneth Sparks (NIOSH), and Timothy Carter, MS (Battelle, Inc.) for laboratory and field assistance with equipment preparation and sample collection; Bureau Veritas North America Laboratories for analytical services; Lian Luo (SRA International) for programming, and Cheryl Estill, MS, PE and Brian Curwin, PhD (NIOSH) for manuscript review prior to submission to the journal.
Footnotes
Disclaimer - The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH), National Institute of Environmental Health Sciences (NIEHS), and Battelle, Inc. It does not represent and should not be construed to represent any agency determination or policy. Mention of company names and internet web sites does not constitute an endorsement by NIOSH, NIEHS, and Battelle, Inc.
Contributor Information
Kevin W. Hanley, Industrywide Studies Branch, Division of Surveillance, Hazard Evaluations and Field Studies, National Institute for Occupational Safety and Health, Cincinnati, Ohio, USA.
Ronnee Andrews, Chemical Exposure and Monitoring Branch, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, Cincinnati, Ohio, USA, RAndrews@cdc.gov.
Steven Bertke, Industrywide Studies Branch, Division of Surveillance, Hazard Evaluations and Field Studies, National Institute for Occupational Safety and Health, Cincinnati, Ohio, USA, SBertke@cdc.gov.
Kevin Ashley, Chemical Exposure and Monitoring Branch, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, Cincinnati, Ohio, USA, KAshley@cdc.gov.
REFERENCES
- 1.Jenkins NT, Eagar TW. Chemical analysis of welding fume particles. Welding J. 2005 Jun;(supplement):87s–93s. [Google Scholar]
- 2.Haynes WM, editor. Handbook of chemistry and physics 94 ed. CRC Press; New York, NY.: 2013. CRC: Properties of elements and inorganic compounds. pp. 4–21. Section 4 ISBN 13:978-1-4665-7117-3. [Google Scholar]
- 3.Pal PK, Samii A, B Calne D. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicol. 1999;20:227–238. [PubMed] [Google Scholar]
- 4.Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catechholamines. Arch Biochem Biophys. 1987;256:638–650. doi: 10.1016/0003-9861(87)90621-7. [DOI] [PubMed] [Google Scholar]
- 5.Oberdoster G, Cherian G. Biological monitoring of toxic metals. Plenum Press; New York: 1988. Manganese. [Google Scholar]
- 6.WHO: Manganese and its compounds: Concise International Chemical Assessment Document. World Health Organization. Vol. 12. CICAD; Geneva: 1999. ISBN 92 4 153012 X. [Google Scholar]
- 7.Chen JY, C Tsao G, Zhao Q, Zheng W. Differential Cytotoxicity of Mn(II) and Mn(III): Special reference to mitochondrial [Fe-S] containing enzymes. Toxicol Appl Pharmacol. 2001;175:160–168. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschner M, Aschner J. Manganese Neurotoxicity: Cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15:333–340. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- 9.Aisen P, Rasa R. R, Redfield AG. The chromium, manganese and cobalt complexes. J Biol Chem. 1969;244:4628–4633. [PubMed] [Google Scholar]
- 10.Sunderman FW. Nasal toxicity, carcinogenicity,and olfactory uptake of metals. Ann Clin Lab Sci. 2001;31:3–24. [PubMed] [Google Scholar]
- 11.Tjalve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotox. 1999;20:181–195. [PubMed] [Google Scholar]
- 12.Olanow CW. Manganese-induced parkinsonism and Parkinson's Disease. Ann NY Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- 13.Taube F. Manganese in Occupational Arc Welding Fumes: Aspects on physiochemical properties, with focus on solubility. Ann Occup Hyg. 2013;57:6–25. doi: 10.1093/annhyg/mes053. [DOI] [PubMed] [Google Scholar]
- 14.Roels H, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys R. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. British J Industrial Med. 1992;49:25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. State-of-the-Science Review: Does manganese exposure during welding pose a neurological risk? J Tox Environ Health, Part B. 2007;10:417–465. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- 16.Aschner M, Guilarte TR. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth JA. Are there common biochemical and molecular mechanisms controlling manganism and parkinsonsism. Neuromolecular Med. 2009;11:281–296. doi: 10.1007/s12017-009-8088-8. [DOI] [PubMed] [Google Scholar]
- 18.Thomassen Y, Ellingsen D, Hetland S, Sand G. Chemical speciation and sequential extraction of Mn in workroom aerosols: Methodology and results from a field study in Mn alloy plants. J Environ Monit. 2001;3:555–559. doi: 10.1039/b104479f. [DOI] [PubMed] [Google Scholar]
- 19.Ellingsen D, Hetland S, Thomassen Y. Manganese air exposure assessment and biological monitoring in the manganese alloy production industry. J Environ Monit. 2003;5:84–90. doi: 10.1039/b209095c. [DOI] [PubMed] [Google Scholar]
- 20.Berlinger B, Naray M, Sajo I, Zaray G. Critical evaluation of sequential leaching procedures for the determination of Ni and Mn species in welding fumes. Ann Occup Hyg. 2009;53(4):333–340. doi: 10.1093/annhyg/mep013. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Bureau of Labor Statistics Division of Occupational Employment Statistics. Occupational Employment and Wages. 2011 [Google Scholar]
- 22.Linnert GE. Welding Metallurgy. 4th ed. Vol. 1. American Welding Society; Miami FL: 1994. ISBN 0-87171-457-4. [Google Scholar]
- 23.Welding Institute: A Welding Engineer's Handbook. The Welding Institute; Cambridge, England: 1976. [Google Scholar]
- 24.Burgess WA. Recognition of Health Hazards in Industry: A Review of Materials and Processes. 2nd ed. John Wiley & Sons; New York, NY: 1995. Industrial operations. ISBN 978-0471-57716-4. [Google Scholar]
- 25.NIOSH: Criteria for a Recommended Standard: Welding, Brazing and Thermal Cutting. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1988. DHHS (NIOSH) Publication No. 88-110. [Google Scholar]
- 26.Harris MK. Welding Safety and Health: A Field Guide for OEHS Professionals. American Industrial Hygiene Association Press; Fairfax, VA: 2002. Health effects of metals, gases, and other agents commonly encountered in welding process. ISBN 978-1-931504-28-7. [Google Scholar]
- 27.Antonini JM. Health effects of welding. Crit Rev Toxicol. 2003;33:61–203. doi: 10.1080/713611032. [DOI] [PubMed] [Google Scholar]
- 28.AWS: Characterization of arc welding fume. American Welding Society; Miami, FL: 1983. [Google Scholar]
- 29.Hewett P. The particle size distribution, density, and specific surface area of welding fumes from SMAW and GMAW mild and stainless steel consumables. Am Ind Hyg Assoc J. 1995;56:128–135. doi: 10.1080/15428119591017150. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer AT, Biswas P. Characterization of the aerosols resulting from arc welding processes. J Aerosol Sci. 2001;32:993–1008. [Google Scholar]
- 31.Jenkins NT, Pierce WM, Eagar TW. Particle size distribution of gas metal and flux core arc welding fumes. Welding J, October supplement. 2005:156s–163s. [Google Scholar]
- 32.ACGIH: Documentation of the Threshold Limit Values and Biological Exposure Indices. Supplement. 7th ed. American Conference of Government Industrial Hygienists; Cincinnati, OH: 2008. 2008. [Google Scholar]
- 33.ATSDR: Toxicological profile for manganese. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry Syracuse Research Corp.; Atlanta, GA: 2012. Contract 200-2004-09793. [PubMed] [Google Scholar]
- 34.Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardif R, Smargiassi A, Martine L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- 35.Korczynski RE. Occupational health concerns in the welding industry. Appl Occup Env Hyg. 2000;15:936–45. doi: 10.1080/104732200750051175. [DOI] [PubMed] [Google Scholar]
- 36.Antonini JM, B Santamaria A, Jenkins NT, Albini E, Lucchini R. Fate of manganese associated with the inhalation of welding fumes: potential neurological effects. Neurotoxicol. 2006;27(3):304–310. doi: 10.1016/j.neuro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicol. 2006a;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: Neurological and neurophysiological sequelae. Neurotoxicol. 2006b;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Rodier J. Manganese poisoning in Moroccan miners. Brit J Ind Med. 1955;12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowler RM, Roels H, Nakagawa S. Dose-effect relationships between manganese exposure and neurological neuropsychological and pulmonary function in confined space welders. Occup Environ Med. 2007;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wastensen G, Sallsten G, Pettersen RB, Barregard L. Neuromotor function in ship welders after cessation of manganese exposure. Int Arch Occup Environ Health. 2012;85:703–713. doi: 10.1007/s00420-011-0716-6. [DOI] [PubMed] [Google Scholar]
- 42.Lees-Haley PR, Greiffenstein MF, Larrabee GJ, Manning EL. Methodological problems in the neuropsychological assessment of effects of exposure to welding fumes and manganese. Clin Psychol. 2004;18:449–464. doi: 10.1080/1385404049052419. [DOI] [PubMed] [Google Scholar]
- 43.Olanow CW. Manganese parkinsonism and Parkinson's disease. Ann NY Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- 44.Jancovik J. Searching for a relationship between manganese and welding and Parkinson's disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- 45.Compendium of Policy Documents and Statements. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1992. NIOSH: NIOSH Recommendations for Occupational Safety and Health. DHHS (NIOSH) Publication No. 92-100. [Google Scholar]
- 46.OSHA: Permissible Exposure Limits. U.S. Department of Labor, Occupational Safety and Health Administration. U.S. Government Printing Office; Washington D.C.: 2015. Code of Federal Regulations, 29 CFR 1910.1000, Table Z-1-A. [Google Scholar]
- 47.ACGIH . Threshold limit values for chemical substances and physical agents and biological exposure indices. American Conference of Government Industrial Hygienists; Cincinnati, OH: 2015. 2015 TLVs® and BEIs®. [Google Scholar]
- 48.Lauwerys R, Roels H, Genet P, Toussaint G, Bouckaert A, De Cooman S. Fertility of maleworkers exposed to mercury vapor or to manganese dust: A questionnaire study. Am J Ind Med. 1985;7:171–176. doi: 10.1002/ajim.4700070208. [DOI] [PubMed] [Google Scholar]
- 49.Roels H, Lauwerys R, Buchet J, Genet P, Sarhan M, Hanotiau I, DeFays M, Bernard A, Stanescu D. Epidemiological survey among workers exposed to manganese: Effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11:307–327. doi: 10.1002/ajim.4700110308. erratum Am J Ind Med 12:119-120. [DOI] [PubMed] [Google Scholar]
- 50.ISO: Health and safety in welding and allied processes -- Sampling of airborne particles and gases in the operator's breathing zone - Part 1: Sampling of airborne particles. International Organization for Standardization; Geneva, Switzerland: 2011. ISO 10882-1. [Google Scholar]
- 51.Schlecht PC, O'Connor PF, editors. NIOSH: NIOSH manual of analytical methods (NMAM®) 4th ed. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 2003. DHHS (NIOSH) Publication 94–113 (Aug 1994); 1st Suppl Publication 96–135, 2nd Suppl Publication 98–119; 3rd Suppl 2003–154. [ www.cdc.gov/niosh/docs/2003-154/] [Google Scholar]
- 52.Jin Y, Hein M, Deddens J, Hines C. Analysis of log-normally distributed exposure data with repeated measures and values below the limit of detection using SAS. Ann Occ Hyg. 2011;55(1):97–112. doi: 10.1093/annhyg/meq061. [DOI] [PubMed] [Google Scholar]
- 53.Goller JW, Paik NW. A comparison of iron oxide fume inside and outside of welding helmets. Am Ind Hyg Assoc J. 1985;46:89–93. doi: 10.1080/15298668591394455. [DOI] [PubMed] [Google Scholar]
- 54.Lui D, Wong H, Quinlan P. Welding helmet airborne fume concentrations compared to personal breathing zone sampling. Am Ind Hyg Assoc J. 1995;56:280–283. doi: 10.1080/15428119591017123. [DOI] [PubMed] [Google Scholar]
- 55.Harris MK, Ewing WM, Longo W, DePasquale C, Mount MD, Hatfield R, Stapleton R. Manganese exposure during shielded metal arc welding (SMAW) in an enclosed space. J Occup Environ Hyg. 2005;2:375–82. doi: 10.1080/15459620591007736. [DOI] [PubMed] [Google Scholar]