Abstract
Objective:
To investigate the cerebral gray matter volume alterations in unilateral sudden sensorineural hearing loss patients within the acute period by the voxel-based morphometry method, and to determine if hearing impairment is associated with regional gray matter alterations in unilateral sudden sensorineural hearing loss patients.
Study Design:
Prospective case study.
Setting:
Tertiary class A teaching hospital.
Patients:
Thirty-nine patients with left-side unilateral sudden sensorineural hearing loss and 47 patients with right-side unilateral sudden sensorineural hearing loss.
Intervention:
Diagnostic.
Main Outcome Measure:
To compare the regional gray matter of unilateral sudden sensorineural hearing loss patients and healthy control participants.
Results:
Compared with control groups, patients with left side unilateral sudden sensorineural hearing loss had significant gray matter reductions in the right middle temporal gyrus and right superior temporal gyrus, whereas patients with right side unilateral sudden sensorineural hearing loss showed gray matter decreases in the left superior temporal gyrus and left middle temporal gyrus. A significant negative correlation with the duration of the sudden sensorineural hearing loss (R = −0.427, p = 0.012 for left-side unilateral SSNHL and R = −0.412, p = 0.013 for right-side unilateral SSNHL) was also found in these brain areas. There was no region with increased gray matter found in both groups of unilateral sudden sensorineural hearing loss patients.
Conclusions:
This study confirms that detectable decreased contralateral auditory cortical morphological changes have occurred in unilateral SSNHL patients within the acute period by voxel-based morphometry methods. The gray matter volumes of these brain areas also perform a negative correlation with the duration of the disease, which suggests a gradual brain structural impairment after the progression of the disease.
Keywords: Contralateral auditory cortex, Unilateral sudden sensorineural hearing loss, Voxel-basedmorphometry
Sudden sensorineural hearing loss (SSNHL), commonly known as sudden deafness, can be associated with tinnitus and vertigo and is defined as mostly unilateral decline of hearing function of more than 30 dB in at least three sequential frequencies over 3 days or less (1). The incidence of SSNHL was reported to range from 3.9 to 27.5 per 100,000 per year and is considered to be an otologic emergency (2–4). Although some pathophysiological mechanisms have been proposed, the precise cause of SSNHL is still unknown (5). Hearing loss is usually unilateral and has no side preference, and only less than 5% of the SSNHL patients were reported to have bilateral involvement (1,6). What is worse is that it has been reported that the hearing function of the SSNHL patients will not be improved even after high-quality appropriate therapy (7), which indicates that perhaps there already exists some type of irreversible brain structural damage in these SSNHL patients.
Brain structural alterations have been reported in patients with auditory impairment such as tinnitus, unilateral hearing loss, or deafness in auditory and nonauditory areas (8–15). However, the results of SSNHL studies vary because of some inhomogeneous experimental factors such as the sample age, disease duration, and the side of the hearing impairments. For example, Yang et al. (15) investigated regional brain structural alterations in 14 patients with 14.2 years of right-sided unilateral sudden hearing loss and found decreased gray matter volume in the bilateral posterior cingulate gyrus and precuneus, left superior/middle/inferior temporal gyrus, right parahippocampal gyrus, and lingual gyrus compared with healthy controls, which suggests that chronic unilateral sudden hearing loss could induce brain morphological changes. Boyen et al. (12) found GM increases in the superior and middle temporal gyrus and decreases in the superior frontal gyrus, occipital lobe, and hypothalamus in 16 hearing loss patients compared with 24 healthy controls. Although there were already some studies that reported the brain atrophy and impaired microstructure induced by unilateral sudden sensorineural hearing loss (15–17), whether these changes already exist during the acute period is still unknown. The inconsistencies between previous studies of unilateral SSNHL and the lack of the brain structure alterations in the acute period prompted us to set up a new voxel-based morphometry (VBM) study to gain more insight into the neuroanatomical differences that are related to unilateral SSNHL.
The aims of this study were the after: (1) to investigate the cerebral gray matter volume alterations in unilateral SSNHL patients within the acute period by the VBM method, and (2) to determine whether hearing impairment is associated with regional gray matter alterations in unilateral SSNHL patients. To the best of our knowledge, no previous studies have examined the gray matter volume changes and uncovered the correlation between these changes and clinical indexes such as disease duration, hearing level, and tinnitus handicap inventory scores in unilateral SSNHL patients. We hope that our results will complement previous studies and further the understanding of unilateral SSNHL pathophysiology.
MATERIALS AND METHODS
Subjects
This study included data collected from two groups (left- and right-side unilateral SSNHL) of patients and a healthy control group, with a total of 191 right-handed subjects. The 86 patients were recruited at the outpatient Otorhinolaryngology Department of Union Hospital, which is a tertiary class A teaching hospital of Tongji Medical College, Huazhong University of Science and Technology, and 105 age-matched right-handed healthy adults were recruited at the Radiology Department of Union Hospital from 2013 to 2015. All of the subjects had the racial category of Chinese Han. The left-side unilateral SSNHL group comprised 39 (19 males) patients, and the right-side unilateral SSNHL group comprised 47 (27 males) patients. The healthy control groups consisted of 105 (47 males) subjects. Pure-tone audiometry was performed with a clinical audiometer using seven different octave frequencies (0.125 0.25, 0.5, 1, 2, 4, and 8 kHz) to measure the pure-tone average (PTA) and reflect the hearing level. All of the unilateral SSNHL patients were included if the PTA hearing threshold at the octave frequencies 0.5, 1, and 2 kHz was higher than 30 dB in the unilateral hearing loss side ear. All of the healthy control group subjects had normal hearing (defined as hearing loss of not more than 25 dB hearing loss at any of the seven frequencies). Additionally, individuals with noisiform or pulsatile tinnitus, Ménière's disease, intralabyrinthine hemorrhage (18), otosclerosis, chronic headache, psychiatric illnesses, head injury, neurosurgery, neurological disorders such as brain tumors, and individuals being treated for mental disorders were excluded from the study, to obtain a more homogeneous sample (19).
Because most of the SSNHL patients also had tinnitus, we assessed the tinnitus handicap by a Dutch translation of the Tinnitus Handicap Inventory (THI) questionnaire (20). This questionnaire consists of 25 questions that examine the functional, emotional and catastrophic reactions to tinnitus by the patients’ self-report. Higher total scores (ranging from 0 to 100) represent a higher impact of the tinnitus on everyday life for the unilateral SSNHL patients. We also assess the handedness by the Edinburgh Inventory questionnaire (21) of all of the subjects. Further details about the demographic information of the subjects can be observed in Table 1.
TABLE 1.
Summary of the demographic and clinical data
| Demographic | Control | Left SNHHL | Right SNHHL | p Value |
| Number (n) | 105 | 39 | 47 | N/A |
| Sex (male/female) | 47/58 | 19/20 | 27/20 | 0.914a |
| Age (yr) | 39.6 + 16.0 | 38.7 + 12.6 | 41.1 + 14.1 | 0.749b |
| Education level (yr) | 10.3 + 4.2 | 9.8 + 3.8 | 10.1 + 3.5 | 0.878b |
| Disease duration (d) | N/A | 8.7 + 3.9 | 8.6 + 4.6 | 0.921c |
| PTA of left ear (dB HL) | 13.2 + 1.5 | 63.7 + 27.5 | 16.3 + 5.2 | <0.001b |
| PTA of right ear (dB HL) | 12.9 + 1.2 | 15.8 + 4.0 | 60.2 + 31.8 | <0.001b |
| THI score (0–100) | N/A | 48.4 + 27.8 | 52.5 + 25.9 | 0.477c |
PTA, pure-tone average; SNHHL, sudden sensorineural hearing loss; THI: tinnitus handicap inventory.
ap value was obtained using a Pearson χ2 test (two-tailed).
bp value was obtained using one-way ANOVA (two-tailed).
cp value was obtained using the independent-sample t test (two-tailed).
This study was approved by the Tongji Medical College of Huazhong University of Science and Technology medical ethics committee. All of the subjects were informed about the purpose of the study before giving their written consent in accordance with Chinese legislation.
Data Acquisition
All of the patients were scanned before any drug treatment. The imaging experiments were performed using a 3-T MRI system (Siemens Trio Tim, Erlangen, Germany), which was equipped with a 12-channel head coil. The patients were asked to lay still in a supine position and cover their ears with headphones to remove noise during the scanning. A foam cushion was used to fix the head to reduce motion artifacts produced by head movements. Anatomical images were acquired by a three-dimensional high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence. The sequence parameters were: repetition time (TR) = 2250 ms, echo time (TE) = 2.26 ms, inversion time (TI) = 900 ms, flip-angle = 9o, voxel size = 1.0 × 1.0 × 1.0 mm3, field of view (FOV) = 256 mm × 256 mm, slice thickness = 1.00 mm; matrix = 256 × 256; and 176 sagittal slices covering the whole brain. A t2-spc-rst-tra-iso (T2-weighted) sequence was also acquired to evaluate the status of the peripheral auditory system. The t2-spc-rst-tra-iso parameters were as follows: TR = 1000 ms, TE = 132 ms, slice thickness = 0.5 mm, slice number = 64, flip-angle = 120°, FOV = 200 mm × 200 mm, and averages = 2. The MR images were diagnosed independently by two radiologists (one with 4 yr of experience, and the other with more than 20 yr of experience). Patients with abnormal MR signals such as otitis media, acoustic neuroma, and brain tumors were excluded from the subsequent analysis.
Data Preprocessing
Data processing was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) within the SPM8 software package (22) (SPM8, http://www.fil.ion.ucl.ac.uk/spm) running under MATLAB (Math Works, Natick, MA, U.S.A.). The origin location of each participant's structural image was manually set to the anterior commissure. Then, these structural images were corrected for bias-field inhomogeneity, registered using linear (12-parameter affine) and nonlinear transformations, and segmented into gray matter, white matter, and cerebro-spinal fluid within the same generative model (23). The segmented images were then iteratively registered by the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra toolbox (DARTEL) to improve the intersubject registration (24). In addition, a template for the group of individuals was created in this step. This template image was transformed to MNI stereotactic space using affine and nonlinear spatial normalization with intensity modulation by the Jacobian determinant of the deformation flow field computed for each image to account for volume changes that resulted from the normalization process (25). For the modulated normalized option, we used “nonlinear only,” to result in an analysis of the relative differences in the regional gray matter volume, which was corrected for the individual brain size. The resulting gray matter images were transformed into MNI space and smoothed with an isotropic Gaussian kernel of 10-mm full width at half maximum (FWHM), to exploit the partial volume effects and increase the signal-to-noise ratio.
Statistical Analysis
To detect the differences among the three groups of subjects, a one-way analysis of covariance (ANCOVA) was performed with sex, age, and education as covariates. A family-wise error (FWE) with a confidence threshold of p < 0.05 was used to correct for multiple comparisons. To determine the differences between each of the groups, a post hoc Tukey pair-wise comparison was conducted between patient groups and healthy control groups with the FWE corrected (p < 0.05 and extent threshold 33 voxels). A correlation analysis was also performed in the patient groups between the regional gray matter volume and the duration of the disease as well as the PTA and THI in the brain areas with the gray matter alterations.
RESULTS
All of the subjects who were included in the study are right handed. Demographic and clinical data for the subjects in the right- and left-side unilateral SSNHL groups are shown in Table 1. No significant differences were found in sex, age, education level, disease duration, or Tinnitus Handicap Inventory (THI) score among the cohorts. The pure-tone average (PTA) of the left ear and the PTA of the right ear were significantly different among the three groups.
Compared with healthy control groups, the patients with left-side unilateral SSNHL had significant gray matter reductions in the right middle temporal gyrus (rMTG) and right superior temporal gyrus (rSTG) (Fig. 1A). We also found a significant negative correlation (R = −0.427, p = 0.012) in these brain areas with the duration of SSNHL (Fig. 1B). Patients with right-side unilateral SSNHL showed decreased gray matter in the left superior temporal gyrus (lSTG) and left middle temporal gyrus (lMTG) compared with the healthy control group (Fig. 2A). This study also showed a significant negative correlation (R = −0.412, p = 0.013) in these brain areas with the duration of the right-side unilateral SSNHL (Fig. 2B). There is no significant correlation between the regional gray matter volume and the PTA and THI scores in both groups of patients. In addition, there was no region that had increased gray matter that was found in either group of unilateral SSNHL patients, nor do any other brain areas had an alteration of gray matter except the contralateral auditory cortex.
FIG. 1.
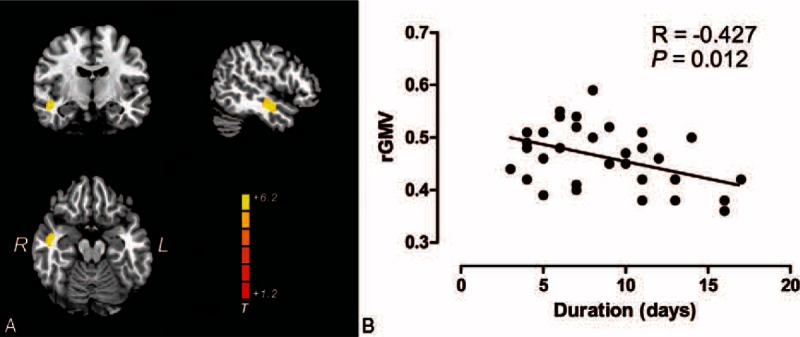
A, Brain regions where there were significant reductions in gray matter volume in left-side unilateral sudden sensorineural hearing loss patients (n = 39) relative to healthy controls (n = 105), highlighted in yellow (T = 6.12; p < 0.05, FWE corrected). B, Scatterplots show the pattern of correlation between the mean gray matter volume for the clusters identified in (A) and the disease durations. L indicates left; R, right; rGMV, regional gray matter volume.
FIG. 2.
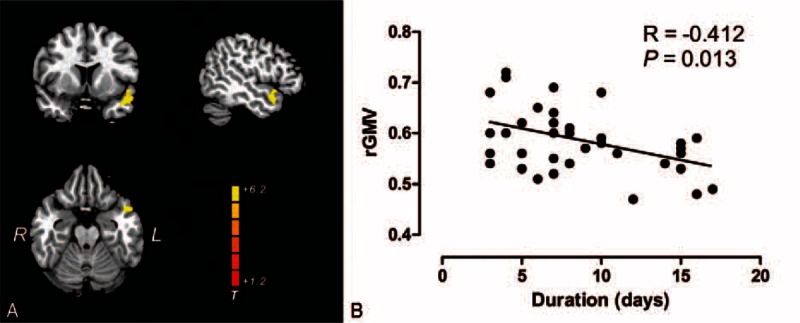
A, Brain regions where there were significant reductions in the gray matter volume in right-side unilateral sudden sensorineural hearing loss patients (n = 47) relative to healthy controls (n = 105), highlighted in yellow (T = 5.65; p < 0.05, FWE corrected). B, Scatterplots show the pattern of correlation between the mean gray matter volume for the clusters identified in (A) and the disease durations. L indicates left; R, right; rGMV, regional gray matter volume.
DISCUSSION
In the present study, we investigated the brain morphological alterations in a large sample of unilateral SSNHL patients using the voxel-based morphometry method. Compared with the healthy control group, left-side unilateral SSNHL patients showed a gray matter reduction in the right auditory cortex (rMTG, rSTG), whereas right-side unilateral SSNHL patients showed a gray matter reduction in the left auditory cortex (lMTG, lSTG). This interesting result suggested two meanings. On the one hand, cortical morphological alterations already existed in unilateral SSNHL patients within the acute period. On the other hand, these alterations mainly occur in the contralateral auditory cortex. For the first point, a previous study has demonstrated activity-dependent transient and selective structural changes in gray matter induced in human adults after 3 months of training (26). Subsequent research from multiple groups has also found cortical changes in gray matter that was already detectable after repetitive nociceptive input over a period of 8 days (27) and after 7 days of visuo-motor training (28). Furthermore, a shorter time frame of as early as within 5 days of induced gray matter changes in the auditory cortex was reported after repetitive transcranial magnetic stimulation (29), and a significant GM increase occurred after 3 days of training in the right ventral striatum (30). Considering the duration (days) of the SSNHL patients in our experiment, we are not surprised with the alteration of the gray matter found in this study because brain morphological alterations could indicate corresponding brain dysfunctions that are related to unilateral SSNHL.
Another notable result of this study is that the location of the decreased gray matter is on the contralateral auditory cortex. To the best of our knowledge, there seems to be a paucity of information in the literature on lateralized brain structural alterations in patients with unilateral SSNHL; in contrast, there was a clear laterality effect of primary auditory cortex in functional studies (31–34). Musiek et al. (35) reported an interesting patient in which a patient had a specific hearing loss that was lateralized distinctly to the right ear, which was contralateral to a stroke that involved the left hemisphere, with neural compromise limited primarily to the left Heschl's gyrus. The underlying mechanism of this contralateral cortex impairment could be related to the misrepresentation of the intensity at the cortical level (35), given that our patients showed no abnormality in the peripheral auditory system. The perception of loudness or intensity at the cortical level could be affected by the interaction between excitatory and inhibitory neurons (35,36). If more excitatory fibers are damaged than inhibitory fibers, then the perception of loudness would decrease. As a result, the neuronal response to intensity from this imbalanced damaged area in one hemisphere will be reduced compared with the other.
Moreover, Yang et al. (15) reported a study in which patients with a 14-year right-sided unilateral hearing loss showed decreased gray matter volume in the left superior/middle/inferior temporal gyrus, which is consistent with our findings in the right-side unilateral SSNHL groups. However, they also reported decreased gray matter volume in nonauditory brain regions such as posterior cingulate gyrus, precuneus, and right parahippocampal gyrus, which is reported in other studies also (37,38). It is reported that these areas were associated with additional disabilities and complications in unilateral hearing loss patients, such as spatial processing, tinnitus, visual processing, and semantic memory (12,39–41). Although some of our unilateral SSNHL patients also presented with some symptoms such as tinnitus and vertigo, we found no other brain area alterations except in the contralateral auditory cortex. We speculate that this finding is because these concomitant symptoms are secondary to the unilateral SSNHL. Given that our patients only have days of duration and the strict statistical correction method that we used (FWE), the impairment of the brain structure after these concomitant symptoms could be very slight and still undetectable, in such a way that no significant changes showed. Increased gray matter could represent a compensatory mechanism that is secondary to the difficulties induced by unilateral SSNHL. We found no brain areas that showed a significant increase of gray matter. We speculate that the brain needs more time to trigger a compensatory system from other cerebrum areas and to accumulate the gray matter alteration to a detectable level.
We also found a significant negative correlation between the gray matter of the altered areas and the disease duration. This finding could suggest that the impairment of the gray matter in unilateral SSNHL has a cumulative effect after the progress of the disease; the longer the disease, the lower the level of the regional gray matter volume. Hence, early intervention and prompt treatment are especially important for the treatment of unilateral SSNHL. Some previous studies (42,43) also showed a correlation between the gray matter in the auditory areas and tinnitus distress (THI scores). However, some of these studies about the correlations are inconsistent; for example, Leaver et al. (42) found a positive correlation between tinnitus distress and the gray matter in auditory areas, whereas Schecklmann et al. (43) reported a negative correlation in their tinnitus study. We found no significant correlation in our study, which is consistent with a previous study (9). The differences might be because of the heterogeneity in sample clinical characteristics such as age, sex, hearing function, and education level as well as methodical differences. In the future, the selection of a larger sample of homogeneous subjects and more accurately matched healthy control groups might help us to uncover the relationship more exactly.
In our data, only the unilateral SSNHL patients were included in this study, and thus, it is not clear whether there is the same result about the gray matter alterations in bilateral SSNHL patients because some studies have reported different clinical characteristics and treatment results between bilateral and unilateral SSNHL patients (6,44,45). The use of MP-RAGE sequence at 3T scanner may partially introduce the central brightening artifact (46). In addition, some unilateral SSNHL patients had some other clinical symptoms, such as tinnitus and vertigo, other than hearing loss. We cannot exclude the compound effect of these clinical characteristics on the brain structure. Furthermore, longitudinal studies with larger samples are needed to provide more information about the alterations of the gray matter with the disease progression of unilateral SSNHL, to obtain a higher statistical power and smaller within-subjects variability (29).
In summary, we found that detectable decreased contralateral auditory cortical morphological changes have occurred in unilateral SSNHL patients within the acute period by voxel-based morphometry methods. The gray matter volumes of these brain areas also perform a negative correlation with the duration of the disease, which suggests a gradual brain structural impairment after the progression of the disease. These findings could help to better characterize unilateral SSNHL and provide insights for the potential pathophysiology of unilateral SSNHL, which will ultimately assist in providing a better diagnosis and more accurate and effective treatment of unilateral SSNHL. It is hoped that this study will alert audiologists to not only focus on the abnormal in the peripheral auditory system but also be aware of the alteration of the gray matter in the cerebral cortex that the unilateral SSNHL patients might have.
Footnotes
This research was supported by the National Natural Science Foundation of China (No. 81171386, 30770623) and Hubei Key Laboratory Foundation of Molecular Imaging (No. 2008-69) as well as the Hubei Natural Science Foundation (No. 2009CDB008).
The authors disclose no conflicts of interest.
REFERENCES
- 1.Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet 2010; 375:1203–1211. [DOI] [PubMed] [Google Scholar]
- 2.Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otol Neurotol 2013; 34:1586–1589. [DOI] [PubMed] [Google Scholar]
- 3.Jourdy DN, Donatelli LA, Victor JD, et al. Assessment of variation throughout the year in the incidence of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2010; 31:53–57. [DOI] [PubMed] [Google Scholar]
- 4.Nosrati-Zarenoe R, Hansson M, Hultcrantz E. Assessment of diagnostic approaches to idiopathic sudden sensorineural hearing loss and their influence on treatment and outcome. Acta Otolaryngol 2010; 130:384–391. [DOI] [PubMed] [Google Scholar]
- 5.Arslan N, Oguz H, Demirci M, et al. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otol Neurotol 2011; 32:393–397. [DOI] [PubMed] [Google Scholar]
- 6.Oh JH, Park K, Lee SJ, et al. Bilateral versus unilateral sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 2007; 136:87–91. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn M, Heman-Ackah SE, Shaikh JA, et al. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif 2011; 15:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanneste S, Van De Heyning P, De Ridder D. Tinnitus: a large VBM-EEG correlational study. PLoS One 2015; 10:e0115122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlau M, Rauschecker JP, Oestreicher E, et al. Structural brain changes in tinnitus. Cereb Cortex 2006; 16:1283–1288. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Andermann M, Wengenroth M, et al. Reduced volume of Heschl's gyrus in tinnitus. Neuroimage 2009; 45:927–939. [DOI] [PubMed] [Google Scholar]
- 11.Leaver AM, Renier L, Chevillet MA, et al. Dysregulation of limbic and auditory networks in tinnitus. Neuron 2011; 69:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyen K, Langers DR, de Kleine E, et al. Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hear Res 2013; 295:67–78. [DOI] [PubMed] [Google Scholar]
- 13.Melcher JR, Knudson IM, Levine RA. Subcallosal brain structure: Correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear Res 2013; 295:79–86. [DOI] [PubMed] [Google Scholar]
- 14.Profant O, Skoch A, Balogova Z, et al. Diffusion tensor imaging and MR morphometry of the central auditory pathway and auditory cortex in aging. Neuroscience 2014; 260:87–97. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Chen HJ, Liu B, et al. Brain structural and functional alterations in patients with unilateral hearing loss. Hear Res 2014; 316:37–43. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Lee SH, Lee YJ, et al. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport 2004; 15:1699–1703. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Wang J, Wu C, et al. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: Changes in radial diffusivity and diffusion anisotropy. J Magn Reson Imaging 2008; 28:598–603. [DOI] [PubMed] [Google Scholar]
- 18.Cervantes SS, Barrs DM. Sudden Sensorineural Hearing Loss Associated With Intralabyrinthine Hemorrhage. Otol Neurotol 2015; 36:e134–e135. [DOI] [PubMed] [Google Scholar]
- 19.Schneider P, Andermann M, Wengenroth M, et al. Reduced volume of Heschl's gyrus in tinnitus. Neuroimage 2009; 45:927–939. [DOI] [PubMed] [Google Scholar]
- 20.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 1996; 122:143–148. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971; 9:97–113. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner J. SPM: A history. Neuroimage 2012; 62:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26:839–851. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113. [DOI] [PubMed] [Google Scholar]
- 25.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14 (1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 26.Draganski B, Gaser C, Busch V, et al. Neuroplasticity: Changes in grey matter induced by training. Nature 2004; 427:311–312. [DOI] [PubMed] [Google Scholar]
- 27.Teutsch S, Herken W, Bingel U, et al. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage 2008; 42:845–849. [DOI] [PubMed] [Google Scholar]
- 28.Driemeyer J, Boyke J, Gaser C, et al. Changes in gray matter induced by learning–revisited. PLoS One 2008; 3:e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May A, Hajak G, Ganssbauer S, et al. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cereb Cortex Jan 2007; 17:205–210. [DOI] [PubMed] [Google Scholar]
- 30.Hamzei F, Glauche V, Schwarzwald R, et al. Dynamic gray matter changes within cortex and striatum after short motor skill training are associated with their increased functional interaction. Neuroimage 2012; 59:3364–3372. [DOI] [PubMed] [Google Scholar]
- 31.Reznik D, Henkin Y, Schadel N, et al. Lateralized enhancement of auditory cortex activity and increased sensitivity to self-generated sounds. Nat Commun 2014; 5:4059. [DOI] [PubMed] [Google Scholar]
- 32.Santosa H, Hong MJ, Hong KS. Lateralization of music processing with noises in the auditory cortex: An fNIRS study. Front Behav Neurosci 2014; 8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamison HL, Watkins KE, Bishop DV, et al. Hemispheric specialization for processing auditory nonspeech stimuli. Cereb Cortex 2006; 16:1266–1275. [DOI] [PubMed] [Google Scholar]
- 34.Tervaniemi M, Hugdahl K. Lateralization of auditory-cortex functions. Brain Res Brain Res Rev 2003; 43:231–246. [DOI] [PubMed] [Google Scholar]
- 35.Musiek F, Guenette L, Fitzgerald K. Lateralized auditory symptoms in central neuroaudiology disorder. J Am Acad Audiol 2013; 24:556–563. [DOI] [PubMed] [Google Scholar]
- 36.Phillips DP, Semple MN, Calford MB, et al. Level-dependent representation of stimulus frequency in cat primary auditory cortex. Exp Brain Res 1994; 102:210–226. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Li W, Xian J, et al. Cortical thickness analysis and optimized voxel-based morphometry in children and adolescents with prelingually profound sensorineural hearing loss. Brain Res 2012; 1430:35–42. [DOI] [PubMed] [Google Scholar]
- 38.Meyer M, Toepel U, Keller J, et al. Neuroplasticity of sign language: Implications from structural and functional brain imaging. Restor Neurol Neuros 2007; 25:335–351. [PubMed] [Google Scholar]
- 39.Weisberg J, Koo DS, Crain KL, et al. Cortical plasticity for visuospatial processing and object recognition in deaf and hearing signers. Neuroimage 2012; 60:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tibbetts K, Ead B, Umansky A, et al. Interregional brain interactions in children with unilateral hearing loss. Otolaryngol Head Neck Surg 2011; 144:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronnberg J, Danielsson H, Rudner M, et al. Hearing loss is negatively related to episodic and semantic long-term memory but not to short-term memory. J Speech Lang Hear Res 2011; 54:705–726. [DOI] [PubMed] [Google Scholar]
- 42.Leaver AM, Seydell-Greenwald A, Turesky TK, et al. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci 2012; 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schecklmann M, Lehner A, Poeppl TB, et al. Auditory cortex is implicated in tinnitus distress: A voxel-based morphometry study. Brain Struct Funct Jul 2013; 218:1061–1070. [DOI] [PubMed] [Google Scholar]
- 44.Sano H, Okamoto M, Ohhashi K, et al. Quality of life reported by patients with idiopathic sudden sensorineural hearing loss. Otol Neurotol 2013; 34:36–40. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki S, Sano H, Nishio S, et al. Hearing handicap in adults with unilateral deafness and bilateral hearing loss. Otol Neurotol 2013; 34:644–649. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein MA, Huston J, Ward HA. Imaging artifacts at 3.OT. J Magn Reson Imaging 2006; 24:735–746. [DOI] [PubMed] [Google Scholar]


