Abstract
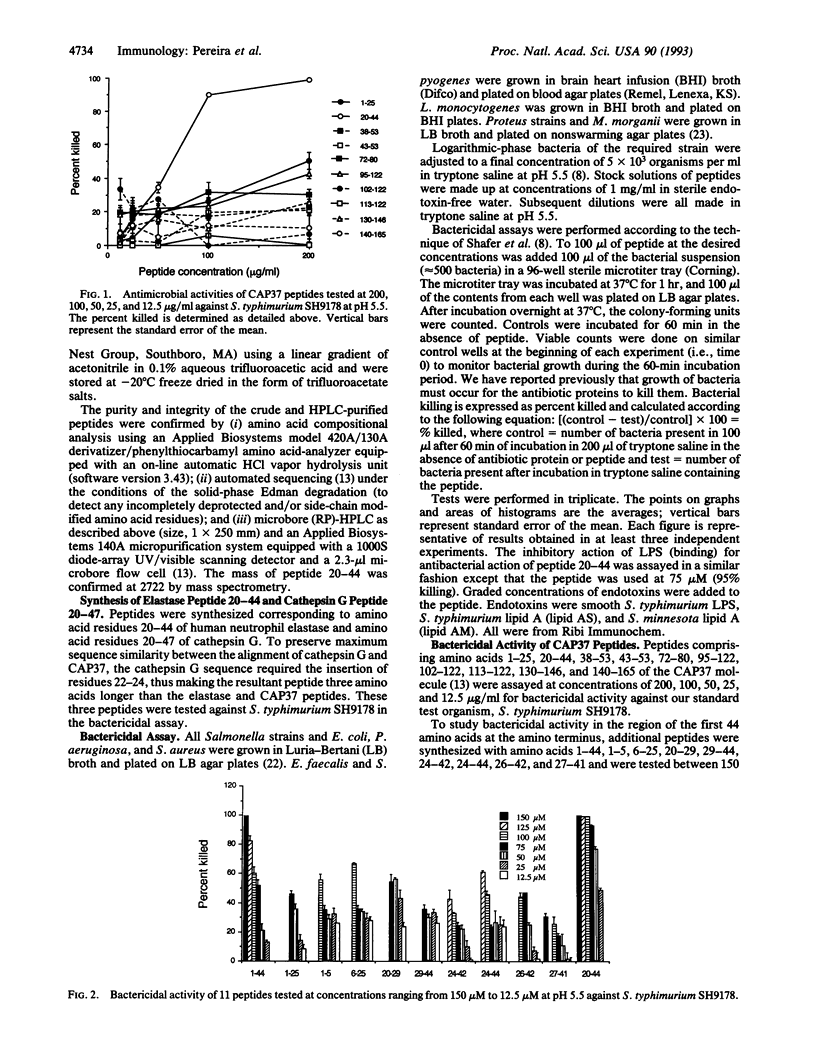
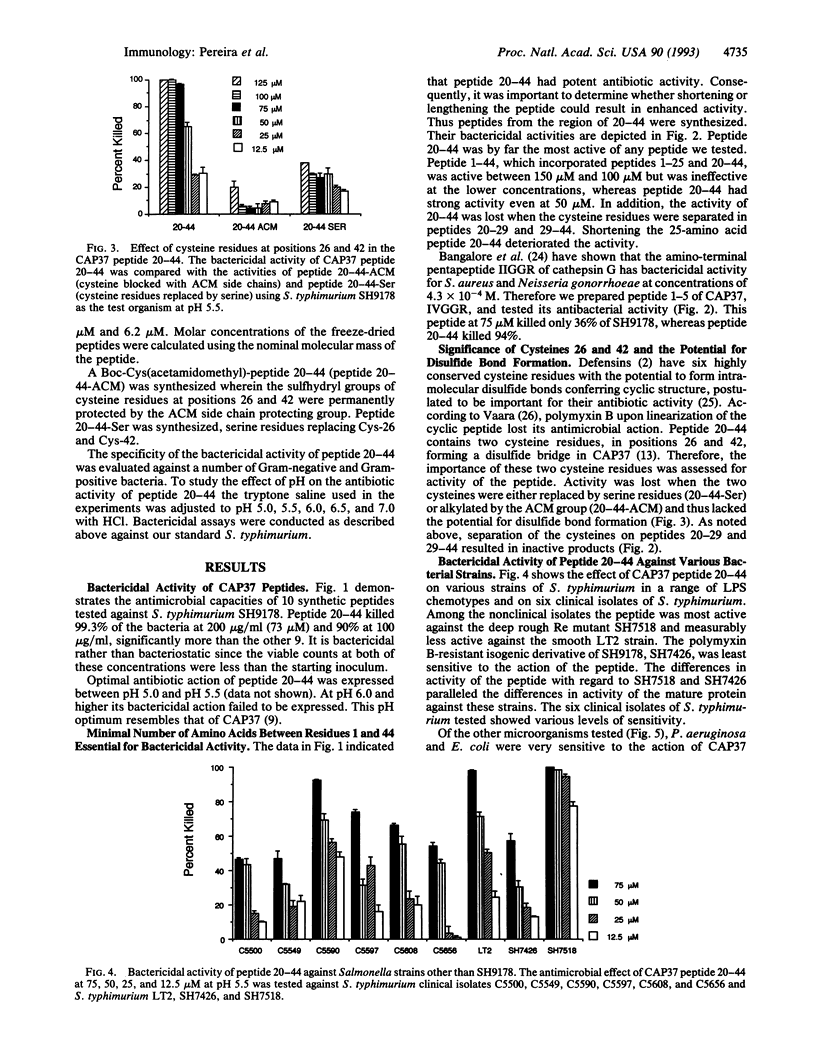
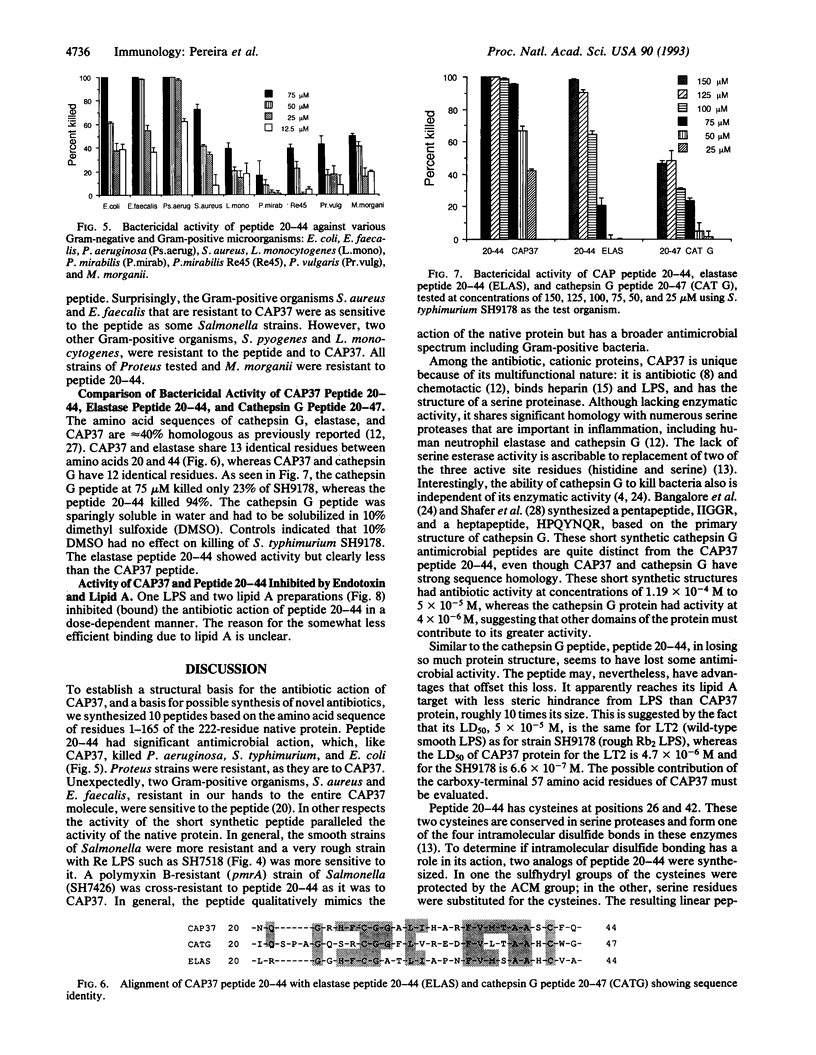
CAP37 (cationic antimicrobial protein of molecular mass 37 kDa) is a multifunctional protein isolated from the granules of human neutrophils. It is antibiotic and chemotactic and binds lipopolysaccharide. A synthetic peptide, amino acid sequence NQGRHFCGGALIHARFVMTAASCFQ, based on residues 20-44 of CAP37 protein mimics its antibiotic and lipopolysaccharide binding action. Peptide 20-44, at the concentrations tested, has antibacterial activity against Salmonella typhimurium, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis, and Staphylococcus aureus. The bactericidal action of the peptide was pH dependent, with maximum activity at pH 5.0 and pH 5.5 and decreased activity at pH 7.0. Various truncations, substitutions, and other modifications in the sequence deteriorate its activity. Free sulfhydryl groups and/or disulfide bridge formation are required for optimum antibiotic activity, since substitution of serines for, or alkylation of, cysteine residues 26 and 42 eliminates bactericidal activity. Evidently amino acids 20-44 represent an important, perhaps principal, antibacterial domain of CAP37. This peptide should provide new insight into the mechanism of antimicrobial activity of CAP37 and may serve as a model for new, useful, synthetic antibiotics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. P., Melchior M., Campanelli D., Nathan C., Gabay J. E. Complementary DNA sequence of human neutrophil azurocidin, an antibiotic with extensive homology to serine proteases. Biochem Biophys Res Commun. 1991 Jun 14;177(2):688–695. doi: 10.1016/0006-291x(91)91843-2. [DOI] [PubMed] [Google Scholar]
- Bangalore N., Travis J., Onunka V. C., Pohl J., Shafer W. M. Identification of the primary antimicrobial domains in human neutrophil cathepsin G. J Biol Chem. 1990 Aug 15;265(23):13584–13588. [PubMed] [Google Scholar]
- Belas R., Erskine D., Flaherty D. Transposon mutagenesis in Proteus mirabilis. J Bacteriol. 1991 Oct;173(19):6289–6293. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990 Mar;85(3):904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley M. M., Shafer W. M., Spitznagel J. K. Antimicrobial binding of a radiolabeled cationic neutrophil granule protein. Infect Immun. 1987 Jun;55(6):1536–1539. doi: 10.1128/iai.55.6.1536-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H., Ostergaard E., Bayne S., Svendsen A., Thomsen J., Engels M., Wollmer A. Covalent structure of two novel neutrophile leucocyte-derived proteins of porcine and human origin. Neutrophile elastase homologues with strong monocyte and fibroblast chemotactic activities. Eur J Biochem. 1991 Apr 23;197(2):535–547. doi: 10.1111/j.1432-1033.1991.tb15942.x. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986 Apr 18;232(4748):341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Morgan J. G., Sukiennicki T., Pereira H. A., Spitznagel J. K., Guerra M. E., Larrick J. W. Cloning of the cDNA for the serine protease homolog CAP37/azurocidin, a microbicidal and chemotactic protein from human granulocytes. J Immunol. 1991 Nov 1;147(9):3210–3214. [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J., Doerfler M. E., Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55-60 kD bactericidal/permeability-increasing protein of human neutrophils. J Exp Med. 1991 Sep 1;174(3):649–655. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. A., Shafer W. M., Pohl J., Martin L. E., Spitznagel J. K. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990 May;85(5):1468–1476. doi: 10.1172/JCI114593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. A., Spitznagel J. K., Pohl J., Wilson D. E., Morgan J., Palings I., Larrick J. W. CAP 37, a 37 kD human neutrophil granule cationic protein shares homology with inflammatory proteinases. Life Sci. 1990;46(3):189–196. doi: 10.1016/0024-3205(90)90104-y. [DOI] [PubMed] [Google Scholar]
- Pohl J., Pereira H. A., Martin N. M., Spitznagel J. K. Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett. 1990 Oct 15;272(1-2):200–204. doi: 10.1016/0014-5793(90)80484-z. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S. Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide. J Biol Chem. 1989 Mar 5;264(7):4003–4007. [PubMed] [Google Scholar]
- Shafer W. M., Casey S. G., Spitznagel J. K. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect Immun. 1984 Mar;43(3):834–838. doi: 10.1128/iai.43.3.834-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Engle S. A., Martin L. E., Spitznagel J. K. Killing of Proteus mirabilis by polymorphonuclear leukocyte granule proteins: evidence for species specificity by antimicrobial proteins. Infect Immun. 1988 Jan;56(1):51–53. doi: 10.1128/iai.56.1.51-53.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granulocytes. Infect Immun. 1986 Sep;53(3):651–655. doi: 10.1128/iai.53.3.651-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C., Martin L. E. Antigonococcal activity of human neutrophil cathepsin G. Infect Immun. 1986 Oct;54(1):184–188. doi: 10.1128/iai.54.1.184-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Pohl J., Onunka V. C., Bangalore N., Travis J. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J Biol Chem. 1991 Jan 5;266(1):112–116. [PubMed] [Google Scholar]
- Spitznagel J. K. Antibiotic proteins of human neutrophils. J Clin Invest. 1990 Nov;86(5):1381–1386. doi: 10.1172/JCI114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. The outer membrane permeability-increasing action of linear analogues of polymyxin B nonapeptide. Drugs Exp Clin Res. 1991;17(9):437–443. [PubMed] [Google Scholar]
- Wasiluk K. R., Skubitz K. M., Gray B. H. Comparison of granule proteins from human polymorphonuclear leukocytes which are bactericidal toward Pseudomonas aeruginosa. Infect Immun. 1991 Nov;59(11):4193–4200. doi: 10.1128/iai.59.11.4193-4200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]



