Abstract
Background: Recently, the association of immunological checkpoint marker programmed death ligand-1 (PD-L1) and the prognosis of various cancers has always been a research hotspot. The objective of this study is to assess the clinical value of PD-L1 as a novel prognostic biomarker of renal cell carcinoma (RCC). Methods: Medline/PubMed, EMBASE, the Cochrane Library databases and Grey literature were searched up to 30 March 2015 for eligible studies of the association between PD-L1 expression and cancer-specific survival (CSS) in RCC. The risk ratio (RR) and its 95% confidence interval (CI) were calculated from the included studies. Moreover, the odds ratio (OR) was also extracted to evaluate the association between the clinicopathological parameters of participants and PDL1 expression. Results: Five studies involving 1073 patients were included in the meta-analysis. The pooled results showed that positive/higher PD-L1 expression was a negative predictor for CSS with RR of 2.90 (95% CI: 1.64-5.13; Pheterogeneity. ≤ 0.001). Additionally, increased PD-L1 was found to be significantly associated with large tumor size (OR = 2.28, 95% CI: 1.61-3.23; Pheterogeneity. = 0.645), high TNM stage (OR = 4.37, 95% CI: 2.99-6.39; Pheterogeneity. = 0.676), poor nuclear grade (OR = 7.58, 95% CI: 5.26-10.92; Pheterogeneity. = 0.203) and present tumor necrosis (OR = 6.77, 95% CI: 4.73-9.71; Pheterogeneity. = 0.111) in renal cell carcinoma patients. Conclusion: The meta-analysis suggested that PD-L1 could act as a significant biomarker in the worse prognosis and adverse clinicopathologic features of renal cell carcinoma.
Keywords: PD-L1, renal cell carcinoma, prognosis, meta-analysis
Introduction
In 2015, an estimated 61,560 new cases in the United States will be diagnosed with cancers of the kidney and renal pelvis, the vast majority of which are renal cell carcinoma (RCC), with an estimated 14,080 deaths [1]. To date, as RCC appeared to be one of the most therapy-resistant malignancies, responding very poorly or not at all to hormonal therapy, radiotherapy and chemotherapy. This led investigators to focus on the new immunotherapeutic strategies [2]. Immune checkpoint blockade with antibodies that target cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and the programmed cell death protein 1 pathway has shown to mediate tumor shrinkage, extend overall survival and demonstrate promise in a variety of malignancies, including RCC [3]. On the other hand, certain immunological checkpoint markers have been reported [4]. Among them, programmed death ligand-1 (PD-L1) has been the focus of research.
PD-L1, also known as B7 homolog 1 (B7-H1) or CD274, is an important member of the B7/CD28 costimulatory factor superfamily. It is a surface glycoprotein known to be expressed on a majority of tumor cells and other immune cells including conventional CD4+ and CD8+ T cells, dendritic cells (DCs), macrophages and Tregs [5]. Under normal circumstance, PD-L1 is expressed to maintain the homeostasis of immune response. However, tumor cells release some immunosuppressive cytokines such as IFN-γ, TNFα and IL-10 that up-regulate its expression to protect themselves from cytolysis by activated T cells. The co-inhibitory characteristic of PD-L1 molecule is attributed to binding to its receptor, programmed death 1 (PD-1) on tumor specific T cells, which lead to their apoptosis and then provide an immune escape for tumor cells [6]. Accumulating evidence has shown that PD-L1 expression is associated with clinicopathological and immunological factors in various human malignancies including gastric [7], liver [8], colorectal [9], pancreatic [10], breast [11], cervical [12], lung [13], bladder [14], brain [15] and blood cancers [16]. So there is an urgent need to obtain a further understanding of the potential relationship between PD-L1 and prognosis in cancer sufferers.
Moreover, some researchers have published their data with respect to PD-L1 expression and have raised concerns about the efficacy of PD-L1 as a specific prognostic factor in cancers; however, its prognostic role in RCC is still under debate. In this study, we aimed to perform an up-to-date meta-analysis to reveal the prognostic value of PD-L1 in RCC.
Materials and methods
Search strategy
We searched several international databases including Medline/PubMed, EMBASE, the Cochrane Library databases and Grey literature up to 30 March 2015. The key terms employed for literature retrieval included “PD-L1” or “B7-H1” or “CD274” or “B7 homolog 1” or “programmed death ligand-1” and “renal cell cancer” or “renal cell carcinoma” or “renal cell tumor” or “kidney tumor” and “survival” or “outcome” or “prognosis”. To obtain additional relevant manuscripts, conference summaries and reference lists missed in the retrieval were identified. We even contacted the corresponding authors to get additional information if necessary.
Selection criteria
Studies were selected if they met the following criteria: (a) they focused on renal cell carcinoma; (b) all selected cancer patients were pathologically confirmed; and (c) correlation between PD-L1, clinicopathological features and prognosis was described.
Articles were excluded from the analyses based on the following criteria: (a) non-English papers; (b) non-human experiments; (c) review articles, case reports or letters; (d) duplicate publication; and (e) insufficient data to report the risk ratios (RR) and 95% confidence interval (95% CI), or the Kaplan-Meier curve could not be extracted.
Data extraction
All data were extracted by two independent reviewers (FX and GSF). The quality of the selected articles was assessed according to the Newcastle-Ottawa Scale(NOS) [17]. Data tables were generated to extract all relevant data from texts, tables and figures, including: author, year of publication, country, patient number, cancer type, specimen, detection method, analysis method, the cut-off value, risk ratio, duration of follow-up as well as positive rates of PD-L1 overexpression. For articles that only provided survival data in a Kaplan-Meier curve, the RR and its 95% CI were digitized and extracted using the software designed by Jayne F Tierney and Matthew R Sydes [18]. To reach a consensus, any disagreement on a conflicting study was resolved by full discussion.
Statistical analysis
The statistical analysis was performed according to the guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology group (MOOSE) [19]. RR with 95% CI was calculated using Stata SE12.0 (Stata Corporation, TX, USA). Odds ratios (ORs) and their 95% CIs were used to assess correlations between PD-L1 expression and the clinicopathological features of renal cell cancer, including tumor size, TNM stage, nuclear grade and tumor necrosis. The heterogeneity among studies was measured using the Q and I2 tests. A probability value of P < 0.1 and I2 ≥ 50% indicated the existence of significant heterogeneity [20]. If there was no significant heterogeneity among studies, the pooled RRs of each study were calculated by the fixed-effects model. If heterogeneity was indicated, the random-effects model was adopted. The potential for publication bias was assessed using the Begg’s funnel plot and the Egger linear regression test. P value < 0.05 was considered statistically significant. All P values are two-tailed.
Results
Search results
The initial search returned a total of 149 manuscripts utilizing the search strategy above. From the title and abstract review, 144 of the articles were excluded due to non-English papers, non-human experiments, non-renal cell cancer-related studies, non-prognostic researches or non-original articles (e.g., review, letter, case report). Finally, a total of 5 studies were included in the meta-analysis. All of these enrolled studies comprehensively assessed the expression of PD-L1 and the survival rate (Figure 1).
Figure 1.

PRISMA flow chart of the literature search.
Study selection and characteristics
All features of the 5 eligible studies are listed in Table 1 [21-25]. The publication years of the eligible studies ranged from 2004 to 2014. All five studies were conducted in USA. The number of patients in each study ranged from 101 to 306 (mean sample size, 215 patients). The quality of the enrolled studies varied from 5 to 8, with a mean of 7. The clinicopathological features including tumor size, TNM stage, nuclear grade and tumor necrosis were reported in 3 studies. PD-L1 expression levels were measured in tumor tissue or blood. In addition, tissue immunochemistry staining (IHC) for PD-L1 expression was utilized in 4 studies. The remaining one study applied enzyme linked immunosorbent assay (ELISA) to detect circulating PD-L1 expression. The mean length of follow-up ranged from 2 to 11.2 years (Table 1). In all studies, none of the patients received neo-adjuvant radio- or chemotherapy prior to surgery.
Table 1.
Main characteristics of the studies included in this meta-analysis
| Authors | Year | Number of patients | Country | Cancer type | Specimen | Detection method | Analysis method | Cut-off (positive/High expression) | Risk ratio | Follow up (years) | P-value | Quality assess-ment (score) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thompson | 2004 | 196 | USA | RCC | Tissue | IHC | Univariable | ≥ 10% tumor cells staining (37.2%) | 4.53 (1.94-10.56) | 2 (0-4.1) | < 0.001 | 7 |
| Thompson | 2006 | 306 | USA | RCC | Tissue | IHC | Mutivariable | ≥ 5% tumor cells staining (63.1%) | 2.00 (1.27-3.05) | 11.2 (0-15) | 0.003 | 5 |
| Krambeck | 2007 | 298 | USA | ccRCC | Tissue | IHC | Univariable | ≥ 5% tumor cells staining (23.5%) | 4.13 (2.74-6.22) | 11.2 (0-15) | < 0.001 | 8 |
| Frigola | 2011 | 172 | USA | ccRCC | Blood | ELISA | Mutivariable | Median (57.1%) | 1.41 (1.08-1.83) | 3.6 (0.1-7.3) | 0.010 | 7 |
| Choueiri | 2014 | 101 | USA | Non-ccRCC | Tissue | IHC | Univariable | ≥ 5% tumor cell membrane staining (10.9%) | 6.41 (2.17-18.88) | 5 | < 0.001 | 8 |
RCC: Renal cell carcinoma; ccRCC: Clear cell renal cell carcinoma; IHC: Immunohistochemistry; ELISA: Enzyme linked immunosorbent assay.
Main results
As shown in Figure 2, we found that elevated PD-L1 had significant association with an enhanced mortality risk of RCC patients in the random-effects model (combined RR 2.90, 95% CI 1.64-5.13), despite the exhibition of heterogeneity among studies (I2 = 84.9%, P < 0.001). To explore the potential source of heterogeneity among studies, “metareg” STATA command was conducted utilizing variables as year of publication, detection method (IHC vs. ELISA) and analysis method (Univariable vs. Mutivariable). The results showed that no variable included in the meta-regression contributed to the heterogeneity.
Figure 2.

Forest plots of studies evaluating risk ratios (RRs) of PD-L1 for cancer specific survival.
In addition, the relationship between elevated PD-L1 and clinicopathological parameters (reported in at least 3 studies) was explored (Figure 3). In renal cell carcinoma, increased PD-L1 was found to be significantly associated with large tumor size (OR = 2.28, 95% CI: 1.61-3.23; Pheterogeneity. = 0.645) (Figure 3A), high TNM stage (OR = 4.37, 95% CI: 2.99-6.39; Pheterogeneity. = 0.676) (Figure 3B), poor nuclear grade (OR = 7.58, 95% CI: 5.26-10.92; Pheterogeneity. = 0.203) (Figure 3C) and present tumor necrosis (OR = 6.77, 95% CI: 4.73-9.71; Pheterogeneity. = 0.111)(Figure 3D) using fixed effect model. As mentioned above, there was no heterogeneity existed. However, no significant relationship was detected between PD-L1 overexpression and other clinical characteristics in RCC due to limited studies (n ≤ 2).
Figure 3.

Forest plots of studies evaluating the association between PD-L1 and clinical parameters in renal cell carcinoma. A. Tumor size (≥ 5 cm vs. < 5 cm). B. TNM stage (III/IV vs. I/II). C. Nuclear grade (3, 4 vs. 1, 2). D. Tumor necrosis (present vs. absent).
Sensitivity analysis
The selected studies were sequentially removed to investigate whether any single study could have an influence on the pooled results. As shown in Figure 4, the stable pooled RR was found to be not significantly affected by each individual study.
Figure 4.
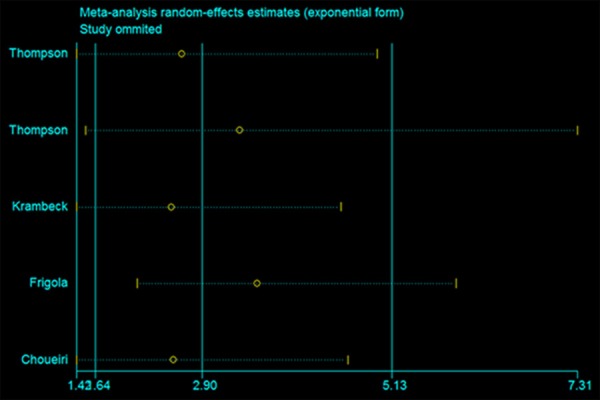
Effect of individual studies on the pooled RRs for PD-L1 and CSS of patients.
Publication bias
The figure of the Bgger’s funnel plot did not show any evidence of obvious asymmetry (P = 0.462; Figure 5). Then, the Egger’s linear regression was performed and publication bias was not detected either (P = 0.135).
Figure 5.

Begg’s funnel plots for all of the included studies reported with CSS.
Discussion
Up to now, the relationship between PD-L1 and the outcome of tumor sufferers remains inconclusive. Our current study chiefly concerned with the prognostic role of PD-L1 in renal cell cancer. To the best of our knowledge, it is the first meta analysis to investigate the clinicopathological feature and prognostic role of PD-L1 in RCC. The pooled RR for cancer-specific survival (CSS) was 2.90 (95% CI: 1.64-5.13; Pheterogeneity. ≤ 0.001), indicating that positive/higher PD-L1 expression significantly predicted poorer CSS compared with negative/lower PD-L1 expression. Significant heterogeneity was observed and could not be eliminated even after using random effect model. Further, meta-regression was performed to investigate the source of heterogeneity. However, none of the variables including year of publication, detection method and analysis method contributed to the heterogeneity in our meta-analysis. Additionally, when the clinicopathological features were considered, the combined odds ratio (OR) was found to be significantly associated with large tumor size, high TNM stage, poor nuclear grade and present tumor necrosis of renal cell carcinoma. However, the association of PD-L1 and other clinicopathological features was explored in few studies enrolled in our analysis. These findings might strengthen the sensitivity and specificity of PD-L1 in predicting the clinical survival of renal cell carcinoma.
Recently, the early clinical experience of large phase I studies targeting PD-L1 pathway with monoclonal antibodies has received substantial attention. A multicenter phase I trial utilized the PD-L1 inhibitor BMS-936559 in patients with RCC in 2012 [26]. Durable tumor regression was noted with an objective response Rate (ORR) observed in 2 of 17 (12%; 95% CI, 2-36) for RCC lasting 4 and 17 months. Seven additional patients (41%) had stable disease lasting at least 24 weeks. Herbst and his colleagues [27] evaluated 175 solid tumors including 56 patients with RCC using the anti-PD-L1 antibody MPDL3280A. The overall ORR was 13%. Responses were seen in both clear-cell and non-clear-cell histology and were observed in patients with tumors expressing high levels of PD-L1, especially when PD-L1 was expressed by tumor-infiltrating immune cells. An additional 30% of patients had stable disease. At 24 weeks, 48% of the patients were alive without disease progression. In an ongoing phase II study, MPDL3280A is being assessed either as monotherapy or in combination with antiangiogenic agents (bevacizumab or sunitinib) in previously untreated RCC (NCT01375842) [28]. Based on these studies, our results suggest that anti-PD-L1 antibodies might be preferentially carried out on patients with renal cell carcinoma in future clinical trials.
Although our results are promising, there are several limitations of this study need to be carefully taken into account. Firstly, as a novel prognostic marker in malignancies, PDL1 just loomed in recent years and nearly half of the sample size was relatively small. Secondly, all of the enrolled studies were from USA, which might contribute to publication bias. Thirdly, a majority of the selected studies measured PD-L1 expression by IHC, the distinct antibodies used, the protocol of staining, the exact cell type and the method of scoring might account for the high variability in the frequencies reported by different authors. On the other hand, we pooled RRs from different studies with a different number of cut off values, which might have caused some of the heterogeneity observed here. Although triple subsets of PD-L1 threshold values revealed the similar results, a baseline referring to PD-L1 overexpression should be set up. Finally, few studies explored the association of patient prognosis and circulating PD-L1 expression, which might provide more valuable evidence than tissue throughout the lives of the cancer patients. Although similar inclusion criteria were employed for each study, potential factors that were not considered might make an impact on our results.
In conclusion, the current evidence firstly shows that an elevated PD-L1 is a negative predictor for survival in renal cell carcinoma. More multicentre studies with larger sample size are needed to present more reliable results of the clinical relevance and precise molecular explanation for the abnormal expression of PD-L1 in the future.
Acknowledgements
This study was supported by the National Science and Technology Support Program of China (2013BAI04B01).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Lee-Gabel L, Nadeau MC, Ferencz TM, Soefje SA. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract. 2015;21:451–67. doi: 10.1177/1078155214538087. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther. 2014;7:1–17. doi: 10.1016/j.hemonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumorspecific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang D, Xu YY, Li F, Xu B, Zhang XG. The role of B7-H1 in gastric carcinoma: clinical significance and related mechanism. Med Oncol. 2014;31:268. doi: 10.1007/s12032-014-0268-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Li G, Meng H, Fan Y, Song Y, Wang S, Zhu F, Guo C, Zhang L, Shi Y. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61:101–108. doi: 10.1007/s00262-011-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX, Zhang F, Gai XD. B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem. 2014;116:1163–1168. doi: 10.1016/j.acthis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Bigelow E, Bever KM, Xu H, Yager A, Wu A, Taube J, Chen L, Jaffee EM, Anders RA, Zheng L. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp. 2013 doi: 10.3791/4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD. Expression of programmed death ligand 1 (PDL1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 13.Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, Aubry MC. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013;14:157–163. doi: 10.1016/j.cllc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Cao YW, Yang XC, Niu HT, Sun LJ, Wang XS, Liu J. Effect of TLR4 and B7-H1 on immune escape of urothelial bladder cancer and its clinical significance. Asian Pac J Cancer Prev. 2014;15:1321–1326. doi: 10.7314/apjcp.2014.15.3.1321. [DOI] [PubMed] [Google Scholar]
- 15.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, Kurscheid S, Hegi ME, Zielinski CC, Marosi C, Hainfellner JA, Preusser M, Wick W. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–75. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, Hyodo H, Shinya E, Takahashi H, Dong H, Tamada K, Chen L, Dan K, Ogata K. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 23.Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, Blute ML, Sebo TJ, Cheville JC, Parker AS, Kwon ED. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749–1756. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
- 24.Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, Bellmunt J, Song J, Carvo I, Lampron M, Stanton ML, Hodi FS, McDermott DF, Atkins MB, Freeman GJ, Hirsch MS, Signoretti S. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25:2178–2184. doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaena RRaD. Immunotherapy in Metastatic Renal Cell Carcinoma: A Comprehensive Review. BioMed Res Int. 2015;2015:367354. doi: 10.1155/2015/367354. [DOI] [PMC free article] [PubMed] [Google Scholar]


