Abstract
Background: Puerarin is an effective ingredient isolated from Radix Puerariae, a leguminous plant. In China, a large number of early studies suggest that puerarin may be used in the treatment of coronary heart disease. In recent years, puerarin injection has been widely used to treat coronary heart disease and angina pectoris. Objective: To systematically evaluate the clinical efficacy and safety of puerarin injection in the treatment of unstable angina pectoris (UAP). Methods: Data were retrieved from digital databases, including PubMed, Excerpt Medica Database (EMBASE), China Biology Medicine (CBM), the Cochrane Library, and Chinese databases. Results: Compared with patients who were treated with conventional Western medicines alone, the patients who were treated with conventional Western medicines in combination with puerarin injection exhibited significant improvements in the incidence of angina pectoris, electrocardiogram findings, nitroglycerin consumption and plasma endothelin levels. Conclusions: Strong evidence suggests that, the use of puerarin in combination with conventional Western medicines is a better treatment option for treating UAP, compared with the use of conventional Western medicines alone.
Keywords: Puerarin injection, unstable angina pectoris, meta-analysis
Introduction
Angina pectoris is a clinical syndrome caused by acute temporary myocardial ischemia and hypoxia due to coronary insufficiency, and episodes of chest pain or discomfort represent the primary manifestations [1]. In the resting state, the appearance or worsening of the above symptoms is diagnosed as unstable angina pectoris (UAP) [2]. UAP is an intermediate state between chronic stable angina pectoris and acute myocardial infarction, with a tendency towards progressive deterioration, and can easily develop into acute myocardial infarction and ischemic sudden death [3]. UAP should be treated immediately once it occurs. Three treatment methods are recommended by the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines: anti-ischemic therapy, anti-platelet therapy and anti-thrombotic therapy. Anti-ischemic therapy includes nitrates, -blockers, angiotensin-converting enzyme (ACE) inhibitors, revascularization and oxygen. Anti-platelet therapy includes aspirin, clopidogrel, and glycoprotein IIb/IIIa receptor antagonists [4,5]. Anti-thrombotic therapy includes low-molecular-weight heparin. The treatment of UAP aims to alleviate ischemia and to prevent serious adverse reactions and their consequences (namely, death, myocardial infarction or re-infarction). Herbal medicine has been broadly employed in the treatment of angina pectoris in China [6].
In China, herbal medicine is often used together with Western medicines to treat UAP. Puerarin is a flavonoid glycoside that is extracted from the root of the leguminous plants Pueraria lobata and Thomson Kudzuvine Root, and its chemical name is 8-β-D-glucopyranosyl-4’,7-dihydroxyisoflavone [7]. A large number of studies suggests that puerarin has the following pharmacological effects on the cardiovascular system: (1) dilating coronary artery to relieve vasospasm, increase coronary blood flow, and thus improve the blood supply to ischemic myocardium [8]; (2) reducing blood pressure, heart rate and myocardial oxygen consumption [9]; and (3) inhibiting platelet aggregation, reducing blood viscosity, and improving microcirculation [10].
Currently, puerarin has been widely used in the treatment of UAP, but the clinical studies on its use are limited by small sample sizes and are of varying quality. For this reason, the present study gathered data from randomized controlled studies on puerarin for the treatment of UAP and evaluated the clinical efficacy and safety of puerarin in an objective and scientific manner to provide strong evidence for the use of puerarin in clinical practice.
Materials and methods
Search strategy
The data were retrieved from digital databases, including PubMed, Excerpt Medica Database (EMBASE), Chinese National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), Chinese Scientific Journal Database (VIP), Wanfang Database and the Cochrane Library. The key words used for the database searches were “puerarin injection”, “unstable angina pectoris”, and “randomized controlled trials”. Any disagreements that occurred during the searching process were resolved via discussion or consultation with a third party.
Inclusion criteria
The experimental group was treated with puerarin injection in combination with conventional Western medicines, and the control group was treated with conventional Western medicines alone. The participants were previously diagnosed with UAP.
Exclusion criteria
The clinical studies in which the comparison was not between an experimental group treated with puerarin injection in combination with conventional Western medicines and a control group treated with conventional Western medicines alone were excluded. The studies involving subjects with concurrent acute myocardial infarction, severe heart failure or liver or functional kidney failure were excluded. Studies involving patients with stable angina pectoris were excluded.
Outcome measures
The primary outcome, mortality (sudden death from acute myocardial infraction and malignant ventricular arrhythmia), was not reported in any studies. The secondary outcome measures were as follows: (1) frequency of acute attacks of angina (e.g., reductions of more than 50% in the frequency of acute angina attack), (2) improvements in electrocardiogram (ECG) findings (e.g., normal resting ECG, or elevated ST segment of 0.5 mV or more, or inverted T wave ≥ 50% or change of flat T wave to upright T wave), (3) dose and incidence of nitroglycerine taken, and (4) levels of plasma endothelin.
Data extraction and quality assessment
A standard quality assessment form [11] was used by two researchers who independently assessed each document that met the inclusion criteria and extracted the data. The extracted data included the following: (1) general information (e.g., title, study authors, and year of publication), (2) participants (e.g., sample size, baseline characteristics and diagnostics), (3) interventions and controls (e.g., dose, route, and treatment duration), (4) outcome measures, and (5) adverse side effects. Cross-checking was performed. Discrepancies were resolved through discussion or consultation with a third researcher. The quality of the methodology adopted in this study was assessed using the risk of bias table provided by the Cochrane Collaboration website, which included six aspects: (1) random sequence generation, (2) allocation sequence concealment, (3) blinding, (4) incomplete outcome data, (5) selective outcome reporting, and (6) other potential sources of bias.
Statistical analysis
The meta-analysis was performed using Stata version 12.0. The χ2 test was used for the heterogeneity test. A random effects model was employed when heterogeneity existed (P < 0.1, I2 > 50%), whereas a fixed effects model was adopted in the absence of heterogeneity (P > 0.1, I2 < 50%). Dichotomous data were assessed by relative risk (RR), and continuous variables were analyzed using weighted mean difference (WMD). The meta-analysis results were compared based on the 95% confidence interval (CI) of these two parameters to examine whether a difference was statistically significant [11]. We aimed to perform sensitivity analyses to explore the influence of each trial on the meta-analysis. A funnel plot was used to assess whether publication bias was present.
Results
Characteristics of the included studies
A total of 149 documents were retrieved. Through review of the abstracts or the full text, 41 studies [12-52] were determined to meet the inclusion criteria, as shown in Figure 1. A total of 2953 subjects were included in these 41 studies, with 1440 subjects in the control group and 1513 subjects in the puerarin injection group. The daily dose of puerarin ranged from 200 mg to 500 mg, which administered intravenously. The treatment duration lasted 7-28 days, with most lasting 14 days. Conventional Western medicines were used in most of the control groups. Puerarin injection was used in the puerarin group in addition to the treatment used in the control group. Most of the studies include reduction in attacks of acute angina attack and improvement on ECG as outcome measures, as shown in Table 1.
Figure 1.
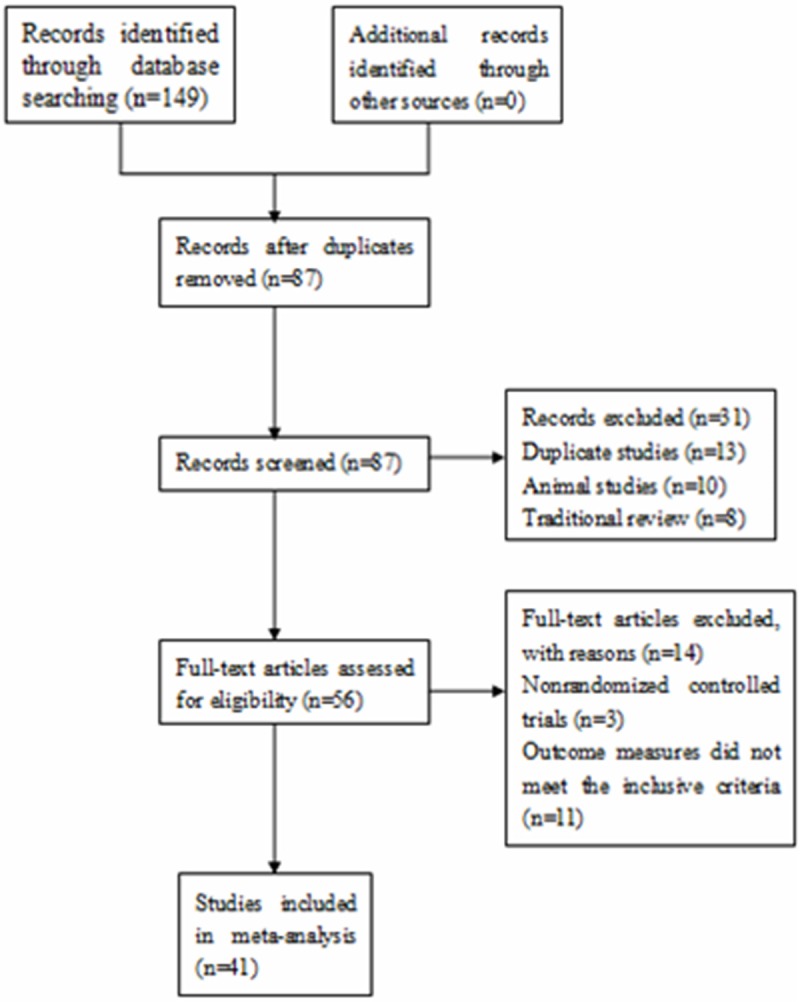
A flow chart of the study selection process.
Table 1.
Characteristics of the randomized control trials included in this study
| Study | Sample (T/C) | Diagnostic standards | Intervention | Control | Duration (days) | Outcome measure |
|---|---|---|---|---|---|---|
| An 2001 [12] | 92 (48/44) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. ET and NO | ||||||
| 5. Systolic pressure, heart rate and oxygen consumption | ||||||
| Bai 2005 [13] | 172 (86/86) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 15 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Bao 2003 [14] | 100 (50/50) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. ET and NO | ||||||
| 5. Systolic pressure, heart rate and oxygen consumption | ||||||
| 6. Haemorheology plasmic viscosity, platelet aggregation rate, fibrinogen | ||||||
| Cai 2002 [15] | 78 (40/38) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 20 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. Blood rheology parameters | ||||||
| 5. Side effects | ||||||
| Chen 2007 [16] | 71 (36/35) | ACC/AHA | Puerarin 300 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Systolic pressure, heart rate | ||||||
| 4. Serum CRP | ||||||
| Chu 2005 [17] | 51 (26/25) | Criteria from the Chinese Society of Cardiology | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Deng 2003 [18] | 61 (32/29) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 15 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Dong 1999 [19] | 74 (38/36) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 20 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. Blood rheology parameters | ||||||
| 5. Side effects | ||||||
| Gao 2005 [20] | 80 (40/40) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Side effects | ||||||
| Guo 2000 [21] | 55 (28/27) | WHO criteria | Puerarin 500mg+ Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Blood rheology parameters | ||||||
| Guo 2007 [22] | 66 (31/35) | ACC/AHA | Puerarin 500 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Side effects | ||||||
| Hu 2004 [23] | 55 (30/25) | ACC/AHA | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. Systolic pressure, heart rate and oxygen consumption | ||||||
| Huang 2002 [24] | 60 (30/30) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Dose and incidence of nitroglycerine | ||||||
| 4. Systolic blood pressure, heart rate | ||||||
| Jiang 2001 [25] | 48 (24/24) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Li 1999 [26] | 74 (38/36) | WHO criteria | Puerarin 300 mg + Western treatment | Western treatment | 20 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. Blood rheology parameters | ||||||
| 5. Side effects | ||||||
| Li 2003 [27] | 102 (58/44) | WHO criteria | Puerarin 400 m + Western treatment | Western treatment | 15 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Li 2004 [28] | 72 (36/36) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 15 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Side effects | ||||||
| Lin 2007 [29] | 60 (30/30) | Criteria from the Chinese Society of Cardiology | Puerarin 200 mg + Western treatment | Western treatment | 15 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Side effects | ||||||
| Liu 2012 [30] | 124 (62/62) | WHO criteria | Puerarin 500mg+ Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Blood rheology parameters | ||||||
| 4. Serum CRP | ||||||
| Long 2004 [31] | 57 (28/29) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Systolic pressure, heart rate and oxygen consumption | ||||||
| 4. Side effects | ||||||
| Lu 2004 [32] | 42 (21/21) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. ET |
| Luo 2000 [33] | 70 (40/30) | WHO criteria | Puerarin 500mg+ Western treatment | Western treatment | 20 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. ET and NO | ||||||
| Meng 2004 [34] | 60 (30/30) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Ren 2007 [35] | 59 (31/28) | ACC/AHA | Puerarin 400 mg + Western treatment | Western treatment | 20 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. Systolic pressure, heart rate and oxygen consumption | ||||||
| Shan 2006 [36] | 89 (46/43) | Criteria from the Chinese Society of Cardiology | Puerarin 500 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| Tang 2004 [37] | 74 (37/37) | Criteria from the Chinese Society of Cardiology | Puerarin 500 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Tao 2006 [38] | 100 (50/50) | Criteria from the Chinese Society of Cardiology | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| Wang 2009 [39] | 86 (44/42) | WHO criteria | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Wang 2011 [40] | 60 (30/30) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Xi 2012 [41] | 76 (38/38) | Criteria from the Chinese Society of Cardiology | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Side effects | ||||||
| Yang 2001 [42] | 46 (26/20) | Criteria from the Chinese Society of Cardiology | Puerarin 500 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Yang 2004 [43] | 100 (52/48) | Criteria from the Chinese Society of Cardiology | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Yang 2005 [44] | 50 (32/28) | WHO criteria | Puerarin 250 mg + Western treatment | Western treatment | 10 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Systolic pressure, heart rate and oxygen consumption | ||||||
| 4. Side effects | ||||||
| Yang 2008 [45] | 68 (36/32) | WHO criteria | Puerarin 300 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Blood rheology parameters | ||||||
| 4. Side effects | ||||||
| Yuan 2003 [46] | 40 (20/20) | WHO criteria | Puerarin 500 mg + Western treatment | Western treatment | 20 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Heart rate and oxygen consumption | ||||||
| Yuan 2006 [47] | 92 (48/44) | Criteria from the Chinese Society of Cardiology | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Side effects | ||||||
| Zhang 2000 [48] | 40 (20/20) | WHO criteria | Puerarin 300 mg + Western treatment | Western treatment | 10 | 1. ET |
| 2. Diastolic function | ||||||
| Zhang 2008 [49] | 80 (40/40) | Criteria from the Chinese Society of Cardiology | Puerarin 500 mg + Western treatment | Western treatment | 28 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Zhao 1998 [50] | 39 (21/18) | ACC/AHA | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| 3. Decrease in usage of nitroglycerine | ||||||
| 4. Systolic pressure, heart rate and oxygen consumption | ||||||
| Zheng 2006 [51] | 60 (30/30) | Criteria from the Chinese Society of Cardiology | Puerarin 400 mg + Western treatment | Western treatment | 14 | 1. Frequency and length of angina attacks |
| 2. ECG improvement | ||||||
| Zhou 2011 [52] | 60 (30/30) | ACC/AHA | Puerarin 500 mg + Western treatment | Western treatment | 7 | 1. Frequency and length of angina attacks |
| 2. ECG improvement |
Risk of bias assessment
The quality of the included studies was assessed based on the following aspects: (1) Randomization: the word “randomized” was mentioned in all the studies, but only two of the studies [49,51] reported the detailed method of randomization; (2) Allocation sequence concealment: no description in any of the documents; and (3) Selective outcome reporting: all of the studies exhibited a low risk. The detailed results are presented in Figure 2 and Table 2.
Figure 2.
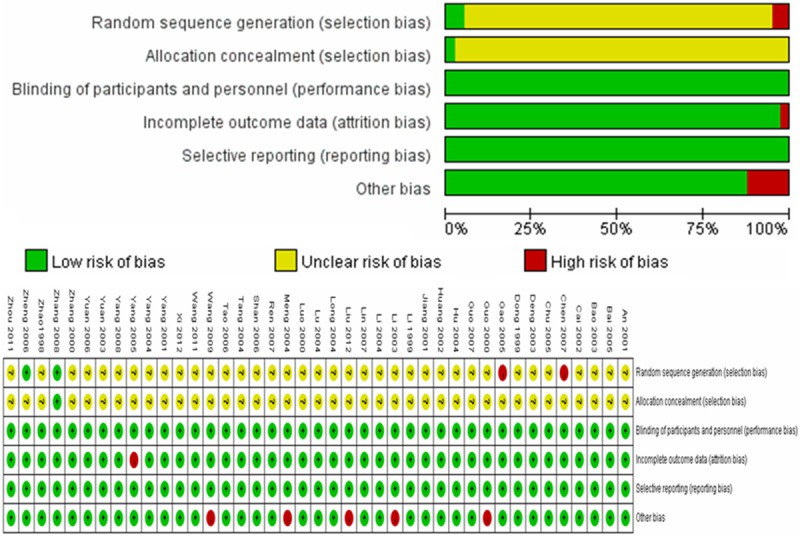
Risk of bias amongst included studies.
Table 2.
Methodological qualities of the included studies
| Study | Risk of bias for randomization | Risk of bias for concealment | Risk of bias for blinding | Risk of bias for incomplete data | Risk of bias for selective outcome reporting | Risk bias for other problem |
|---|---|---|---|---|---|---|
| An 2001 [12] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Bai 2005 [13] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Bao 2003 [14] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Cai 2002 [15] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Chen 2007 [16] | High risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Chu 2005 [17] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Deng 2003 [18] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Dong 1999 [19] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Gao 2005 [20] | High risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Guo 2000 [21] | Unclear | Unclear | Low risk | Low risk | Low risk | High risk |
| Guo 2007 [22] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Hu 2004 [23] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Huang 2002 [24] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Jiang 2001 [25] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Li 1999 [26] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Li 2003 [27] | Unclear | Unclear | Low risk | Low risk | Low risk | High risk |
| Li 2004 [28] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Lin 2007 [29] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Liu 2012 [30] | Unclear | Unclear | Low risk | Low risk | Low risk | High risk |
| Long 2004 [31] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Lu 2004 [32] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Luo 2000 [33] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Meng 2004 [34] | Unclear | Unclear | Low risk | Low risk | Low risk | High risk |
| Ren 2007 [35] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Shan 2006 [36] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Tang 2004 [37] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Tao 2006 [38] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Wang 2009 [39] | Unclear | Unclear | Low risk | Low risk | Low risk | High risk |
| Wang 2011 [40] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Xi 2012 [41] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Yang 2001 [42] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Yang 2004 [43] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Yang 2005 [44] | Unclear | Unclear | Low risk | High risk | Low risk | Low risk |
| Yang 2008 [45] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Yuan 2003 [46] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Yuan 2006 [47] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Zhang 2000 [48] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Zhang 2008 [49] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zhao 1998 [50] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Zheng 2006 [51] | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Zhou 2011 [52] | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
Primary outcomes
After a follow-up period of three months, a study [41] reported two cases of acute myocardial infarction in the control group, where no incidents of myocardial infarction were reported in the experimental treatment group. In this reported study, there were two cases of death (one case due to heart failure and one case due to ventricular fibrillation) in the control group and one death case in the experimental treatment group (heart failure).
Frequency of acute angina attack
Thirty-nine studies [12-31,33-47,49-52] reported the number of angina pectoris attacks in patients with UAP after treatment with puerarin injection. The heterogeneity test indicated that no statistical heterogeneity was present (P = 0.184); thus, a fixed effects model was adopted for analysis. The results indicated that puerarin in combination with conventional Western medicines reduced the number of angina pectoris attacks in UAP patients relative to treatment with conventional Western medicines alone (RR = 1.29, 95% CI: 1.24 to 1.34, Z = 13.07, P < 0.001) (Figure 3).
Figure 3.
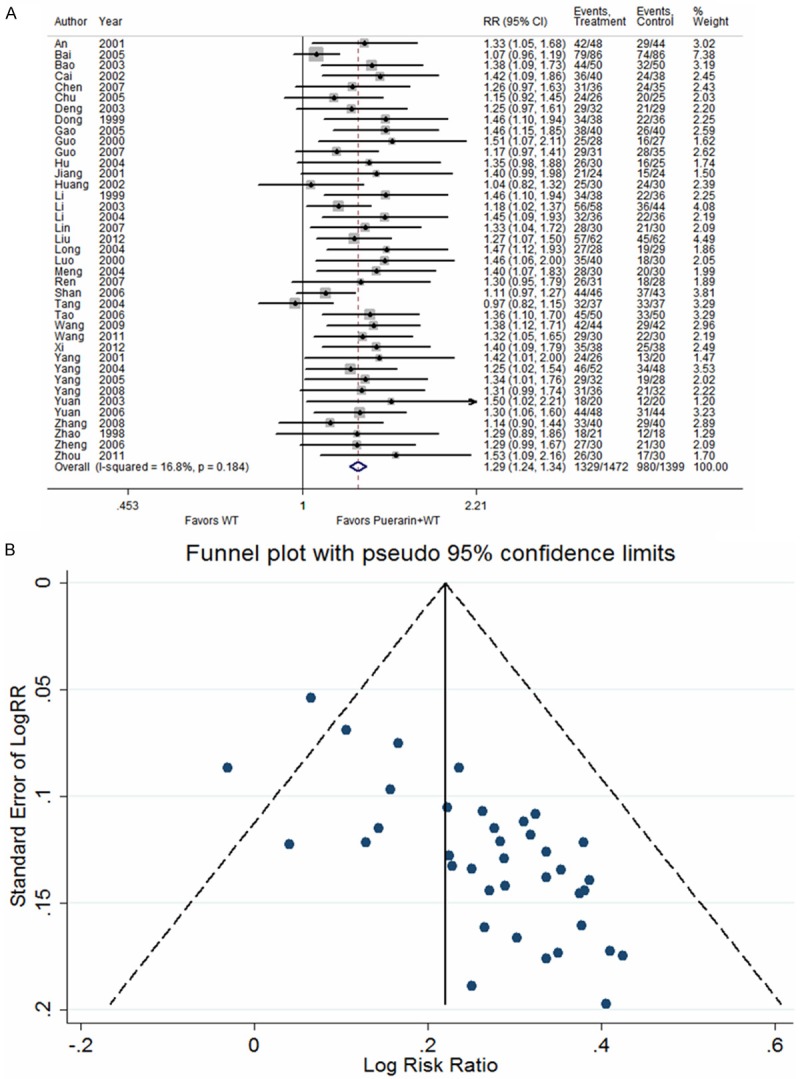
Puerarin injection plus western treatment versus western treatment, Outcome 1 Reduction in attacks of acute angina attack (more than 50%). A. Forest plots; B. Funnel plots.
ECG
Thirty-seven studies [12-14,16-31,34-47,49-52] reported improvements in the ECG of patients with UAP after treatment with puerarin injection. The heterogeneity test indicated no statistical heterogeneity (P = 0.369); thus, a fixed effects model was employed for analysis. The results indicated that puerarin in combination with conventional Western medicines improved ECG in UAP patients more than did conventional Western medicines alone (RR = 1.33, 95% CI: 1.27 to 1.39, Z = 12.30, P < 0.001) (Figure 4).
Figure 4.
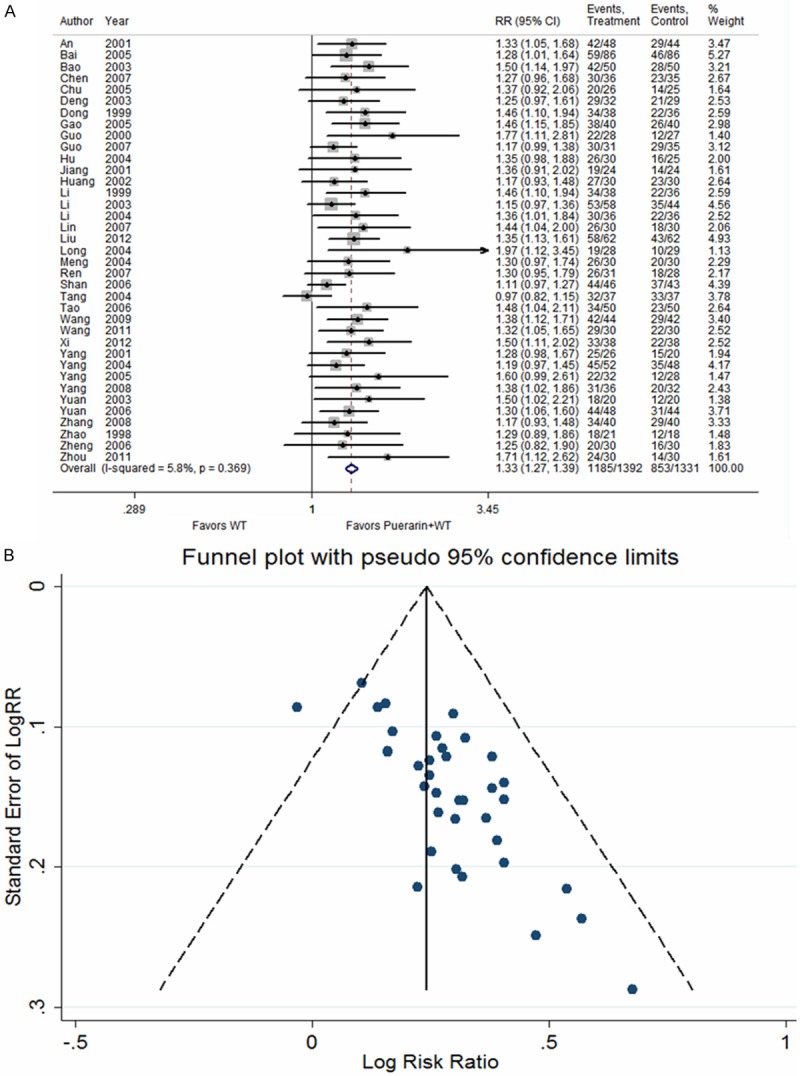
Puerarin injection plus western treatment versus western treatment, Outcome 2 Improvement of ECG. A. Forest plots; B. Funnel plots.
The level of plasma endothelin
Four studies [12,32,33,48] reported the plasma endothelin (ET) levels in patients with UAP after treatment with puerarin injection. The heterogeneity test indicated no statistical heterogeneity (P = 0.545); thus, a fixed effects model was used for analysis. The results demonstrated that puerarin in combination with conventional Western medicines markedly reduced the plasma ET levels relative to patients treated with conventional Western medicines alone (WMD = -11.04, 95% CI: -13.20 to -8.88, Z = 10.03, P < 0.001) (Figure 5).
Figure 5.
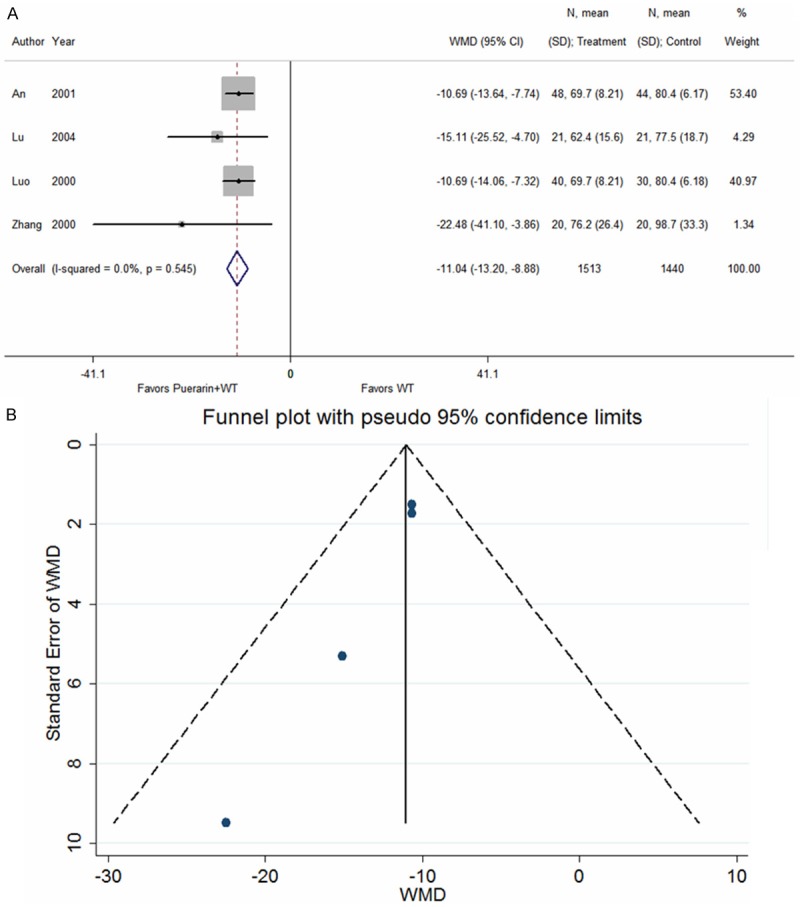
Puerarin injection plus western treatment versus western treatment, Outcome 3 Plasma endothelin concentration (pg/ml). A. Forest plots; B. Funnel plots.
Daily dose of nitroglycerine
Seven studies [15,19,23,26,35,38,50] reported the daily dose of nitroglycerine in patients with UAP after treatment with puerarin injection. The heterogeneity test indicated no statistical heterogeneity (P = 0.804); thus, a fixed effects model was adopted for analysis. The results indicated that the daily dose of nitroglycerin in patients treated with puerarin in combination with conventional Western medicines was significantly reduced relative to patients treated with conventional Western medicines alone (WMD = -0.82, 95% CI: -0.86 to -0.79, Z = 47.32, P < 0.001) (Figure 6).
Figure 6.
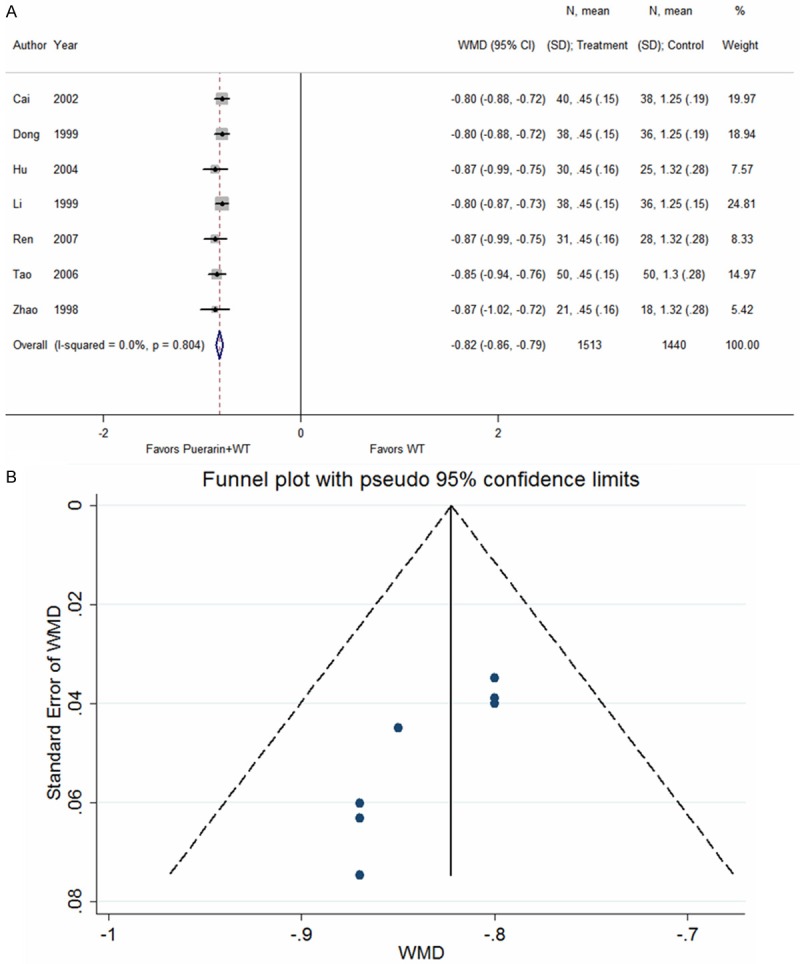
Puerarin injection plus western treatment versus western treatment, Outcome 4 Daily dose of nitroglycerine. A. Forest plots; B. Funnel plots.
Sensitivity analysis
No significant changes were observed in any efficacy indicator after excluding one study at a time.
Publication bias
The funnel plot of the number of angina pectoris attacks and ECG improvement revealed asymmetry, indicating possible publication bias, which is likely related to unpublished negative results, missing follow-up visits, and small sample sizes (Figures 3A, 4A). Vickers found that in China, a large proportion of published studies reported positive results within the complementary medicine fields [53].
Adverse reactions
Among the 41 included studies, 24 studies [13,15,19,20,22,24,26,28,29,31,41,44,45,47] described adverse reactions in detail, and no adverse reactions were observed in 10 studies [18,23,35-38,43,50-52]. The adverse reactions included transient headache and fever [15,19,22,24,26,31,44], dizziness [24], hypotension [13], abdominal distension and nausea [24,28,41,44,45], allergic skin reactions [20,22,29], and sinus bradycardia [47]. All of the adverse reactions were mild, with spontaneous remission and no effect on treatment. No serious adverse reactions were reported. Adverse reactions were not mentioned in the other 17 studies [12,14,16,17,21,25,27,30,32-34,39,40,42,46,48,49].
Discussion
Relative to conventional Western medicines alone, the analysis results from this study demonstrated that the addition of puerarin injection increased treatment efficacy in patients with UAP, resulting in improved ECG outcomes and reduction in the number of angina pectoris attacks, nitroglycerine doses and plasma ET levels. A total of 41 randomized studies including 2953 participants were included. All the RCTs claimed that the positive effect of puerarin injection combined with conventional drugs was better than conventional drugs alone. However, because of the various durations of treatment and diverse reporting methods in included trials, we still could not make firm conclusions. Furthermore, the methodological quality of all studies was limited.
The systematic review of this study has its limitations. First, the quality of the included RCTs is poor. Second, publication and other biases may play an important role. The included studies were all conducted in China. Among these studies, many have small sample sizes and positive findings. Third, only two of the studies [49,51] reported that random sequence was generated by random number table; most studies were not reported. Fourth, blinding of participants and personnel and blinding of outcome assessment were not detailed description. We made attempts to avoid any language or location biases. However, potential publication bias cannot be ruled out.
The systematic review of this study included three diagnosis criterions: “ACC/AHA Guideline for the Diagnosis and Management of Patients with Unstable Ischemic Heart Disease (ACC/AHA)”, “the International society and Federation of Cardiology/World Health Organization (ISFC/WHO)” and “Criteria from the Chinese Society of Cardiology” to diagnose unstable angina patients. ECG improvement, reduction in the number of angina pectoris attacks, nitroglycerine doses and plasma ET levels were used to evaluate the efficacy of puerarin injection for UAP. The main findings of this systematic review were that puerarin injection combined with conventional drugs confirmed that the addition of puerarin injection increased treatment efficacy in patients with UAP, resulting in ECG improvement (RR = 1.33, 95% CI: 1.27 to 1.39, Z = 12.30, P < 0.001) and reduction in the number of angina pectoris attacks (RR = 1.29, 95% CI: 1.24 to 1.34, Z = 13.07, P < 0.001), nitroglycerine doses (WMD = -0.82, 95% CI: -0.86 to -0.79, Z = 47.32, P < 0.001) and plasma ET levels (WMD = -11.04, 95% CI: -13.20 to -8.88, Z = 10.03, P < 0.001).
Several reviews [54,55] on the pharmacological effects of puerarin were detailed summarized. However, Puerarin has numerous effects on the cardiovascular system: (1) Anti-arrhythmia effect: Puerarin inhibits voltage-gated K+ channels and Na+ channels in rat ventricular myocytes, which may partly contribute to its anti-arrhythmic property [56,57]. Inhibition of K+ channels has been shown to prolong the duration of the action potential and the refractory period in cardiomyocytes and hence prevent arrhythmia [58,59]. Blocking Na+ channels can significantly suppress the velocity of cardiac impulse conduction and prolong the refractory period of cardiac excitability, thereby inhibiting arrhythmia progression [60]. (2) Puerarin has been shown to lower blood pressure and decelerate rhythm of the heart, relieve myocardial ischemic damage through blocking the P2X3 signaling transmission and then depressed the aggravated sympathoexcitatory reflex [61]. (3) Vascular dilation to improve microcirculation: Puerarin can also produce a non-endothelium-dependent vasodilator effect, which is primarily achieved through the cyclic adenosine mono-phosphate (cAMP) pathway [62]. Dong’s study showed that puerarin induces an endothelium-independent vasorelaxant effect on rat aortic rings. Its mechanism may involve the reduction in Ca2+ influx through the non-voltage-sensitive calcium channels and the activation of the potassium channels [63]. (4) Protective effects on cardiac hypertrophy: Puerarin attenuates Ang II-induced cardiac hypertrophy by inhibiting activation of the redox-sensitive ERK1/2, p38 and the NF-κB pathways [64]. Puerarin may have an ability to retard the progression of cardiac hypertrophy and apoptosis which is probably mediated by the blockade of PI3K/Akt and JNK signaling pathways [65]. (5) Protective effect on the myocardial ischemia and reperfusion injury: Puerarin has been shown that the acylated modification of phenolic hydroxyl at C-7 in the molecular may improve the cardioprotective activity [66]. Gao et al [67] also revealed that puerarin relieve ischemia-reperfusion injury through promoting the opening of calcium-activated potassium channels and the activation of protein kinase C. Tang et al [68] revealed the protective effects of puerarin in cardiomyocytes from ischemia and reperfusion injury via the protein kinase C epsilon signaling pathway. These may be account for the improvement of puerarin injection for the Treatment of Unstable Angina Pectoris.
In summary, when used in combination with conventional angina pectoris treatment, such as nitrates, β-blockers, ACE inhibitors, anti-platelets, and anti-thrombotics, puerarin injection can further improve treatment efficacy in UAP patients, reducing the incidence of angina pectoris attacks and the required dose of nitroglycerin, improve ECG outcomes and decrease plasma ET levels. As an effective treatment, puerarin does not exhibit significant differences in side effects relative to conventional therapies and therefore should be promoted for clinical application. However, only a limited number of studies were included in this systematic review, and these studies have small samples sizes and are of low quality. Therefore, further review of more rigorous, multi-center, randomized, controlled clinical studies with large sample sizes and long-term follow-up is required to demonstrate the efficacy of puerarin injection and to better guide the clinical treatment of angina pectoris.
Disclosure of conflict of interest
None.
References
- 1.Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979;59:607–609. doi: 10.1161/01.cir.59.3.607. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Morrow DA. Unstable angina: is it time for a requiem? Circulation. 2013;127:2452–2457. doi: 10.1161/CIRCULATIONAHA.113.001258. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Pollack CV Jr, Braunwald E. 2007 update to the ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med. 2008;51:591–606. doi: 10.1016/j.annemergmed.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Braunwald E. Unstable angina and non-ST-segment elevation myocardial infarction: part I. Initial evaluation and management, and hospital care. Ann Emerg Med. 2004;70:525–532. [PubMed] [Google Scholar]
- 6.Tang JL, Liu BY, Ma KW. Traditional Chinese medicine. Lancet. 2008;372:1938–1940. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 7.Maji AK, Pandit S, Banerji P, Banerjee D. Puerarin tuberose: a review on its phytochemical and therapeutic potential. Nat Prod Res. 2014;28:2111–2127. doi: 10.1080/14786419.2014.928291. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Lam TN, Zuo Z. Radix puerariae: an overview of its chemistry, pharmacology, pharmacokinetics and clinical use. J Clin Pharmacol. 2013;53:787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 9.Song XP, Chen PP, Chai XS. Effects of puerarin on blood pressure and plasma renin activity in spontaneously hypertensive rats. Acta Pharmacol Sin. 1988;9:55–58. [PubMed] [Google Scholar]
- 10.Wei SY, Chen Y, Xu XY. Progress on the pharmacological research of puerarin: a review. Chin J Nat Med. 2014;12:407–414. doi: 10.1016/S1875-5364(14)60064-9. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Green S. Cochrane Handbook for Systematic reviews of Interventions. The Cochrane Collaboration. 2011 [Google Scholar]
- 12.An JR, Zhang H, Cai XZ, Zheng Q, Fu Q, Sun Q. Effect observation of puerarin injection for unstable angina pectoris. Chin J Clin Pharmacol Ther. 2001;6:244–245. [Google Scholar]
- 13.Bai ZS, Zhao QR, Zheng GS. Clinical observation of puerarin injection for unstable angina pectoris. J Prac Med Tech. 2005;12:1886–1887. [Google Scholar]
- 14.Bao XM. Observation on curative effects of puerarin injection in the patients with unstable angina pectoris. Medical Journal of Communications. 2003;17:12–13. [Google Scholar]
- 15.Cai HL, Liu YH. Observation on curative effects of puerarin injection in the patients with unstable angina pectoris. Digest of the World Latest Medical Information. 2002;1:351–352. [Google Scholar]
- 16.Chen TH. Clinical observation of 71 cases of puerarin for unstable angina pectoris. Journal of Jinggangshan University. 2007;28:80–81. [Google Scholar]
- 17.Chu JX, Zeng YW. Clinical observation of 26 cases of puerarin injection for unstable angina pectoris. Hunan Journal of Traditional Chinese Medicine. 2005;21:17–18. [Google Scholar]
- 18.Deng MY. Clinical observation of 32 cases of puerarin injection for unstable angina pectoris. Hainan Medicine Journal. 2003;14:14–15. [Google Scholar]
- 19.Dong PS, Liu YH, Song RL. Observation on curative effects of puerarin injection for unstable angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 1999;6:355–356. [Google Scholar]
- 20.Gao DL. Clinical observation of 40 cases of puerarin for unstable angina pectoris. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2005;14:72. [Google Scholar]
- 21.Guo YJ, Cen YW, Zhao HF. Observation on curative effects of puerarin injection in the patients with unstable angina pectoris. Journal of Youjiang Medical College for Nationalities. 2000;5:716–717. [Google Scholar]
- 22.Guo XF, Wu YF, Cao YF. Observation on curative effects of puerarin injection in the patients with unstable angina pectoris. Chinese Journal of Hemorheology. 2007;17:574–575. [Google Scholar]
- 23.Hu FM. Clinical observation of puerarin injection for unstable angina pectoris. Medical Journal of Chinese People Health. 2004;16:196–197. [Google Scholar]
- 24.Huang XF, Lu YL, Fan XN. Clinical observation of puerarin for unstable angina pectoris. Journal of Medicine Theory and Practice. 2002;15:776–777. [Google Scholar]
- 25.Jiang RY. Clinical observation of 48 cases of puerarin injection for unstable angina pectoris. Journal of Xianning Medical College. 2001;15:197–198. [Google Scholar]
- 26.Li Y, Liu YM. Observation on curative effects of puerarin injection in the patients with unstable angina pectoris. Journal of Luoyang Medical College. 1999;17:293–294. [Google Scholar]
- 27.Li HC, Ji SJ. Clinical observation of puerarin injection for unstable angina pectoris. Central Plains Medical Journal. 2003;30:24–25. [Google Scholar]
- 28.Li NX, Cui CL, Ma TJ, Zhang YY. Clinical observation of 36 cases of puerarin for unstable angina pectoris. Chinese Journal of Clinical Pharmacy. 2004;13:69–70. [Google Scholar]
- 29.Lin LZ, Luo F. Clinical observation of 30 cases of puerarin for unstable angina pectoris. Jilin Journal of Traditional Chinese Medicine. 2007;27:14–15. [Google Scholar]
- 30.Liu Y, Guo AW, Lu Q, Geng HH. Effect of puerarin on the high-sensitivity C-reactive protein and haemorheology in patients with unstable angina pectoris. Medical Journal of Communication. 2012;26:452–453. [Google Scholar]
- 31.Long B, Zhou XM, Cao HF, et al. Clinical assessment of puerarin injection for unstable angina pectoris. West China Journal of Pharmaceutical Sciences. 2004;19:234–235. [Google Scholar]
- 32.Lu YH. Effect of puerarin on the plasma endothelin level in patients with unstable angina pectoris. Jiangxi Journal of Traditional Chinese Medicine. 2004;35:20–21. [Google Scholar]
- 33.Luo ZR, Gai XB, Zheng WX. Effects of puerarin on unstable angina pectoris and its coagulant, fibrinolytic and function of endothelial cells. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2000;7:105–106. [Google Scholar]
- 34.Meng QG. Clinical observation of unstable angina treated by puerarin injection its effect on oxidized low-density lipoprotein. Hebei Journal of Traditional Chinese Medicine. 2004;26:779–780. [Google Scholar]
- 35.Ren M, Suo Z, Zhou HT. Clinical observation of puerarin for unstable angina pectoris. Chinese Journal of Information Traditional Chinese Medicine. 2007;14:49–50. [Google Scholar]
- 36.Shan LX, Xu YZ, Wang YF. Observation on curative effects of 89 cases of puerarin for unstable angina pectoris. Chinese Journal of Clinical Healthcare. 2006;9:495–496. [Google Scholar]
- 37.Tang HL, Xiong J. Clinical observation of puerarin injection in the patients with unstable angina pectoris. Journal of Chengdu University of Traditional Chinese Medicine. 2004;27:45–47. [Google Scholar]
- 38.Tao HX. Observation on curative effects of puerarin injection for unstable angina pectoris. Medical Journal of Chinese People Health. 2006;18:703. [Google Scholar]
- 39.Wang PX. Efficacy of puerarin injection for unstable angina pectoris. Chinese Journal of Misdiagnostics. 2009;9:6123–6124. [Google Scholar]
- 40.Wang ZZ, Gu ZC. Observation on curative effects of puerarin injection for unstable angina pectoris. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2011;9:1382. [Google Scholar]
- 41.Xi ZR, Jin XQ. Clinical observation of 38 cases of puerarin for unstable angina pectoris. Journal of Guiyang Medicine College. 2012;37:670–671. [Google Scholar]
- 42.Yang NL. Observation on curative effects of puerarin injection for unstable angina pectoris. Chinese Journal of Rural Medicine and Pharmacy. 2001;8:18. [Google Scholar]
- 43.Yang QK. Observation on curative effects of puerarin injection for unstable angina pectoris. Journal of Modern Medicine Health. 2004;20:2613–2614. [Google Scholar]
- 44.Yang JZ, Zeng YM, Xiong HS. Clinical evaluation of puerarin combining routine drugs in the treatment of unstable angina pectoris. China Pharmacy. 2005;16:848–849. [Google Scholar]
- 45.Yang Z. Clinical observation of puerarin injection for unstable angina pectoris. Youjiang Medicine Journal. 2008;36:21–22. [Google Scholar]
- 46.Yuan ZY, Jin PK. Observation on curative effects of puerarin injection for unstable angina pectoris. Clinical Focus. 2003;18:640–641. [Google Scholar]
- 47.Yuan XH. Clinical observation of 92 cases of puerarin for unstable angina pectoris. Journal of Clinical Emergency Call. 2006;7:310–311. [Google Scholar]
- 48.Zhang SR, Liu GT, Fang JP, Dong L, Liu GT, Hao YM. Effect of puerarin on the endothelin and function of left ventricular in patients with unstable angina pectoris. Information on Traditional Chinese Medicine. 2000;7:47–49. [Google Scholar]
- 49.Zhang PY, Wang ZL, Liu M, Zhang YQ, Li JL, Liang T, Mei FG, Zhao G. Clinical study of puerarin injection for unstable angina pectoris. Journal of Liaoning University of Traditional Chinese Medicine. 2008;10:3–4. [Google Scholar]
- 50.Zhao Z, Yang X, Zhang Y. Clinical study of puerarin in the treatment of patients with unstable angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine. 1998;18:282–284. [PubMed] [Google Scholar]
- 51.Zheng Y, Wang HH. Clinical observation of puerarin for unstable angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2006;13:189–190. [Google Scholar]
- 52.Zhou JL, Ma T, Ni H. Observation on curative effects of puerarin injection for unstable angina pectoris. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2011;9:1258–1259. [Google Scholar]
- 53.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 54.Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 55.Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol. 2011;134:584–607. doi: 10.1016/j.jep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Miao WN, Shen YJ, Zeng XR. Effect of puerarin on K+ channel of isolated ventricular myocytes in guinea pig. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2000;18:155–158. [PubMed] [Google Scholar]
- 57.Zhang GQ, Hao XM, Dai DZ, Fu Y, Zhou PA, Wu CH. Puerarin blocks Na+ current in rats ventricular myocytes. Acta Pharmacol Sin. 2003;24:1212–1216. [PubMed] [Google Scholar]
- 58.Findlay I, Suzuki S, Murakami S, Kurachi Y. Physiological modulation of voltage-dependent inactivation in the cardiac muscle L-type calcium channel: a modelling study. Prog Biophys Mol Biol. 2008;96:482–498. doi: 10.1016/j.pbiomolbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Ravens U, Cerbai E. Role of potassium currents in cardiac arrhythmias. Europace. 2008;10:1133–1137. doi: 10.1093/europace/eun193. [DOI] [PubMed] [Google Scholar]
- 60.Catterall WA. Molecular mechanisms of gating and drug block of sodium channels. Novartis Foundation Symp. 2002;241:206–218. [PubMed] [Google Scholar]
- 61.Liu S, Zhang C, Shi Q, Li G, Song M, Gao Y, Xu C, Xu H, Fan B, Yu S, Zheng C, Zhu Q, Wu B, Peng L, Xiong H, Wu Q, Liang S. Puerarin blocks the signaling transmission mediated by P2X3 in SG and DRG to relieve myocardial ischemic damage. Brain Res Bull. 2014;101:57–63. doi: 10.1016/j.brainresbull.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Yeung DK, Leung SW, Xu YC, Vanhoutte PM, Man RY. Puerarin, an isoflavonoid derived from Radix puerariae, potentiates endothelium-independent relaxation via the cyclic AMP pathway in porcine coronary artery. Eur J Pharmacol. 2006;552:105–111. doi: 10.1016/j.ejphar.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 63.Dong K, Tao QM, Shan QX, Jin HF, Pan GB, Chen JZ, Zhu JH, Xia Q. Endotheliumindependent vasorelaxant effect of puerarin on rat thoracic aorta. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3757–3760. doi: 10.1109/IEMBS.2004.1404054. [DOI] [PubMed] [Google Scholar]
- 64.Chen G, Pan SQ, Shen C, Pan SF, Zhang XM, He QY. Puerarin inhibits angiotensin II-induced cardiac hypertrophy via the redox-sensitive ERK1/2, p38 and NF-κB pathways. Acta Pharmacol Sin. 2014;35:463–475. doi: 10.1038/aps.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan Y, Zong J, Zhou H, Bian ZY, Deng W, Dai J, Gan HW, Yang Z, Li H, Tang QZ. Puerarin attenuates pressure overload-induced cardiac hypertrophy. J Cardiol. 2014;63:73–81. doi: 10.1016/j.jjcc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Feng ZQ, Wang YY, Guo ZR, Chu FM, Sun PY. The synthesis of puerarin derivative and their protective effect on the myocardial ischemia and reperfusion injury. J Asian Nat Prod Res. 2010;12:843–850. doi: 10.1080/10286020.2010.505563. [DOI] [PubMed] [Google Scholar]
- 67.Gao Q, Yang B, Ye ZG, Wang J, Bruce IC, Xia Q. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574:179–184. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Tang L, Liu D, Yi X, Xu T, Liu Y, Luo Y, Yin D, He M. The protective effects of puerarin in cardiomyocytes from anoxia/reoxygenation injury are mediated by PKCε. Cell Biochem Funct. 2014;32:378–386. doi: 10.1002/cbf.3026. [DOI] [PubMed] [Google Scholar]


