Abstract
The influence of bone marrow stem cells on regeneration of spinal cord in rats was investigated. Young adult male Wistar rats were used (n=22). Focal injury of spinal cord white matter at Th10 level was produced using our original non-laminectomy method by means of high-pressured air stream. Cells from tibial and femoral bone marrow of 1-month old rats (n=3) were cultured, labeled with BrdU/Hoechst and injected into cisterna magna (experimental group) three times: immediately after spinal cord injury and 3 as well as 7 days later. Neurons in brain stem and motor cortex were labeled with FluoroGold (FG) delivered caudally from the injury site a week before the end of experiment. Functional outcome and morphological features of regeneration were analyzed during 12-week follow-up. The lesions were characterized by means of MRI. Maximal distance of expansion of implanted cells in the spinal cord was measured and the number of FG-positive neurons in the brain was counted. Rats treated with stem cells presented significant improvement of locomotor performance and spinal cord morphology when compared to the control group. Distance covered by stem cells was 7 mm from the epicenter of the injury. Number of brain stem and motor cortex FG-positive neurons in experimental group was significantly higher than in control. Obtained data showed that bone marrow stem cells are able to induce the repair of injured spinal cord white matter. The route of cells application via cisterna magna appeared to be useful for their delivery in spinal cord injury therapy.
Keywords: Bone marrow cells, cell transplantation, SCI, spinal cord regeneration, therapeutic strategies
Introduction
Spinal cord injuries are still a serious problem of regenerative medicine. The effects of various therapies are up to date unsatisfactory, though many trials are performed, examining, among others, the effectiveness of cellular therapies, including bone marrow stem cell transplants [1].
Characteristic features of stem cells are: ability to undergo unlimited cell divisions (to reconstruct the population) and possibility to differentiate into various cells. There are two sources (types) of stem cells, depending on the phase of ontogenesis: fetal (embryonic stem cells) and adult (somatic stem cells). Another important characteristic of stem cells determines their ability to differentiate into various cell lines. There are toti-, pluri-, multi- and monopotential stem cells. Totipotential stem cells are able to form the whole organism and fetal membranes, in contrast to monopotential stem cells, which can give rise to one particular cell line only [2-4].
In adult animal organisms, most of the stem cells are mono- or multipotential somatic stem cells. These cells are characteristic to particular tissues and they have been already isolated from many body organs [4-6]. In the brain, stem cells are found in specific areas as hippocampal gyrus and periventricular zone. These cells can differentiate into neurons, oligodendrocytes, and astrocytes. In experimental medicine, as well as in clinical practice, two types of stem cells are currently most commonly used: embryonic and bone marrow stem cells. The first ones are obtained from the embryos by isolation of single blastomeres or by cloning [2]. There is also a possibility to reprogram differentiated somatic cells with help of genetic engineering to create induced pluripotential stem cells. Vectors are used in this case to introduce genes (transcription factors) typical for the pluripotential cell (e.g. Oct-4, Klf-4, c-Myc, Nanog) into fibroblasts line [7]. It is known, that embryonic pluripotential stem cells (neural stem cells) can differentiate in the injured spinal cord to form astrocytes, oligodendrocytes or, less frequently, neurons [3,4,6]. Furthermore, it was shown that in contrast to undifferentiated embryonic stem cells, neural and glial embryonic stem cells can be responsible for the remyelination of demyelination foci, caused by different factors like contusion, chemical injury and others [5,8].
Another type of stem cells is mesenchymal stem cells. These cells can be found in bone marrow and many other tissues (e.g. adipose tissue, skin, bones, and blood-including umbilical cord blood) and organs (e.g. liver, lungs). Their number in bone marrow oscillates between 0.001% to 0.01% of all mononuclear cells and decreases with age [2]. These cells have the capacity to differentiate into bone, fat, cartilage and muscle. Differentiation into neuron-like cells expressing markers typical for mature neurons has been also reported [2,8]. Recently, a great deal has been learned about the isolation and characterization of these cells, as well as about ability to control their differentiation. Walker et al. [9], in their fundamental paper, found that that bone marrow-derived mesenchymal stromal cells (MSCs), used as “MSC therapy” following traumatic CNS injury, act as remote “bioreactors” via stimulation of lung macrophages and augmentation of production by the spleen of T regulatory cells, leading to systemic increase in circulating anti-inflammatory cytokines and alteration of the milieu of the central nervous system.
In the presented work, we examined the use of MSCs delivered via cerebrospinal fluid in the repair of focal injury of spinal cord in rats.
Material and methods
All procedures were performed in accordance to the EU animal protection law and were approved by the Local Animal Research Ethics Committee. Twenty five male Wistar C rats were used; twenty two adults (3 month-old, approximately 300 g b.w.) were randomly divided into two groups, control (C; n=10) and experimental (BM; n=12) and three 30-day-old rats were donors of bone marrow-derived stem cells.
Spinal cord injury
The focal spinal cord injury was performed using the device we designed and created, the pressure impactor, producing a precisely controlled air blast [10].
After intraperitoneal anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg), animals were placed on the heated plate and immobilized by means of the head holding bars and spine clamps at the vertebral levels of Th-9 and Th-11 to destroy selectively the segment of Th-10 of the spinal cord. For this purpose, Th-10 vertebra was stabilized and easy accessible for further steps. The skin was then incised over the spinous processes and the vertebral surfaces were exposed dorso-laterally from Th-9 to Th-11. Under the control of stereomicroscope (Nikon, Japan), a small hole (2 mm diameter) was drilled in the Th-10 vertebral arch on the right side. To avoid overheating and thermal lesion, trepan was cooled down with chilled phosphate-buffered saline (PBS). Impactor tip was placed close to the drilled opening and adjusted by means of micromanipulator. Penetration depth of the tip was set up in the way that a contact with the dura mater was established, however without exerting any pressure on the dura. After setting on impact parameters (150 kPa pressure, 0.1 s duration), the air blast system was activated. The “shot” was observed under the stereomicroscope and recorded by the attached camera (Nikon, Japan). After completing the procedure, the hole was secured with a bone wax, muscles were sutured in layers, skin was closed and the wound was treated with a sterile bandage.
To avoid dehydration, all animals were subcutaneously injected with 2 mL of sterile saline. Because the autonomic function of urinary bladder was impaired due to the spinal shock, it was emptied manually twice a day until the recurrence of bladder function. To prevent pain, paracetamol (suspension 120 mg/5 mL) was dissolved in drinking water (an average drug dosage of 5 mg/kg b.w.).
Bone marrow stem cell culture
Stem cells were obtained from red bone marrow isolated from femoral and tibial bones of three 30-day-old Wistar C rats. Prior to stem cells isolation animals were sacrificed by overdose of with Avertin (Tribromoethanol). Diaphysis of the bone was cut in sterile conditions in order to open the marrow cavity. Bone marrow was pushed out of the bone with a syringe with No. 9 needle containing alfa-MEM medium (Sigma-Aldrich, Germany). The marrow was dispersed by pushing it several times through the same needle containing stem cell culturing medium MesenCult (Stem Cell Technologies Inc., Canada). Obtained bone marrow suspension was transferred to T75 culture bottles (75 cm2 surface area). After 24 hours of culturing in temp of 37°C, in air with 5% CO2 content, the culturing medium with non-adherent cells was removed. Adherent cells were rinsed with phosphate buffered saline (PBS) and supplied with fresh MesenCult medium. When the cells covered whole surface of the culture bottles, they were dissociated with AccuMax solution (PAA, Austria), containing plant protease and passaged in culturing bottles with twice as large surface area. Cultured cells (one passage each time) were used in the experiment or were frozen for later use. Cell banking solution contained 70% of alfa-MEM medium without antibiotics, 20% of non heat inactivated bovine fetal serum and 10% of DMSO (dimethyl sulfoxide). Cells were chilled gradually, spending 24 h in temperatures of -20°C and -80°C to be finally frozen in liquid nitrogen where they were stored.
BrdU and Hoechst labeling
To enable visualization BM cells after their transplantation into the spinal cord, their nuclei were labeled with BrdU. Twenty four hours before transplantation, BrdU (Sigma, Germany) at the final concentration of 10 mg/mL was added to the culture of the bone marrow stem cells. The excess of tracer was washed out with PBS and cells were suspended in fresh culture medium to obtain approximately 300.000 cells in 10 μL.
In addition, for the cells devoted for transplantation, the cell culture was treated with Hoechst 33342 nuclear dye (Sigma, USA; 5 µg/ml in DMEM) for 1 hour at 37°C before cell harvest and then washed for several times in PBS. The fluorescent activity of these cells was checked before transplantation by viewing a sample in fluorescent microscope.
The cells pellet was resuspended in fresh culture medium to obtain approximately 300.000 cells in 10 μL.
Examination of cell culture purity
Stem cells isolated from rat bone marrow created a mixed population of adherent cells. Characteristic of adherent rat red bone marrow cells was determined with fluorescence-activated cell sorter (FACS) Aria BD (USA). Cell surface antigens, allowing for identification of specific cell populations, were detected by fluorochrome-conjugated antibodies. Low-differentiated stem cells are characterized by the presence of alpha-chemokine receptor specific for stromal-derived-factor-1, CXCR4. Hematopoietic stem cells, as well as mesenchymal stem cells, present transmembrane sialomucin protein CD34. Anti-CXCR4 antibody was conjugated with allophycocyanin (APC) and anti-CD34 antibody was labeled with phycoerythrin conjugated with cyanine dye Pe-Cy7 (both- Chemicon, USA). CD45 and Lin antigens (labeled with mix of antibodies against CD11b/c, γ/δTCR i αβTCR), are commonly used hematopoietic cell line markers. The mixture of these antibodies, conjugated with Pe-Cy7, was used for the identification purposes.
Analysis with flow cytometer showed presence of cells with various surface antigens. Most of the cells (~82%) in examined population were mesenchymal cells. Ten percent were CXCR4-positive, similarly like in other studies, showing that only small percentage of mesenchymal stem cells express this protein [11]. Only small fraction (3.5%) of CD34+ cells was found, and 55.1% of cells were CD45- and Lin-negative while 33.3% possessed these antigens, thus were of hemopoietic line (Table 1).
Table 1.
Percentage of different cell populations in mixed culture of rat bone marrow stem cells
| Surface antigen | Cell % in culture | Cell characteristics |
|---|---|---|
| CD34+ | 3.5 | mesenchymal/hematopoietic |
| CXCR4+ | 10 | low differentiated mesenchymal |
| CXCR4+/CD34+ | 4.6 | low differentiated mesenchymal/hematopoietic |
| CXCR4-/CD34- | 81.9 | mesenchymal |
| CD45+/Lin+ | 33.3 | hematopoietic |
| CD45- /Lin- | 55.1 | non-hematopoietic |
Cells transplantation
We decided to deliver cells via cerebrospinal fluid (Figure 1), what should provide the most physiologic way of their migration and protect the injured spinal cord from an extra trauma during direct delivery of these cells into the spinal cord parenchyma.
Figure 1.
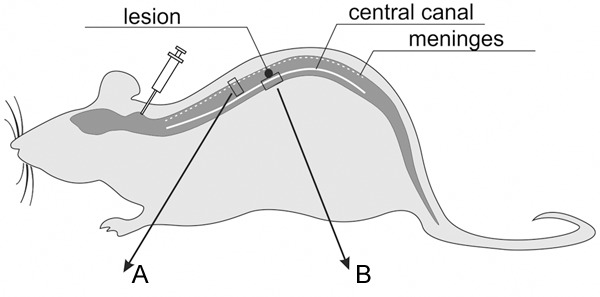
Schematic cells spreading along the spinal cord after their application into the cisterna magna. A and B areas of the spinal cord presented in Figure 3.
The cannulation of cisterna magna (CM) and the application of MSCs were performed according to the technique of Solomon et al. [12] with our own modifications [13]. The animals were anesthetized by intraperitoneal injection of Ketamine (100 mg/kg). The rat’s head was immobilized in the stereotactic apparatus (Narishige, Japan), and the body slumped freely at the operative table, at right angle to the barrel. Parietal bone, occipital bone, the arch of the atlas and atlanto-occipital membrane were exposed after the medial incision of the skin and muscles in the parieto-occipital region. The 0.8 mm trephination hole was drilled sagittally over the parieto-occipital suture. Then the cannula (Venocath-18, Abbot) was placed via this hole into the CM (Figure 2A) and attached to the parietal bone with cyano-acrylic glue. The correct position of the cannula was achieved by observing the localization of its end in the CM through the atlanto-occipital membrane and by noting free minor CSF outflow through the cannula. The trephination hole was then protected from the CSF leakage with bone wax and cyano-acrilic glue (Figure 2B).
Figure 2.
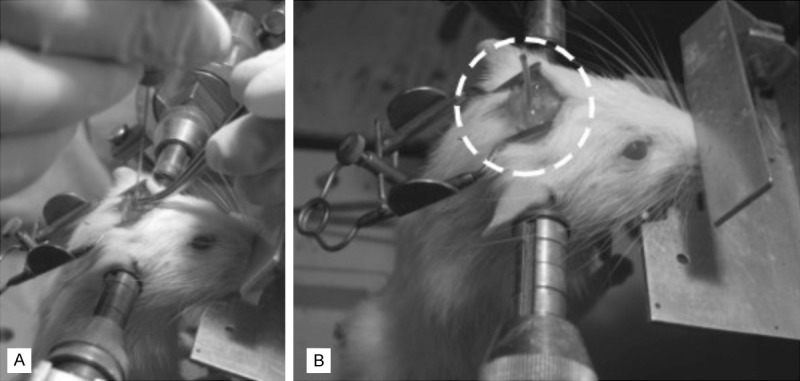
A. Cannulation of cisterna magna; B. Implanted cannula prior to bone marrow cells transfer.
Ten microliters of BM cells suspension containing approximately 300.000 cells suspended in fresh medium (BM group) or 10 µl saline (C group) were slowly injected through the previously inserted cannula immediately after spinal cord injury. This procedure was repeated 3 and 7 days later. In order to confirm successful delivery of transplanted cells into the injured spinal cord, spinal cord sections collected from 2 animals from BM cells group 24 h post transplantation were stained to visualize exogenous stem cells in the spinal cord.
Assessment of locomotor function
During 12 weeks postoperatively, animals were observed and analyzed regarding development or regression of neurological deficits. Behavioral observations included gait analysis (foot print test) and open field test according to the BBB (Basso, Beattie and Bresnaham) scale [14].
Foot print test
Foot print test was carried out in 1st, 4th, 7th, and 12th week postoperatively. Animals were tested on a 100 cm long and 7 cm wide runway with side walls and transparent bottom. The walk of a rat through the runway was recorded with digital camera and automatically analyzed frame by frame (Catwalk XT 8.1, Noldus, Holandia). Each animal was tested three times consecutively. Framed foot images were analyzed regarding the foot rotation angle (the angle made by two lines connecting the third toe and the stride line at the center of the paw) of the right hind paw (ipsilateral to the injury site), and interlimb coordination (smallest distance between the middle point of the hind paw and forepaw on the same side). Normal rotation angle is about 8° and after trauma increases up to 30°. Interlimb coordination is about 1.5 cm in healthy rat and increases to 3 cm or more after injury [15].
BBB open field test
Test was carried out in classical way on the plexiglass surface [14]. During the test, motor function of joints was analyzed to asses the stepping ability of the animal. Additionally, general coordination and stability of the body were evaluated. The value range was 0-22; 0 was related to complete lack of motor capability, and 22 indicated the best score in this aspect.
Neuroanatomical tracing
Retrograde neuroanatomical tracing was used to determine the extent to which supraspinal neurons regrew axons to reach spinal cord segments caudal to the injury. Four animals in each group were randomly selected for retrograde tracing. One week before the end of experiment, after anesthesia as above, the spinal cord was exposed by laminectomy below the injury site, and 2 microcrystals of Fluorogold (FG) (Fluorochrome Inc., Englewood, NJ) were placed bilaterally inside the spinal cord 10 mm caudally from injury site. For quantification of the number of FG-positive neurons present in brain stem (red nucleus) and primary motor cortex on the last day of experiment, animals were rapidly perfused with a bolus of cold PBS, and whole brains were carefully dissected, dehydrated in 15% sucrose, embedded in TissueTek (Sakura, Japan), frozen and then cut coronally into 10 µm sections. Every sixth slide was analyzed, giving 18-22 sections per animal. The sections were viewed under UV excitation (at 365 nm wave specific for FG) in fluorescent microscope using appropriate filter (Labophot 2, Nikon, Japan) and photographed. The number of cells from each section was then summed for each rat and the number of cells per mm2 was calculated and finally averaged for whole group.
Morphological evaluation
MRI analysis
MR images were obtained at the Institute of Nuclear Physics PAN (Cracow, Poland) using 4.7 T research MRI scanner, equipped with MARAN DRX digital console (Resonance Instruments, UK). Measuring probe-head with surface RF coil dedicated for rat spinal cord investigations was used. Animals were anesthetised with halothane (2%) mixed with air (60%/1.2 L/min) and oxygen (40%/0.8 L/min) Physiological conditions of the animal (temperature, breath rate and cardiac rate) were continuously monitored during experiment using MR-compatible Small Animal Monitoring & Gating System (SA Instruments, Inc., USA).
Spin echo pulse sequence was used for acquiring T2 weighted and diffusion weighted MR images in sagittal and axial projections. The following parameters of the spin echo sequence were applied: echo time (TE=32 ms), repetition time (TR=3 s), field of view (FOV=2.5 cm), image matrix size 128×128, slice thickness of 1.5 mm. The signal was accumulated (NA=6) in order to improve signal to noise ratio (SNR).
Diffusion weighted images were obtained with additional diffusion sensitive gradients (b=1000 s/mm2, δ=5 ms and Δ=23 ms) added to spin echo sequence in transversal and longitudinal directions to the spinal cord axis.
In order to minimize motion artifacts, all measurements were synchronized with animal breath cycle.
Histology
One (3 animals per group) or 12 weeks (3 animals per group) after injury, rats were re-anesthetized and perfused transcardially with 300 mL PBS (pH 7.4) followed by 300 mL of 4% paraformaldehyde solution in the same buffer. Fragments of spinal cord (~2 cm) containing the injury area were dissected and dehydrated in 20% sucrose in PBS for 24 h at 4°C, embedded in TissueTek (Sakura, Japan), frozen, and cut sagittally into 10 µm sections mounted on SuperFrost Plus slides (Menzel Glaeser, Germany) in 10-section-step manner. For immunostaining, the frozen section were air dried at room temperature for 10 min. and washed with PBS for 10 min. Slides were subjected to hematoxylin-eosin staining, 1% toluidine blue staining or to immunohistochemical labeling, and mounted in VectaShield with DAPI (Vector, USA). Sections were examined under confocal laser scanning microscope FluoView (Olympus, Japan). The images were digitally stored and then analyzed.
To examine the ability of transplanted BM cells to survive in host spinal cord, 1 and 11 weeks following last transplantation, the number of Hoechst/BrdU-positive BM cells was determined for the 3 animals at each time point by counting all labeled cells present at the epicenter and nearest surrounding of injury site and expressed as number of cells per mm2.
In the same group of animals, one week after transplantation maximal distance of expansion of implanted BM cells in injured spinal cord was measured 1 week after last transplantation as a maximal distance covered by Hoechst/BrdU-positive BM cells from the epicenter of the lesion into the spinal cord parenchyma.
Twelve weeks after injury we also measured the sizes of the lesion center (cavity area) using Image Pro Plus software to calculate automatically manually outlined contour (area borders) of the cavity lesion.
Nestin immunostaining for visualization of neuronal precursors
The sections were blocked with 1% BSA, 5% skim milk and 0.3% Triton X-100 in PBS for 1 h at room temperature, and the primary antibody was applied in the same blocking solution overnight at 4°C. We used anti-rat rabbit anti-nestin antibody (1:100; Chemicon, UK). Then, slides were washed 3 times in PBS and incubated with secondary goat anti-rabbit IgG antibodies conjugated with Alexa 488 (1:400; Molecular Probes, USA). Mounted in VectaShield (Vector, USA) sections were examined under confocal laser scanning microscope FluoView (Olympus, Japan). The images were digitally stored and then analyzed.
BrdU-positive cells visualization
The sections were washed with PBS incubated in 2N HCL and 1% Triton X-100 in PBS for 15 min. at room temperature, and wash in sodium borate in PBS for 10 min. The slides were blocked with 1% BSA, 5% skim milk and 0.3% Triton X-100 in PBS for 1 h at room temperature and incubated with a mouse anti-BrdU antibody (1:100; BD-Immuno; Oxford, UK). In the same blocking solution overnight at 4°C. Slides were then washed 3 times in PBS and incubated with goat anti-mouse antibody conjugated with Alexa 594 (1:400; Molecular Probes, USA). Finally, sections were examined under confocal laser scanning microscope FluoView (Olympus, Japan). The images were digitally stored and then analyzed.
Maximal distance of expansion of transplanted cells in the injured spinal cord was measured at 2 weeks after operation and survival of transplants (number of cells seen in the epicenter of the injury) was examined 2 and 12 weeks following surgery (1 or 11 weeks after last cell transplantation).
Statistical analysis
ANOVA or nonparametric Kruskal-Wallis ANOVA or Student’s test were used. Significance level was set at P≤0.05. All data are expressed as means ± SD.
Results
Surviving and migration of transplanted cells within injured spinal cord
After grafting, BM cells (identified after Hoechst/BrdU-positive nuclei) were observed on the surface of the injured spinal cord and then they propagated along meninges in the direction of white matter. Later (1 week after last [third] injection), transplanted MSCs penetrated spinal cord parenchyma deep inside the spinal cord interior close to the central canal, very near to the injury region. Some of these cells invaded the other side of the spinal cord-not affected by injury (Figure 3). Their number decreased with time -1 week following last transplantation we found 967.2±331.7 cells/mm2, while at the end of experiment, it was 71.7±47.1 cells/mm2 in analyzed fragments of spinal cord.
Figure 3.
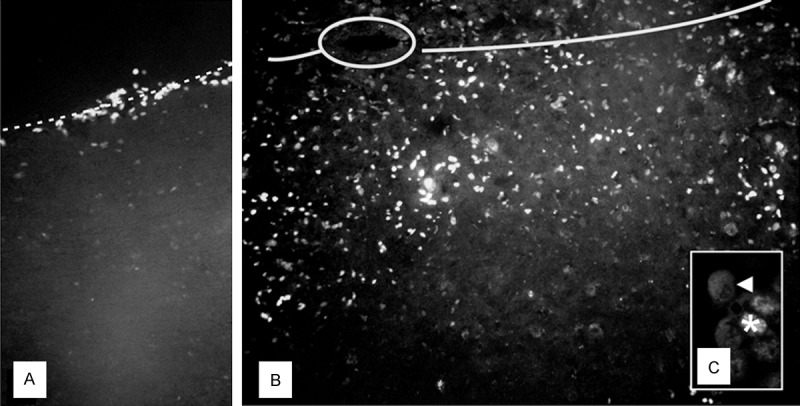
Fluorescent microscope images of rat spinal cord, 7 days after injury and triple application of bone marrow stem cells: A. Cells (nuclei labeled with Hoechst) migrating along the spinal meninges (dash line); B. Cells crossing the middle line of the spinal cord (solid line, central canal-circled); C. Double-labeled (asterisk-BrdU; arrowheadnestin) implanted cells. Magnification: A, B. 100×; C. 200×.
Behavioral analysis
Immediately after SCI, 17 animals (74%) were monoplegic. Rats in the experimental group presented significant improvement of locomotor performance when compared to the control group. The footprint pattern of animals one week after SCI showed strongly compromised both the interlimb coordination and the angle of rotation (2.5±0.42 and 23.4±1.7°, respectively). From 7th week after SCI, the experimental group displayed a significant reduction in the angle of rotation in hindlimb placement compared with the control group (12.3±1.4 versus 18.9±1.1 at the 12th week following injury) (P<0.05) (Figure 4). From 4th week after injury, the BM group showed also a significant (P<0.05) improvement in the interlimb coordination, reaching at the end of the experiment value of 1.53±0.11 in contrast to the control animals (2.19±0.21) (Figure 5). In addition, significant functional deficit, as measured with the BBB average score, was noted in all animals from the control group (10.1±0.3) when compared to the experimental one (16.3±0.7) (P<0,05) (Figure 6).
Figure 4.
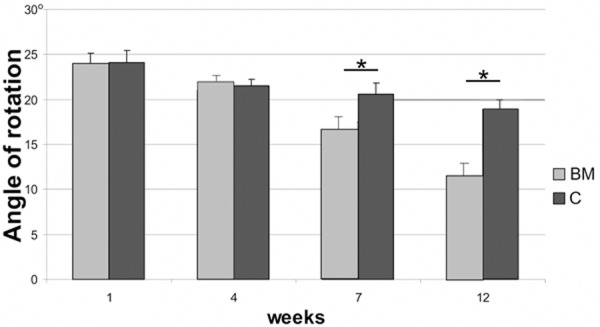
Angle of rotation of the hind paw after spinal cord injury in BM and C groups, respectively. Asterisks indicate statistical significance (P≤0.05).
Figure 5.
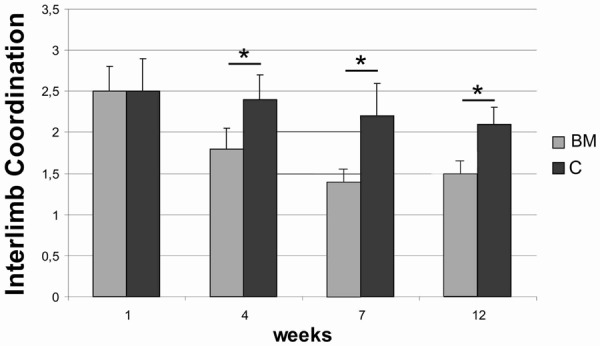
Inter-limb coordination after spinal cord injury in BM and C groups, respectively. Asterisks indicate statistical significance (P≤0.05).
Figure 6.
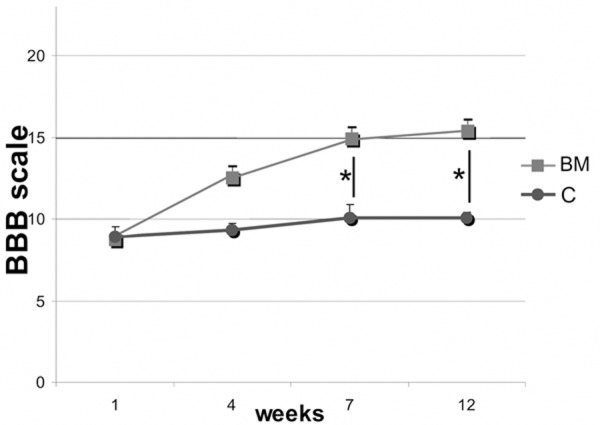
BBB open field score after spinal cord injury in BM and C groups, respectively. Asterisks indicate statistical significance (P≤0.05).
Magnetic resonance imaging
MR images showed no post-traumatic cyst development in the white matter of spinal cord in the BM group, while in the control group changes resembling typical post-traumatic syringomyelia were frequently found (Figure 7). Comprehensive analysis of diffusion data from white matter gives quantitative description of injury in terms of the spatial change of the diffusion parameters as shown on Figure 8. The transverse diffusion gradients of the white matter (perpendicular to the spinal cord axis) showed no difference between BM and C group, while the longitudinal diffusion gradients were slightly, however non-significantly, higher in the BM group when compared to the control (P>0.05). These values were close to the reference values measured in healthy animals.
Figure 7.
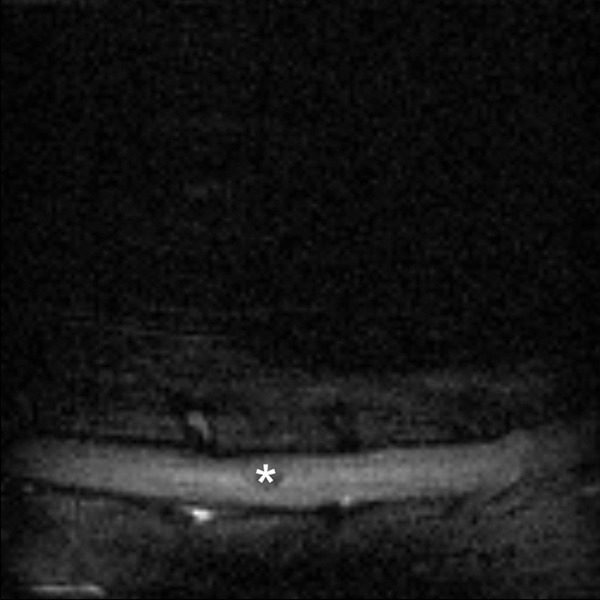
MR sagittal image of control rat’s spinal cord 12 weeks after injury; post-traumatic cyst (asterisk) visible in the central part.
Figure 8.
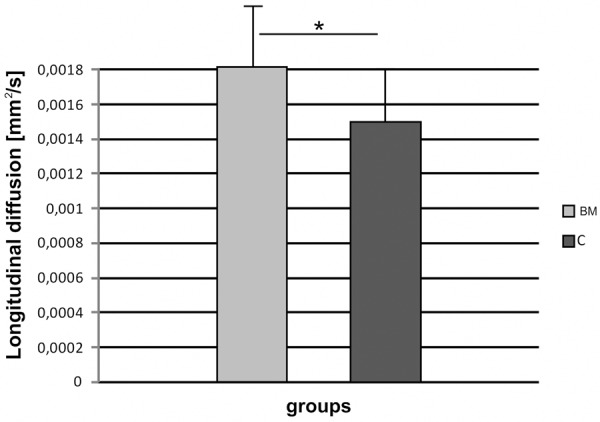
Longitudinal diffusion gradients of the spinal cord white matter in BM and C groups, respectively.
Assessment of cavity area
The average size of the lesion center (cavity area) was also significantly smaller in the experimental group (0.12±0.14 mm²) than in the control one (1.02±0.21 mm²) (P<0.05) (Figure 9).
Figure 9.
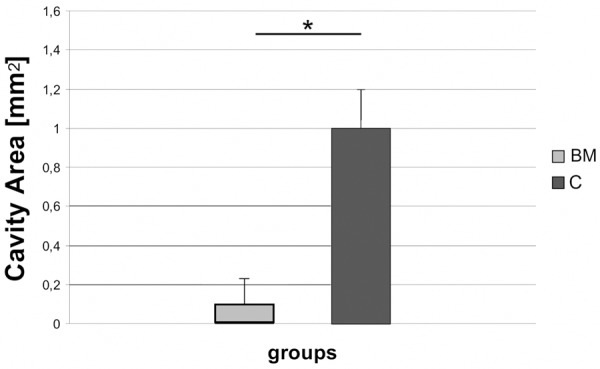
Areas of lesion cavities, 12 weeks after spinal cord injury in BM and C groups, respectively. Asterisk indicates statistical significance (P≤0.05).
Neuroanatomical tracing
Number of the brain stem and primary motor cortex FG-positive neurons (Figure 10) in BM group (248.1±105.1) was significantly higher than in the control (14.3±3.3) (P<0.05).
Figure 10.
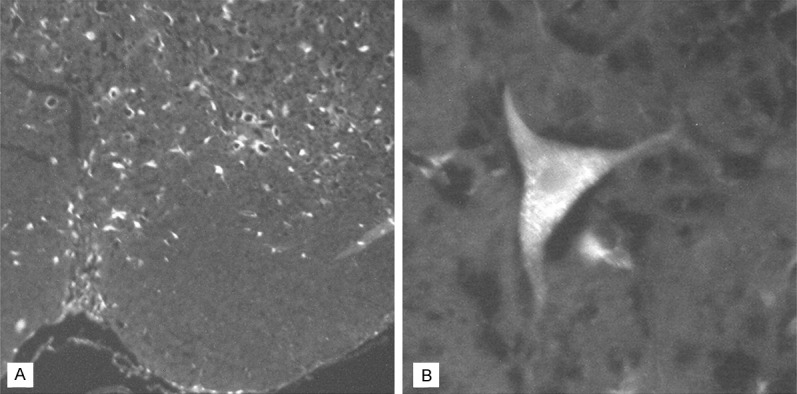
FluoroGold-positive cells in the rat’s brain (BM group), 12 weeks following spinal cord injury and bone marrow cells transplantation. A. Brain stem cells. Magnification 100×; B. Motor cortex neuron. Magnification 400×.
Neuronal precursors’ number
Some transplanted bone marrow cells differentiated towards neurons, as confirmed by BrdU/nestin double labeling. Number of such double labeled cells found one week following transplantation was 41.4±23.7 mm², while after 11 weeks it reached significantly smaller value of 7.1±1.3 mm².
Discussion
We showed that mixed population of bone marrow derived stem cells delivered via cerebrospinal fluid may improve morphologic as well as functional deficits of injured spinal cord in rats.
A surprising finding of our study was that relatively small heterogeneous population of bone marrow stem cells repeatedly grafted via cerebrospinal fluid can overpass some limitation of current therapy of spinal cord injury in animals. This refers to elaboration of optimal delivery rout of grafted cells and their successful survival, migration, integration and finally differentiation within the host tissue as well as their putative regenerative influence on injured white matter. Unlike many other studies, we focus on therapy of focal injury of white matter of spinal cord consisting predominantly of axons, not neuronal cell bodies.
The mechanism of an influence of bone marrow derived stem cells on an injured spinal cord of adult mammals is complex and has not been entirely explored. There are probably two possibilities: limitation of the extent of injury in the traumatic spinal cord, which seems to be more probable, or strictly neuro-regenerative effect leading to both cell survival and regrowth of their axons. It is possible that both abovementioned mechanisms overlap and the truth lies somewhere between. It seems possible, that bone marrow-derived stem cells through differentiating into oligodendrocytes restore the population of these cells, lost due to spinal cord injury. Demyelinated axons are very sensitive to further neuro-degradation and their prompt remyelination by oligodendrocytes is necessary to limit their progressing decomposition. The latter, in turn, can result in propagation of the damaged region to next parts of the spinal cord, not affected directly by the damage or merely injured [16]. Eventually, the white matter of the spinal cord becomes severely damaged and a classic post-traumatic cyst is formed, which is observed both in humans and rats [17]. Bone marrow derived stem cells may also influence the formation of new intercellular connections what lead to spinal cord plasticity as well as stimulation of axonal growth [4,18].
In our experiment, we decided not to use selected homogenous population but mixed bone marrow cells. It made possible to reconstruct the entire spectrum of supposed activities of bone marrow stem cells in injured spinal cord. There are experiments showing that mixed populations of stem cells, composed usually of two or more cell lineages, transplanted in the injured spinal cord present higher vitality, differentiate better and cause more significant improvement of the functional outcome than any one of them used separately [1].
Currently, a tendency can be noted to examine this kind of complex therapies, including mixed cell therapies that are usually more effective than a mono-therapy [19]. Another reason to apply a mixed population of bone marrow derived stem cells are problems concerning the direction of their differentiation in the recipient. For instance, it was proven that highly purified mouse hematopoietic bone marrow-side population (SP) of stem cells [20] and CD34+ positive bone marrow cells [21] showed no ability to transdifferentiate and restore neurons or astroglia. Contrary, another type of hematopoietic bone marrow-side populations of stem cells, identified as Sca-1+, c-kit+ and Lin-, relatively easily differentiated into oligodendrocytes and astrocytes [22,23]. The whole bone marrow derived stem cells, allo- [24] and xenogeneic [21], differentiated easily into glial cells and replaced the population of lost oligodendrocytes, retaining the ability to remyelinate damaged axons of the spinal cord, as opposed to hematopoietic stem cells that were not able to remyelinate the axons. An example that well illustrates this strategy is combined application of glial cells of various origins, namely olfactory bulb cells with adult Schwann cells, into the injured spinal cord. It permitted achievement of better functional tests results than separate application of these cells [25].
The abovementioned differences indicate that bone marrow contains populations of stem cells not completely described and considerably more diverse that one can suspect [26]. It is probable that the direction of differentiation of bone marrow derived stem cells is determined, apart from mutual cellular interactions, by other factors, including external ones, like for instance the nature of the spinal cord injury itself [27]. It is difficult to predict the behaviour of implanted stem cells in conditions where the white matter of the spinal cord was damaged, that took place in our model.
It is suspected that one of the most crucial characteristics of bone marrow derived mesenchymal stem cells that determines their main functions and clearly distinguishes them from other types of stem cells is their ability to form “bands of cells” similar to bands of Büngner present in the peripheral nervous system and connecting edges of post-traumatic changes (cysts) [4,28]. Possibly, in our experiment, the almost complete lack of visible post-traumatic changes in the spinal cord in the BM group is related to an inflow and filling of the regions of potential cysts by these cells with a possible differentiation later into other spinal cord cell populations.
The ability of the implanted cells to penetrate into the area of post-traumatic changes and, first and foremost, invading the region of the glial scar in the rostral spinal segments is crucial for the recovery of the lost nerve functions. Interesting studies in this matter were carried out by Lu et al. [29]. They showed a possibility of penetrating even several week-old post-traumatic cyst with regenerating spinal axons after application of mesenchymal stem cells into the cyst in rats.
A favorable feature of mesenchymal stem cells that facilitates their application in regenerative medicine is also their low immunogenicity or complete lack of it, what allows to eliminate immunosuppression in case of their application [17,30]. Moreover, in a number of papers it was indisputably proven that application of bone marrow derived mesenchymal stem cells has a favorable influence on functional recovery (usually test BBB was applied) of the injured spinal cord. This effect was not related to cells differentiation into specific populations of cells and did not depend on spinal cord injury model [28,31-33]. In in vitro conditions, whole bone marrow stem cells caused a significant improvement of nerve conduction [24]. In another study, behavioral tests showed an improvement in motor functions, despite the fact that among implanted cells only a small group differentiated into oligodendrocytes and astrocytes [23].
Only partial recovery of motor functions observed in experimental animals after MSC therapy, despite the lack of obvious morphological changes in the injured spinal cord, can be explained that even almost complete reconstruction of a structure does not always restore accurate function in the CNS. From experiments with other cells used in SCI therapy, Felts and Smith found that remyelination of spinal cord axons by Schwann cell is permanent, fully effective and in general restore almost normal impulse conduction in them [34]. It is also known, however, that normal conduction in remyelinated axon can be disturbed as a consequence of membrane ion channels rearrangement [35].
Results presented above show positive influence of bone marrow derived stem cells on injured spinal cord white matter. The route of delivery of these cells via cerebrospinal fluid also appeared to be a useful method. However, the precise mechanisms of action of these cells in the repair of the nervous system require further examination.
Acknowledgements
Work was supported with Medical University of Silesia grants No.: KNW-1-030/P/1/0 and KNW-1-026/P/2/0.
Disclosure of conflict of interest
None.
References
- 1.Oudega M, Gautier SE. Spinal cord repair strategies: Schwann cells, neurotrophic factors, and biodegradable polymers. Biomed Rev. 1999;10:75–88. [Google Scholar]
- 2.Barrilleaux B, Phinney DG, Prockop DJ, O’Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 3.Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter CP, Holmström NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisén J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Hammang JP, Archer DR, Duncan ID. Myelination following transplantation of EGFresponsive neural stem cells into a myelin-deficient environment. Exp Neurol. 1997;147:84–95. doi: 10.1006/exnr.1997.6592. [DOI] [PubMed] [Google Scholar]
- 9.Walker PA, Shah SK, Jimenez F, Aroom KR, Harting MT, Cox CS Jr. Bone marrow-derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery. 2012;152:790–793. doi: 10.1016/j.surg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Marcol W, Slusarczyk W, Gzik M, Larysz-Brysz M, Bobrowski M, Grynkiewicz-Bylina B, Rosicka P, Kalita K, Weglarz W, Barski JJ, Kotulska K, Labuzek K, Lewin-Kowalik J. Air gun impactor-a novel model of graded white matter spinal cord injury in rodents. J Reconstr Microsurg. 2012;28:561–568. doi: 10.1055/s-0032-1315779. [DOI] [PubMed] [Google Scholar]
- 11.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 12.Solomon RA, Antunes JL, Chen RY, Bland L, Chien S. Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke. 1985;16:58–64. doi: 10.1161/01.str.16.1.58. [DOI] [PubMed] [Google Scholar]
- 13.Jedrzejowska-Szypułka H, Larysz-Brysz M, Kukla M, Snietura M, Lewin-Kowalik J. Neutralization of interleukin-1beta reduces vasospasm and alters cerebral blood vessel density following experimental subarachnoid hemorrhage in rats. Curr Neurovasc Res. 2009;6:95–103. doi: 10.2174/156720209788185669. [DOI] [PubMed] [Google Scholar]
- 14.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 15.Karimi-Abdolrezaee S, Eftekhapour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blight AR. Miracles and molecules-progress in spinal cord repair. Nat Neurosci. 2002;5(Suppl):1051–1054. doi: 10.1038/nn939. [DOI] [PubMed] [Google Scholar]
- 17.Bunge RP, Puckett WR, BecerrA JL, Marcillo A, Quencer RM. Observation on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 18.Myckatyn TM, Mackinnon SE, McDonald JW. Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol. 2004;12:343–358. doi: 10.1016/j.trim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Geremia N, Bao F, Pniak A, Rossoni M, Brown A. Schwann cell co-culture improves the therapeutic effect of bone marrow stromal cells on recovery in spinal cord-injured mice. Cell Transplant. 2010;20:1065–1086. doi: 10.3727/096368910X544906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cell in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koda M, Okada S, Nakayama T, Koshizuka S, Kamada T, Nishio Y, Someya Y, Yoshinaga K, Okawa A, Moriya H, Yamazaki M. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport. 2005;16:1763–1767. doi: 10.1097/01.wnr.0000183329.05994.d7. [DOI] [PubMed] [Google Scholar]
- 23.Koshizuka S, Okada S, Okawa A, Koda M, Murasawa M, Hashimoto M, Kamada T, Yoshinaga K, Murakami M, Moriya H, Yamazaki M. Transplanted hematopoetic stem cell from marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2004;63:64–72. doi: 10.1093/jnen/63.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, Bunge MB. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- 26.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 27.Enzmann GU, Benton RL, Talbott JE, Cao Q, Whittemore SR. Functional consideration of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006;23:479–495. doi: 10.1089/neu.2006.23.479. [DOI] [PubMed] [Google Scholar]
- 28.Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004;15:1105–1108. doi: 10.1097/00001756-200405190-00004. [DOI] [PubMed] [Google Scholar]
- 29.Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Lu P, Jones L, Tuszynski MH. BDNF expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 33.Wu SF, Suzuki Y, Ejiri Y, Noda T, Bai H, Kitada M, Kataoka K, Ohta M, Chou H, Ide C. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal. J Neurosci Res. 2003;72:343–351. doi: 10.1002/jnr.10587. [DOI] [PubMed] [Google Scholar]
- 34.Felts PA, Smith KJ. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Res. 1992;574:178–192. doi: 10.1016/0006-8993(92)90815-q. [DOI] [PubMed] [Google Scholar]
- 35.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cells responses adjacent to injury epicentrum cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]


