Abstract
Purpose: Postoperative nausea and vomiting (PONV) is a common complication after gynecological surgeries. This meta-analysis was conducted to evaluate the efficacy of dexmedetomidine on PONV after gynecological surgeries. Methods: Three main electronic databases including Pub Med, Embase and Cochrane Central Register of Controlled Trials for randomized controlled trials (RCTs) were searched by two researchers independently. The metaanalysis was completed using Review Manager. Results: Eleven RCTs with 692 patients were included in this metaanalysis. Dexmedetomidine a bridged postoperative nausea [Risk Ratio (RR)=0.59, 95% confidence interval (CI): 0.44 to 0.79] and vomiting [RR=0.48, 95% CI: 0.36 to 0.64] compared with placebo. Despite of higher incidence of intra operative bradycardia [RR 2.87, 95% CI 1.08 to 7.58] and hypotension [RR 4.26, 95% CI 1.43 to 12.69], we found significant decrease in postoperative shivering [RR 0.23, 95% CI 0.13 to 0.40] and pruritus [RR 0.40, 95% CI 0.17 to 0.93] in dexmedetomidine group, as well as the pain scores [standard mean difference (SMD)-0.96, 95% CI-1.37 to-0.54]. Significant reductions in the need for intraoperative fentanyl (RR 0.10, 95% CI 0.01-0.76, I2 0%), antiemetic (RR 0.62, 95% CI 0.39-0.99, I2 0%) and postoperative analgesic (RR 0.18, 95% CI 0.08-0.42, I2 0%) were also elicited. Conclusions: The current meta-analysis exhibits that dexmedetomidine is superiority to placebo in attenuating the incidence of PONV, postoperative shivering, pruritus, as well as the pain scores in patients undergoing gynecological surgeries. Still, the potential cardiovascular complications should be taken seriously.
Keywords: Dexmedetomidine, gynecological surgery, PONV, meta-analysis
Introduction
Postoperative nausea and vomiting (PONV) are common issues after sedation or anesthesia, which may give rise to unwished admission or postponed hospital discharge [1]. Moreover vomiting can stress wounds, cause electrolyte imbalance and aggravate bleeding [2]. The incidence of PONV is even higher especially after gynecologic surgery, ranging from 24% to 75% [3], even up to 90% [4] after gynecologic laparoscopic operation. This is probably related to the fact that females confer a two-fold to fourfold risk of PONV [4,5]. Three other main risk factors for PONV have been identified: history of motion sickness or PONV; nonsmoking status and use of opioids [6].
Dexmedetomidine, a strong and highly selective α2-adrenoceptor agonist, possesses diverse properties like sedative, analgesic [7], sympatholytic and amnestic [8], and has no activity on the γ-aminobutyric acid (GABA) system [9]. It has been widely used during perioperative period. Recently, researches focusing on the effect of dexmedetomidine on PONV have been performed, while, the controversy remains ongoing for inconsistent results reported in different literatures.
Hitherto, no such meta-analysis has been conducted to combine related data. So we performed this estimate to investigate the antiemetic effect of dexmedetomidine in patients undergoing gynecological surgeries.
Methods
Since nausea and vomiting are two distinguishing phenomena, researches should report and assess the variables independently [10]. However, nausea and vomiting are usually co-existence in a patient, the occurrence of postoperative nausea (PON) is noticeably parallel to PONV, thus some researches do not struggle to distinguish the two variables [11]. So, if only PONV was reported in the trials, we regard the PONV variables as a substitute for PON [10]. The data near 24-hour postoperative were collected, since 24-hour interval was most commonly used to measure antiemetic effect [10].
Search strategy
Cochrane Central Register of Controlled Trials (CENTRAL), Embase and PubMed were systematically searched by two researchers (L.X. and Z.M.) independently. The search strategy involved the following terms: (dexmedetomidine) and (gynecological or female) and (nausea, vomiting, or PONV) and (surgery, operation, or surgical procedure). The studies retrieval was updated on May 7, 2015 without language limitation. The reference lists of relevant reviews, original reports and case reports were also checked.
Study selection and data retrieval
Studies had to satisfy all the following pre-established criteria for inclusion into the review: (1) trail: randomized controlled trials (RCTs); (2) patients: adults underwent gynecologic surgery; (3) interventions: dexmedetomidine versus placebo; (4) outcome: postoperative nausea or vomiting. Studies were excluded if they conformed to the following criteria: (1) trail: non-randomized controlled trials (NRCTs), animal experiments, review articles; (2) patients: children or underwent other surgeries; (3) interventions: agent/combinational agents (including dexmedetomidine) versus agent/combinational agents; (4) outcome: relevant data could not be obtained from the original author. (5) duplications or abstracts only.
Data extraction including: name of the first author, publication year, interventions, patients, type of anesthesia and surgery, number of nausea and vomiting cases and total patients, operation time, funding (Table 1). The eligibility of included studies was assessed by two reviewers (GXY and LX) independently. Any of disputes about this meta-analysis were resolved punctually by discussion among all of the authors.
Table 1.
Characteristics of the included trials
| Author | Year | Patients | Type of anesthesia | Type of surgery | Trail | Dosage regimen | Comparisons | Total | Nausea | Vomiting | Operation time (Mean±SD or median, min.) | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nie17 | 2014 | adults | SA | elective caesarean delivery | E | S | dexmedetomidine IV 0.5 ug/kg | 40 | 1 | 0 | 40.2±7.5 | no |
| placebo IV | 38 | 2 | 0 | 39.2±6.7 | ||||||||
| Almarakbi20 | 2014 | adults | PNB | abdominal hysterectomy | I | L | dexmedetomidine PNB | 25 | 1 | - | 72.6±7.5 | no |
| Placebo PNB | 25 | 2 | - | 74.5±9.1 | ||||||||
| Wu22 | 2013 | adults | GA | laparoscopic surgery | E | S | dexmedetomidine IV 1.0 ug/kg | 40 | 1 | 1 | 94.62±5.28 | |
| placebo IV | 40 | 5 | 4 | 92.16±6.36 | ||||||||
| Shin24 | 2013 | adults | GA | laparoscopically assisted vaginal hysterectomy, total abdominal hysterectomy, ovarian surgery | I | S | dexmedetomidine IV 1.0 μg/kg | 21 | 2 | 0 | - | no |
| placebo IV | 21 | 3 | 0 | |||||||||
| Lee27 | 2013 | adults | GA | laparoscopically assisted vaginal hysterectomy | I | L | dexmedetomidine IV | 28 | 1 | - | - | |
| placebo IV | 29 | 8 | - | |||||||||
| Kim a29 | 2013 | adults | GA | uterine artery embolization | I | C | dexmedetomidine IV 0.2 ug/kg/h | 25 | 8 | 8 | 43±8 | no |
| placebo IV | 25 | 5 | 18 | 42±8 | ||||||||
| Kim b28 | 2013 | adults | GA | modified radical mastectomy | I | S | dexmedetomidine IV 0.5 ug/kg | 46 | 18 | - | 120 | no |
| placebo IV | 46 | 26 | - | 118 | ||||||||
| Ohtani39 | 2011 | adults | GA | open gynecological abdominal surgery | I | C | dexmedetomidine IV | 16 | 3 | - | 250±66 | |
| placebo IV | 16 | 2 | - | 233±69 | ||||||||
| Massad49 | 2009 | adults | GA | elective diagnostic laparoscopic surgeries | I | C | dexmedetomidine IV 0.5 ug/kg/h | 42 | 8 | 5 | 30.5±3.1 | no |
| placebo IV | 39 | 15 | 8 | 28.4±2.2 | ||||||||
| Elvan53 | 2008 | adults | GA | elective total abdominal hysterectomy | I | L | dexmedetomidine IV | 40 | 2 | - | 78.3±19.7 | no |
| placebo IV | 40 | 2 | - | 81.9±28.2 | ||||||||
| Gurbet59 | 2006 | adults | GA | total abdominal hysterectomy | I | L | dexmedetomidine IV | 25 | 6 | - | 101±25 | no |
| placebo IV | 25 | 15 | - | 109±25 |
GA: general anesthesia, SA: spinal anesthesia, PNB: peripheral neural blockade, IV: intravenous, I: induction, E: end, S: single dose, L: loading dose followed by continuous infuse, C: continuous infuse. ① Science and Technology Program of Guangdong Province, Research Project of Commission on Innovation and Technology of Guangzhou (2011KP304), Youth Foundation of The Third Affiliated Hospital of Guangzhou Medical University (2010Y05). ② Supported by Wonkwang University. ③ Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to N.O. (No. 21791429) and E.M. (No. 22659362).
Qualitative assessment
Two authors (G.X.Y. and Z.M.) independently evaluated the quality of the included trials in accordance with the guideline recommended by the Cochrane Collaboration. Six categories were assessed including randomization sequence generation, blinding method, allocation concealment, incomplete outcome data, selective reporting and other bias. The first three categories were regarded as “key domains”, and each category contained three levels: high risk, unclear risk, and low risk. The risk of bias of each study was assessed according to the levels of the three key domains: high risk (high risk of bias for one or more key domains), unclear risk (unclear risk of bias for one or more key domains), and low risk (low risk of bias for all key domains).
Statistical analysis
Compared with placebo, the efficacy of dexmedetomidine on nausea and vomiting, adverse events, and need for rescue agents was estimated with Risk Ratio (RR) and 95% confidence intervals (CI); pain scores were assessed by pooled Standard Mean Difference (SMD). The overall effect was assessed by Z test and P<0.05 was deemed statistically significant. I2 statistic was used to evaluate heterogeneity. I2≤50% meant low risk heterogeneity, and a fixed-effect model would be applied, otherwise, a random-effect model would be employed.
Sensitivity analysis was performed to test the reliability of these results by eliminating the data of high-risk studies. Subgroup analyses were conducted according to the type of gynecologic surgical procedure (laparotomy or laparoscopy), route of administration (intravenous infusion or peripheral neural blockade), dosage regimen (single dose or loading dose followed by continuous infusion) and administration time of dexmedetomidine (during anesthesia induction or at the end of surgery).
Potential publication bias were evaluated by Egger’s Test and Begg’s Test with Stata® (Version 13.0.; Stata Corp, TX, USA), meanwhile statistical analyses were accomplished using Review Manager (Rev Man®) (Version 5.2.; The Cochrane Collaboration, Oxford, UK).
Result
Study selection
Systematically search of Pub Med, Em base, CENTRAL and reference lists generated 795 articles (Figure 1). A total of 202 duplications were removed at first, and then 569 trials were discarded for not relevant to our study according to the tittles and abstracts. Twenty four papers were fully read, while [13] of which had no related endpoints. Finally, [11] trials [12-22] met the selection criteria and included in the meta-analysis.
Figure 1.
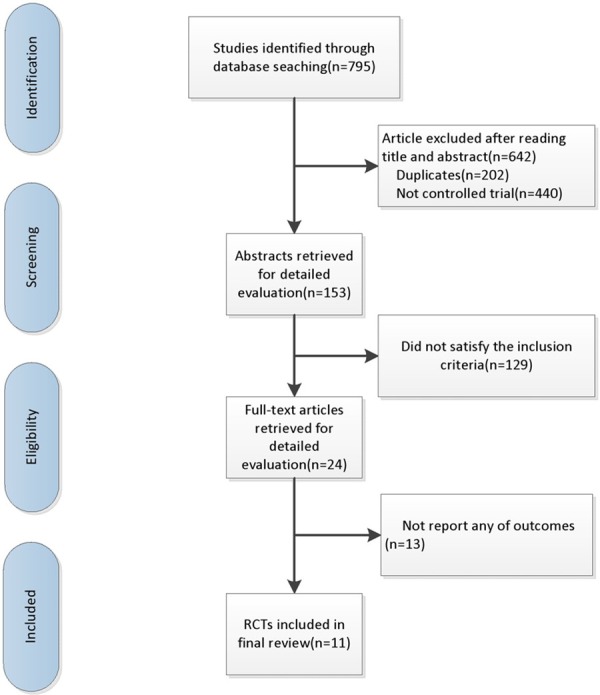
Search flow diagram for studies included in the meta-analysis.
Study characteristic
All [12-22] the included studies explored the efficacy of dexmedetomidine on nausea, and five [12,14,15,18,20] of them involved the influence of dexmedetomidine on vomiting compared with placebo. Five studies [14,16,18,21,22] reported the adverse events, including shivering [14,16,21], pruritus [18,22], headache [14,18], hypotension [16,18] and bradycardia [16,18]. There are six trials [13,15,18,20-22] reporting the rescue agents including intra operative use of ephedrine [15,21] and fentanyl [15,21], postoperative use of antiemetics [13,18] and analgesics [18,20,22]. Only six [12-14,16,19,20] of the included articles clearly mentioned the status of funding, three [14,16,19] of which were supported by institutional foundation, and three studies [12,13,20] declared no financial support (Table 1).
The methodological quality of the included studies
Ten [12,13,15-22] of the 11 included trials provided a detailed description of randomization. Seven studies [12,15,17-20,22] applied double-blinded method; eight trials [12,13,15,18-22] reported the use of allocation concealment. None of the studies had incomplete outcome (attrition bias) and each of the study reported all the end points mentioned in the Methods section (reporting bias). Meanwhile, other bias did not exist in all these studies. An overview of the risk of bias was showed in Figure 2.
Figure 2.
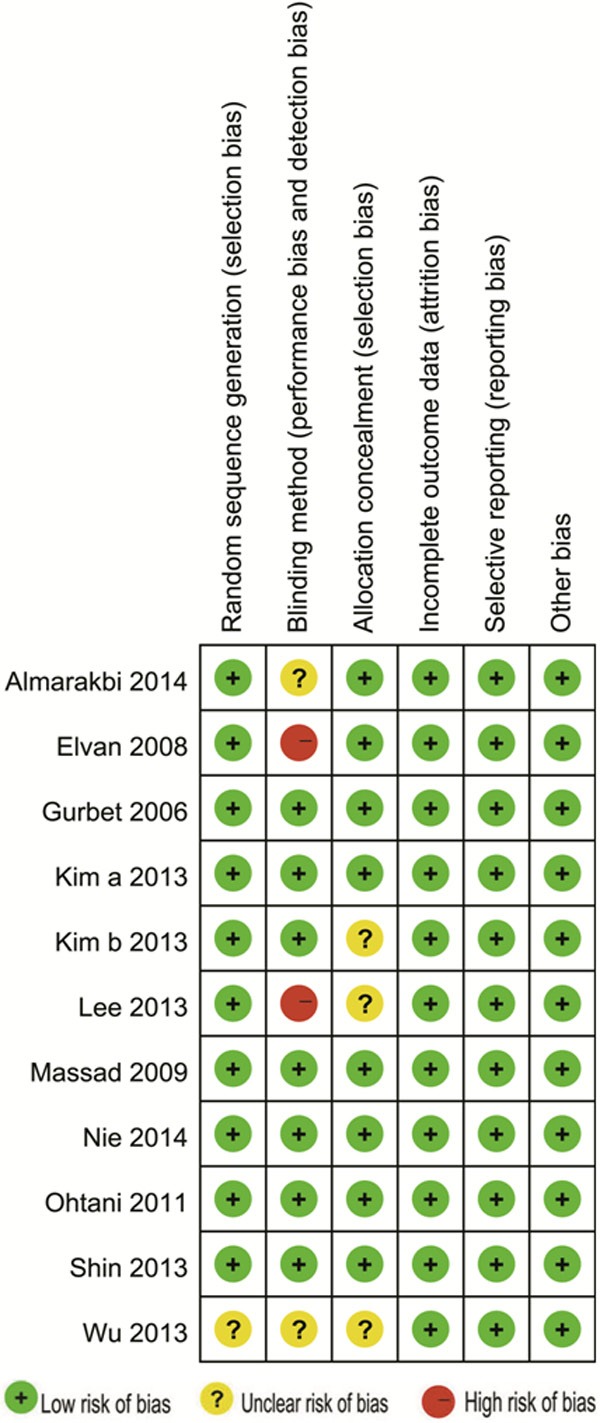
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Results of meta-analysis
PONV
Nausea
Eleven trials [12-22], comprising 692 patients, researched the efficacy of dexmedetomidine on averting nausea, the incidence of nausea (RR 0.59, 95% CI 0.44-0.79; I2 5%) in the dexmedetomidine group was significantly lower than the placebo group (Figure 3).
Figure 3.
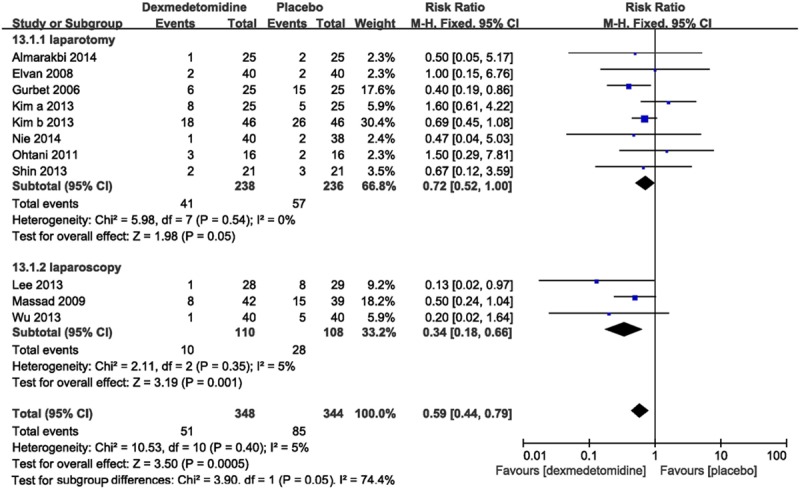
Forest plot showing RR (with 95% CI) for postoperative nausea comparing dexmedetomidine with placebo in a fixed effect model.
Subgroup analysis showed that, in dexmedetomidine group, the incidence of nausea after gynecologic operation was decreased both in laparotomy and laparoscopy, and when dexmedetomidine was infused intravenous or at the time of anesthesia induction; this reduction still existed when different regimens (single dose or loading dose followed by continuous infusion) were used (Table 2).
Table 2.
Subgroup analysis of efficacy of dexmedetomidine on nausea and vomiting
| Comparison | Number of studies | Dexmedetomidine | Placebo | RR (95% CI) | I2 | References |
|---|---|---|---|---|---|---|
| Nausea | ||||||
| Route of administration/initiated trails/dosage regimen | ||||||
| Intravenous infusion | 10 | 50/323 | 83/319 | 0.60 (0.44, 0.80) | 14% | [12,14-22] |
| Peripheral neural blockade | 1 | 1/25 | 1/25 | 0.50 (0.05, 5.17) | - | [13] |
| Induce | 9 | 49/268 | 78/266 | 0.62 (0.46, 0.84) | 13% | [13,15-22] |
| End | 2 | 2/80 | 7/78 | 0.28 (0.06, 1.31) | 0% | [13,15-22] |
| Single dose | 4 | 22/147 | 36/145 | 0.61 (0.40, 0.93) | 0% | [12,14,15,17] |
| Loading dose followed by continuous infusion: | 4 | 10/118 | 27/119 | 0.37 (0.20, 0.71) | 0% | [13,16,21,22] |
| Vomiting | ||||||
| Route of administration/initiated trails/dosage regimen | ||||||
| Intravenous infusion | 5 | 14/168 | 30/163 | 0.46 (0.27, 0.77) | 0% | [12,14,15,18,20] |
| Peripheral neural blockade | - | - | - | - | - | - |
| Induce | 3 | 13/88 | 26/85 | 0.49 (0.28, 0.84) | 0% | [15,18,20] |
| End | 2 | 1/80 | 4/78 | 0.25 (0.03, 2.14) | - | [12,14] |
| Single dose | 3 | 1/101 | 4/99 | 0.25 (0.03, 2.14) | - | [12,14,15] |
| Loading dose followed by continuous infusion: | - | - | - | - | - | - |
Egger’s Test (P=0.631) and Begg’s Test (P=0.350) suggested that no significant publication bias existed in the comparisons of nausea between dexmedetomidine and placebo.
Vomiting
Vomiting was detected in five trails [12,14,15,18,20] with 331 patients. The incidence of postoperative vomiting (RR 0.48, 95% CI 0.36-0.64; I2 0%) was significantly lower in dexmedetomidine group than placebo group (Figure 4).
Figure 4.
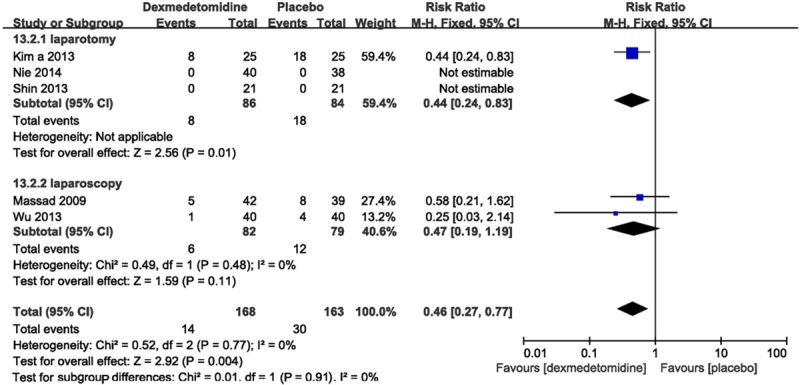
Forest plot showing RR (with 95% CI) for postoperative vomiting comparing dexmedetomidine with placebo in a fixed effect model.
Subgroup analysis imparted an efficacy of dexmedetomidine on vomiting when dexmedetomidine was infused intravenous or at the time of anesthesia induction (Table 2).
Side effects
Cardiovascular complications
Two studies [16,18] reported intra operative cardiovascular complications, including bradycardia and hypotension. The pooled analysis showed the statistically higher incidence of intraoperative bradycardia (RR 2.87, 95% CI 1.08-7.58, I2 0%) and hypotension (RR 4.26, 95% CI 1.43-12.69, I2 0%) using dexmedetomidine compared with placebo.
Shivering
There were three trials [14,16,21] reporting postoperative shivering. Compared with placebo, a statistically significant reduction in shivering (RR 0.23, 95% CI 0.13-0.40; I2 0%) was exposed in patients receiving dexmedetomidine.
Pruritus
Two studies [18,22] assessed postoperative pruritus. The pooled analysis showed a significant decrease (RR 0.40, 95% CI 0.17-0.93; I2 16%) in this side effect in dexmedetomidine group.
Headache
Postoperative headache was involved in two studies [14,18], the pooled estimate did not excluded a statistical reduction in headache (RR 1.33, 95% CI 0.31-5.74; I2 16%) in patients receiving dexmedetomidine compared with placebo (Figure 5).
Figure 5.
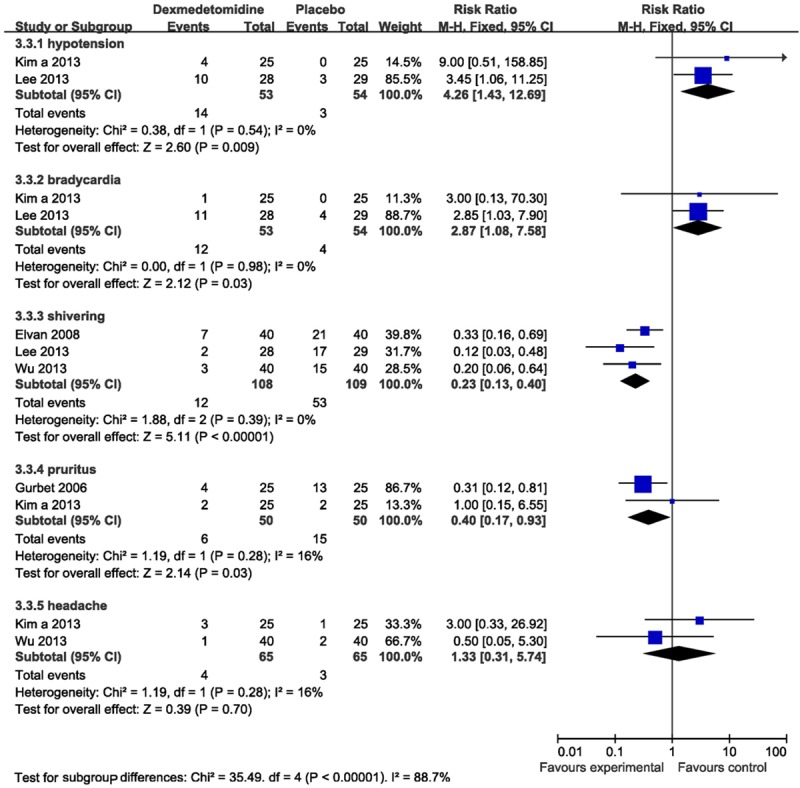
Forest plot showing RR (with 95% CI) for perioperative side effects comparing dexmedetomidine with placebo in a fixed effect model.
Rescue agents
Intraoperative rescue ephedrine
Two trials [15,21] reported the need for rescue ephedrine. The pooled analysis did not show a significant increase need for a rescue ephedrine (RR 2.60, 95% CI 0.63-10.77, I2 0%) during the surgery.
Intraoperative rescue fentanyl
Two studies [15,21] assessed the need for use of intraoperative rescue fentanyl, which was used as the only rescue analgesic drug. The pooled estimate indicated a significant reduction in the need for rescue fentanyl (RR 0.10, 95% CI 0.01-0.76, I2 0%).
Postoperative rescue antiemetic
Two studies [13,18] reported the need for postoperative rescue antiemetic, including ondansetron and metoclopramide. The pooled analysis showed a statistic diminution in the need for rescue antiemetic (RR 0.62, 95% CI 0.39-0.99, I2 0%).
Postoperative rescue analgesic
Three studies [18,20,22] assessed the need for a rescue analgesic, like morphine, propacetamol and tramadol. The pooled estimate suggested a significant decrease in the need for rescue analgesic postoperatively (RR 0.18, 95% CI 0.08-0.42, I2 0%) (Figure 6).
Figure 6.
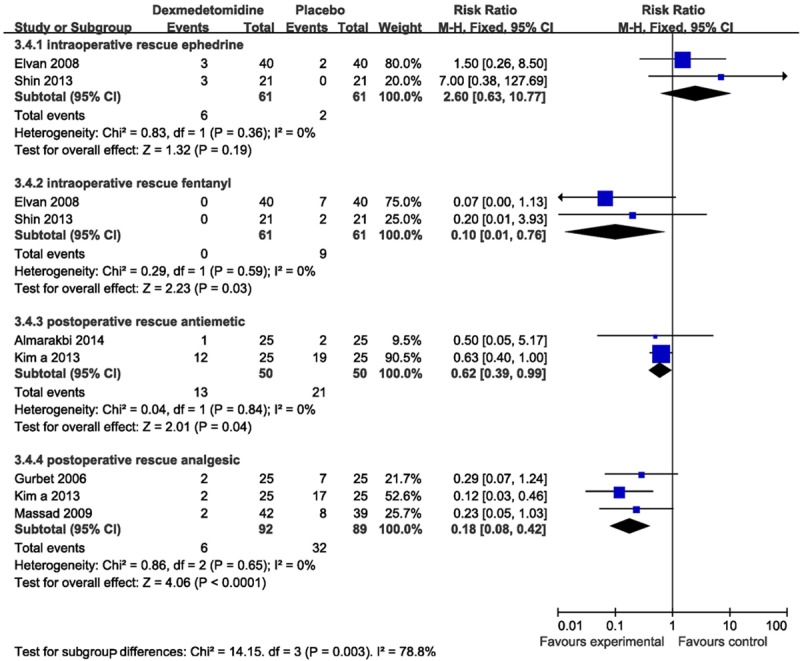
Forest plot showing RR (with 95% CI) for rescue agents comparing dexmedetomidine with placebo in a fixed effect model.
Pain scores
Two trials [15,16] measured the available pain scores using a visual analog scale. The result showed a reduction in the pain score (SMD-0.96, 95% CI-1.37--0.54, I2 0%) in dexmedetomidine group compared with placebo (Figure 7).
Figure 7.
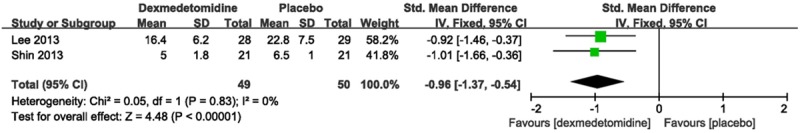
Forest plot showing RR (with 95% CI) for pain scores comparing dexmedetomidine with placebo in a fixed effect model.
Sensitivity analysis
Upon the studies with high risk were excluded by sensitivity analysis, there was no significant difference in results from overall pooled estimates across all outcomes above.
Discussion
Gynecologic surgery as a common surgical operation has a high risk of PONV, resulting in serious consequences. Despite of massive researches over the past few decades, PONV remains an extremely momentous challenge because of its complex mechanisms [23]. So we cry for an effective way to prevent PONV after gynecologic procedure.
Through this meta-analysis, we demonstrated that: (1) Dexmedetomidine showed superiority to placebo in the prevention of postoperative nausea and vomiting. Not only intravenous administration of dexmedetomidine, but also both single dose and loading dose followed by continuous infusion could reduce the incidence of nausea, but we did not exclude the efficacy of single dose on vomiting. (2) Administration of dexmedetomidine reduces adverse events, such as postoperative shivering and pruritus, while cardiovascular complications were increased. (3) Intra operative administration of dexmedetomidine diminishes the need for the rescue analgesic and antiemetic, but not intraoperative ephedrine. (4) Postoperative pain scores were decreased with intravenous administration of dexmedetomidine.
Although the biologic basis remains obscure, this beneficial antiemetic effect may be explained by direct antiemetic properties of α2 agonists. Nausea and vomiting could be induced by relatively high catecholamine concentrations, a reduction of sympathetic tone might explain the antiemetic efficacy of dexmedetomidine [20].
It is the first time to shed light on the efficacy of dexmedetomidine in gynecologic surgery on nausea and vomiting by a meta-analysis of RCTs. Most of the included trials were well designed and assessed as low risk, meanwhile studies with high risk were excluded by sensitivity analysis. Moreover all these studies within groups were in the absence of heterogeneous which increased the accuracy of outcomes.
However, this meta-analysis still has some in adequacies. Studies included in subgroups are still too little to guarantee the convincing results. Only six trials revealed the source of their funding, and we did not know if the others were supported by companies or industries, which may slant the outcomes towards the best light of drug. Therefore, more well-designed and large-scale trials are expected to confirm these findings.
In conclusion, this current meta-analysis suggested that administration of dexmedetomidine may reduce the incidence of postoperative nausea and vomiting, shivering, pruritus, as well as the pain scores. Besides the routine usage for sedation and analgesia, our results provided evidence for the extension of clinical value of dexmedetomidine. However, when we use dexmedetomidine, the potential cardiovascular complications should be considered consciously.
Disclosure of conflict of interest
None.
References
- 1.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Rashiq S, Bray P. Relative value to surgical patients and anesthesia providers of selected anesthesia related outcomes. BMC Med Inform Decis Mak. 2003;3:3. doi: 10.1186/1472-6947-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikawa K, Takao Y, Nishina K, Shiga M, Maekawa N, Obara H. Optimal dose of granisetron for prophylaxis against postoperative emesis after gynecological surgery. Anesth Analg. 1997;85:652–656. doi: 10.1097/00000539-199709000-00030. [DOI] [PubMed] [Google Scholar]
- 4.McCracken G, Houston P, Lefebvre G Society of Obstetricians and Gynecologists of Canada. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. 2008;30:600–607. 608–616. doi: 10.1016/s1701-2163(16)32895-x. [DOI] [PubMed] [Google Scholar]
- 5.Raphael JH, Norton AC. Antiemetic efficacy of prophylactic ondansetron in laparoscopic surgery: randomized, double-blind comparison with metoclopramide. Br J Anaesth. 1993;71:845–848. doi: 10.1093/bja/71.6.845. [DOI] [PubMed] [Google Scholar]
- 6.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Y, Chen GZ, Dong YL, Wang W. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9:e93114. doi: 10.1371/journal.pone.0093114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Lu Y, Huang Y, Jiang H. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Paediatr Anaesth. 2014;24:863–874. doi: 10.1111/pan.12391. [DOI] [PubMed] [Google Scholar]
- 9.Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71:1481–1501. doi: 10.2165/11207190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002;46:921–928. doi: 10.1034/j.1399-6576.2002.460801.x. [DOI] [PubMed] [Google Scholar]
- 11.Visser K, Hassink EA, Bonsel GJ, Moen J, Kalkman CJ. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with is oflurane-nitrous oxide: postoperative nausea with vomiting and economic analysis. Anesthesiology. 2001;95:616–626. doi: 10.1097/00000542-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Nie Y, Liu Y, Luo Q, Huang S. Effect of dexmedetomidine combined with sufentanil for postcaesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol. 2014;31:197–203. doi: 10.1097/EJA.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 13.Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: A prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–166. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Huang H, Zeng J, Li B, Lei X, Chen Y. [Effect of dexmedetomidine in preventing shivering after general anesthesia for laparoscopic surgery: a randomized, single-blinded, and placebo-controlled trial] . Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:611–614. [PubMed] [Google Scholar]
- 15.Shin HW, Yoo HN, Kim DH, Lee H, Shin HJ, Lee HW. Preanesthetic dexmedetomidine 1 microg/kg single infusion is a simple, easy, and economic adjuvant for general anesthesia. Korean J Anesthesiol. 2013;65:114–120. doi: 10.4097/kjae.2013.65.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64:301–307. doi: 10.4097/kjae.2013.64.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim b SH, Oh YJ, Park BW, Sim J, Choi YS. Effects of single-dose dexmedetomidine on the quality of recovery after modified radical mastectomy: a randomised controlled trial. Minerva Anestesiol. 2013;79:1248–1258. [PubMed] [Google Scholar]
- 18.Kim a SY, Chang CH, Lee JS, Kim YJ, Kim MD, Han DW. Comparison of the efficacy of dexmedetomidine plus fentanyl patient-controlled analgesia with fentanyl patient-controlled analgesia for pain control in uterine artery embolization for symptomatic fibroid tumors or adenomyosis: a prospective, randomized study. J Vasc Interv Radiol. 2013;24:779–786. doi: 10.1016/j.jvir.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani N, Yasui Y, Watanabe D, Kitamura M, Shoji K, Masaki E. Perioperative infusion of dexmedetomidine at a high dose reduces postoperative analgesic requirements: a randomized control trial. J Anesth. 2011;25:872–878. doi: 10.1007/s00540-011-1239-8. [DOI] [PubMed] [Google Scholar]
- 20.Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30:1537–1541. [PubMed] [Google Scholar]
- 21.Elvan EG, Oc B, Uzun S, Karabulut E, Coskun F, Aypar U. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. Eur J Anaesthesiol. 2008;25:357–364. doi: 10.1017/S0265021507003110. [DOI] [PubMed] [Google Scholar]
- 22.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–652. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 23.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, Bergese SD, Candiotti KA, Chan MT, Davis PJ, Hooper VD, Lagoo-Deenadayalan S, Myles P, Nezat G, Philip BK, Tramèr MR Society for Ambulatory Anesthesia. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]


