Abstract
Objective: To investigate the neuro-protective effects of dexmedetomidine (dex) on I/R-induced spinal injury and potential mechanisms. Methods: sprague-Dawley rats in the treatment group received intraperitoneal injections of 25 mg/kg dexmedetomidine, MC stabilizer cromolyn (100 mg/kg), MCs stimuliser compound 48/80 (80 mg/kg), PBS at 24 h befor IR. Underwent 5 minutes of aortic occlusion via median sternotomy, functional scores were recorded at 12, 24, 36 and 48 hours after reperfusion. Additionally, 3 mice underwent sham surgery with sternotomy and dissection of the aorta and subclavian artery with no occlusion. Spinal cords were examined for protein kinase B (AKT), CREB, and brain-derived neurotrophic factor (BDNF) following treatment alone or ischemia-reperfusion surgery. Collected the serum to observe the expression of pro-inflammation cytokines (TNF-α, INF-γ and IL-1β) and anti-inflammation cytokines (TGF-β, IL-10 and IL-6). Then the MCs were harvested to test the expression surface molecular of FcεR and MCs’ degranulation. Results: Pretreated the rats with dexmedetomidine has higher neurologic function at all time points after I/R injury. We collected the serum of rats then detected the pro-inflammation cytokines TNF-α, INF-γ and IL-1β levels and anti-inflammation cytokinses TGF-β, IL-10 and IL-6 levels, found that the pro-inflammation cytokines of dexmedetomidine group was decreased whereas the anti-inflammation cytokinses was increased. At the same time the protect protein of AKT, CREB and mRNA BDNF were increased. They had the same results with cromolyn group, and opposite with the compound 48/80 group. We pretreated MCs with dexmedetomidine in vitro, and found that the activity surface molecular of MCs was down-regulation, and MCs degranulation was decreased. Conclusion: We thus demonstrate a possible mechanism by which dexmedetomidine alleviates spinal cord I/R injury through blocking the MCs degranulation.
Keywords: Dexmedetomidine, mast cell, degranulation, ischemia-reperfusion injury
Introduction
The ischemic insult to the spinal cord during thoracoabdominal aortic interventions continues to have devastating consequences in up to one fifth of high-risk patients [1]. Ischemia-reperfusion (IR) injury is complex and poorly understood. Neurons are the most vulnerable cells to ischemia and coupled with their inability to regenerate, spinal cord I/R is a significant threat to patients. The current management protocol for spinal ischemia is limited to supportive medical care. Recanalization following ischemia is the most effective method for treatment of acute spinal infarct and correction of hypoxia, however, paradoxically could cause severe spinal ischemia-reperfusion (I/R) injury [2,3]. Due to the lack of efficient neuroprotective therapies, the I/R injury is still a major medical problem which urgently needed to be further studied. It is therefore of major important to prevent or alleviate I/R-induced injury in recanalizing patients, and perioperative treatment may be a potent protective strategy for the ischemic neuronal injury [4].
Dexmedetomidine (DEX) is an α-2 adrenoreceptor agonist and is used as a sedative and an analgesic especially in a neurointensive care unit [5,6]. Dexmedetomidine has been found to be very effective as a premedication agent because of its sympatholytic, analgesic, anxiolytic, and sedative effects [7]. Previous studies have demonstrated that dexmedetomidine exhibited antiapoptotic and anti-inflammatory effects apart fromits anesthetic features [8,9]. In cerebral neurons, clonidine and other α-2 agonists have been shown to promote phosphorylation of neuroprotective pathways and up-regulation of the PI3K AKT pathway. Cyclic AMP response-element binding protein (CREB) is a transcription factor that is critical for neuron survival after ischemia supporting its role inthe induction of ischemic tolerance. Among other functions, CREB induces expression of prosurvival proteins brain derived neurotrophic factor (BDNF) [10]. Moreover, studies in animals have reported organ protective effects of dexmedetomidine in ischemia-reperfusion injury [11,12]. Dexmedetomidine promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion [13]. But the mechanism is not understand.
Mast cells (MCs) participate in I/R injury in many organs, such as the heart, brain [14] and intestine. MCs take effect mainly through releasing chemicals stored within the MC granules, which include pro-inflammation cytokines and anti-inflammation cytokines, such as histamine [15], interleukin [16], and MC tryptase [17], and chymase [18]. Stablizing the MCs membrane could alleviate the organs I/R injury. So we hepothesis that dexmedetomidine could stabilize the MCs’ membrane and increase the protective signal protein to alleviate the spinal I/R injury.
Material and methods
Animals
All rats were purchased from Schleck Experimental Animals Co. (Shanghai, China). All rats were housed in a Specific pathogen free facility and used in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals of the Chinese Academy of Sciences.
Cell preparation
Rat bone marrow-derived mast cells were obtained as described previously [19]. The purity of MCs was assessed by measuring the expression of c-kit (CD117) and FCεRIα using flow cytometry. MCs were used at a purity of 95%.
Flow cytometry
MCs pretreated for 24 h with: PBS, 30 μg/kg dexmedetomidine, MCs stabilizer cromolyn (100 μg/kg), MCs stimuliser compound 48/80 (80 μg/kg), MCs were incubated with FcεRI-PE antibodies for 30 min on ice. Flow cytometry analysis was performed using a FACS Calibur flow cytometer (BD BioSciences) equipped with Cell Quest software.
Mast cell degranulation assay
After treating MCs with PBS, dexmedetomidine, cromolyn, compound 48/80 for 24 h, MCs were preincubated with anti-DNP-IgE (100 ng/mL) for 24 h then challenged using DNP (200 ng/mL). After 1 h, conditional media from the IgE/Ag degranulated MCs was added to the culture system. After solubilization with 0.5% Triton X-100 in Tyrode’s buffer, the enzymatic activity of β-hexosaminidase in both supernatants and cell pellets was measured using p-nitrophenyl-N-acetyl-β-D-glucosaminide in 0.1 M sodium citrate pH 4.5 at 37°C for 60 min. The reaction was stopped by addition of 0.2 M glycine pH 10.7 and the released p-nitrophenol was measured at 405 nm. The extent of degranulation was calculated as the p-nitrophenol absorbance of the supernatant divided by the total absorbance of the supernatant and detergent-solubilized cell pellet.
Aortic cross clamping: IR surgery
Rats were anesthetized using 2% isoflurane and placed in the supine position. Surgery was performed under normothermic conditions. The aortic arch was exposed using a cervicothoracic approach as previously described [20]. Disruption of arterial flow to the spinal cord was achieved by placing vascular clamps on the aortic arch distal to the left common carotid artery and the subclavian artery for 5 minutes. To confirm at least 90% reduction in distal flow, a laser Doppler blood flow monitor (Moor Instruments, Devon, United Kingdom) was placed over the left femoral artery.
Medication administration
Rats in the treatment group received intraperitoneal injections of 25 mg/kg dexmedetomidine, MC stabilizer cromolyn (100 mg/kg) MCs stimuliser compound 48/80 (80 mg/kg) or PBS (n = 5) at 24 h befor IR. Additionally, 3 mice underwent sham surgery with sternotomy and dissection of the aorta and subclavian artery with no occlusion.
Functional scores
The Basso Mouse Scale for locomotion, which ranges from a score of 0 for complete paraplegia to a score of 9 for normal function, was used to quantify hind-limb function in mice after ischemia. Function was scored at 12, 24, 36, and 48 hours after reperfusion.
Quantitative polymerase chain reaction
Measurements BDNF (brain-derived neurotrophic factor) transcripts from treated and untreated spinal tissue without undergoing IR surgery were performed. Total RNA was isolated using the RNAqueous-4 PCR kit (Ambion, Austin, Tex) per the manufacturer’s instructions. Real-time polymerase chain reactions using iQ mastermix (Bio-Rad, Hercules, Calif) on Bio-Rad CFX detection system (Hercules, Calif) were performed in duplicate using 50 ng complementary DNA. FAM-labeled probe/primer sets used to detect BDNF (Mm04230607_s1) were synthesized by Invitrogen (Grand Island, NY). The housekeeping gene β-actin (Mm00607939_s1) was also assayed for each sample using 5 ng complementary DNA. Cycle parameters used 95°C for 3 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Expression levels were calculated using the delta-delta Ct method relative to β-actin.
Protein extraction and western blotting
Spinal cords were removed following pretreatment or IR surgery. Briefly, Protein extracts were obtained using lysis buffer (50 m MTris HCl pH 8.0, 120 mM NaCl, 0.25% Nonidet P40, 0.1% SDS, protease inhibitors PMSF, aprotinin, leupeptin, and pepstatin (Roche) at a final concentration of 10 ng/mL, 1 mM DTT). A total of 60 mg of each protein sample was subjected to 15% SDS-PAGE and blotted onto a nitrocellulose membrane (Amersham Pharmacia Biotech, USA). Membranes were blocked with 5% non-fat dry milk in TBS-Tween (0.5%) and probed with either rabbit anti-Rat cyclic AMP response-element binding Protein (CREB) monoclonal antibody (2 mg/mL; Abcam) or rabbit anti-Rat β-actin monoclonal antibody (Bioworld, USA) followed by HRP-conjugated anti-rabbit IgG antibody (Amersham Pharmacia Biotech). Immunoreactive protein bands were detected with the ECL detection kit (Amersham Pharmacia Biotech).
Cytokines detection
Serum were harvested and bioactive TNF-α, INF-γ, IL-1β, TGF-β, IL-10 and IL-6 were quantified by ELISA (eBiosciences) according to the manufacturer’s instructions.
Statistical analysis
All statistical analysis was carried out using SPSS9.13 for Windows and expressed as the mean ± SEM. One-way analysis of variance (ANOVA) was performed to determine the statistical significance of the data. Each sample was tested for homogeneity of variance with the Student-Newman-Keuls multiple comparison test for two-variable analysis. If the variance was not homogeneous, nonparametric statistical tests were performed. Differences were considered significant at the P<0.05 level.
Results
Functional outcomes
Mice in the sham group had no observable functional deficit at any time point in the postoperative period. Ischemia with 5 minutes of aortic occlusion produced a significant functional deficit in all mice. Twenty-four hours of pretreatment with dexmedetomidine, PBS, cromolyn, compound 48/80 resulted in a significant neurologic function (P<.05) at all time points postoperatively (Figure 1).
Figure 1.
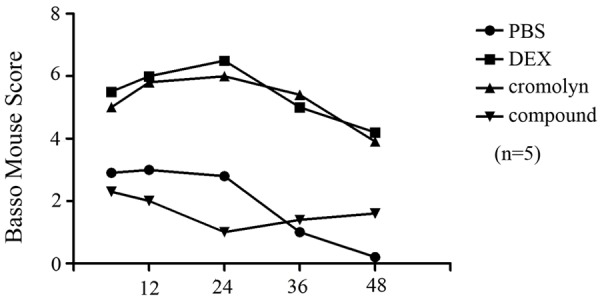
Time (h) following the ischemia. Following ischemia-reperfusion (IR) surgery with 5 minutes of aortic occlusion a significant functional deficit in hind-limb function was observed in all rats. 24 hours of pretreatment with dexmedetomidine (IR+ Dex) and cromolyn (IR+ cromolyn) resulted in significantly higher neurologic function at all time-point postoperatively. The rats pretreated with compound 48/80 had opposite result. Error bars represent mean ± SEM. *P<0.05.
Effect of dexmedetomidine on serum of rats TNF-α, INF-γ and IL-1β levels
To determine whether treatment with dexmedetomidine could reduce inflammation cytokines of MCs degranulation. ELISA assay was performed to evaluate the I/R induced changes in the protein levels of TNF-α, INF-γ and IL-1β, which in part, responsible for the pathogenesis of many inflammatory diseases. Results presented here showed that spinal I/R led to a significant increase in the TNF-α, INF-γ and IL-1β levels at 24 h time point. Perioperative treatment with dexmedetomidine, MCs stabilizer cromolyn both significantly inhibited I/R injury induced up-regulation of TNF-α, INF-γ and IL-1β production, there were no significant (Figure 2). The group of stimulate MCs degranulation compoud 48/80 prompted the I/R injury. This suggested that dexmedetomidine might stabilize the MC membrane and displayed better anti-inflammatory effect in I/R model rats.
Figure 2.
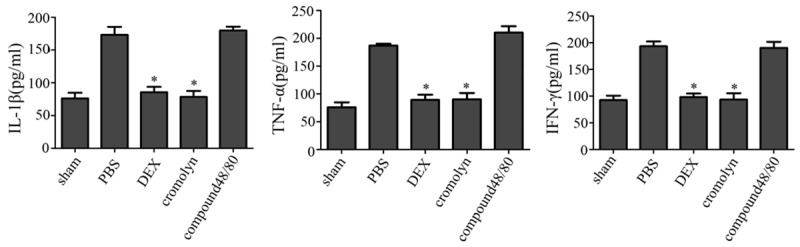
The expression of pro-inflammation cytokines. Dexmedetomidine suppressed I/R induced upregulation of TNF-α, INF-γ and IL-1β levels expressions in spinal. ELISA was performed to detect the serum expression of TNF-α, INF-γ and IL-1β levels 24 h following I/R insult (n = 5; *P<0.05).
Effect of dexmedetomidine on serum of rats TGF-β, IL-10 and IL-6 levels
MCs degranulation could release pro-iflammation cytokines, whereas resting MCs could release anti-inflammation cytokines. To determine whether treatment with dexmedetomidine could stabilze MCs membrane, we detected the anti-inflammation cytokines TGF-β, IL-10 and IL-6 which released by MCs (Figure 3). ELISA assay was performed to evaluate after I/R. The results showed the rats pretreated with dexmedetomidine and cromolyn that the TGF-β, IL-10 and IL-6 levels were high after I/R injury, the group of compoud 48/80 had opposite result. So, this result suggested pretreated with dexmedetomidine made MCs release anti-inflammation cytokines to protect the spinalcord from I/R injury.
Figure 3.
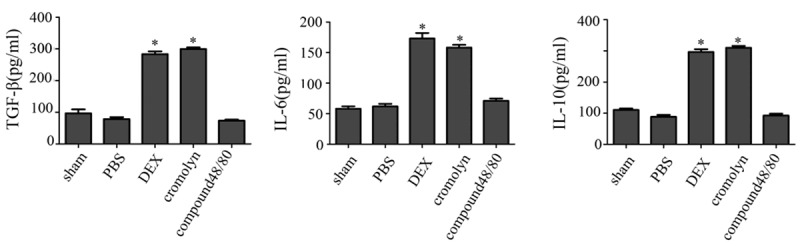
The expression of anti-inflammation cytokines. Dexmedetomidine induced upregulation of TGF-β, IL-10 and IL-6 levels expressions in spinal to alleviate the spinal I/R injury. ELISA was performed to detect the serum expression of TGF-β, IL-10 and IL-6 levels 24 h following I/R insult (n = 5; *P<0.05).
Pretreatment and survival signaling
AKT and CREB phosphorylation are protect protein signaling after I/R injury of neural system. Spinal cord homogenates were analyzed for AKT and CREB phosphorylation. Following pretreatment with dexmedetomidine and cromolyn there were a robust and significant (*P<0.05) increase in AKT and CREB phosphorylation (Figures 4, 5). To determine if CREB phosphorylation resulted in a subsequent production of neuroprotective proteins with dexmedetomidine treatment, spinal cord tissue was tested by quantitative polymerase chain reaction for production of BDNF. Pretreatment with dexmedetomidine resulted in a significant BNDF when compared with the group of compound 48/80 and PBS (Figure 6). Dexmedetomidine treatment resulted in statistically significant elevations in BDNF expression following I/R surgey. There was no significant with the group of cromolyn.
Figure 4.
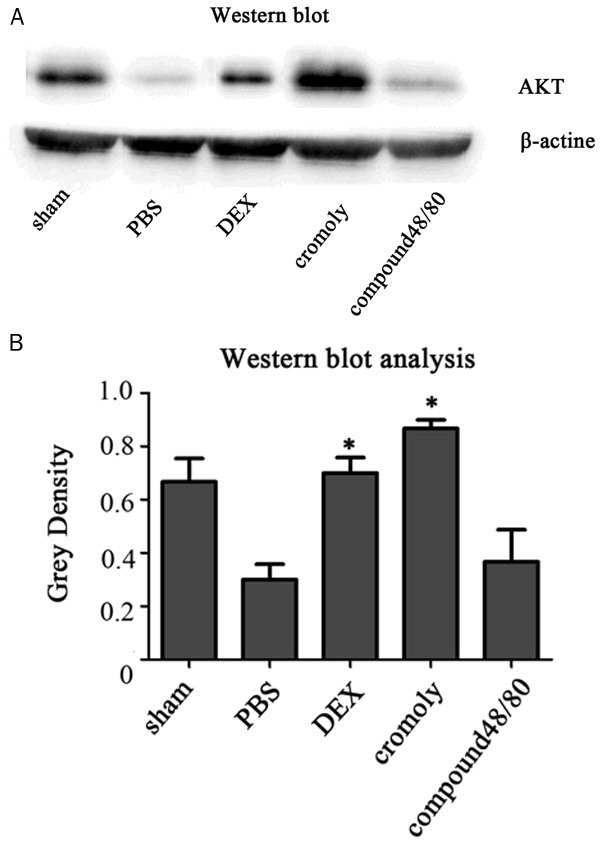
The protein of AKT expression. Dexmedetomidine induced enhancement in spinal AKT expression. A. Western blots assay was performed to evaluate AKT protein expression in the spinal 24 h following I/R insult. B. Bands corresponding to AKT and β-actin protein were quantified densitometrically. Results are expressed as the ratio of full-length AKT to β-actin. Data are means ± SD of three independent experiments performed in triplicate or a representative result. *P<0.05.
Figure 5.
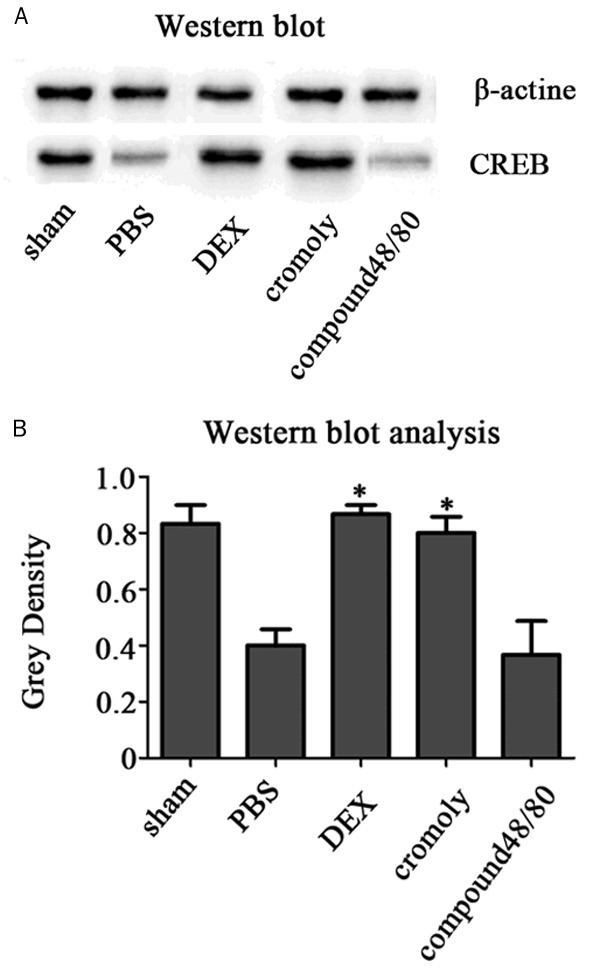
The protein of CREB expression. Dexmedetomidine induced enhancement in spinal CREB expression. A. Western blots was performed to evaluate CREB protein expression in the spinal 24 h following I/R insult. B. Bands corresponding to CREB and β-actin protein were quantified densitometrically. Results are expressed as the ratio of full-length CREBto β-actin. Data are means ± SD of three independent experiments performed in triplicate or a representative result. *P<0.05.
Figure 6.
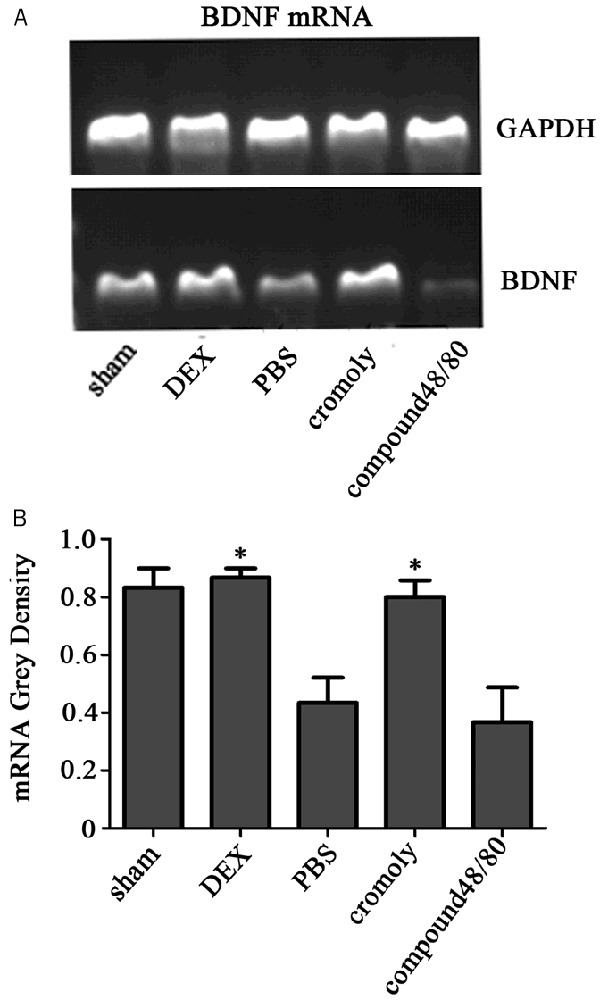
The mRNA of BDNF expression. A. Quantitative polymerase chain reaction for production of brain-derived neurotrophic factor (BDNF) transcripts was performed. Pretreatment with dexmedetomidine resulted in a significant up-regulation BNDF messenger RNA expression. B. Bands corresponding to BDNF and GAPDH were quantified densitometrically. Data are means ± SD of three independent experiments performed in triplicate. *P<0.05.
Dexmedetomidine down regulation the FcεR of MCs
To examine the indirect effect of dexmedetomidine on MCs, we extracted the rat MCs from bone marrow (MCs in spinal and bone marrow are homologous), then, pretereated with PBS, dexmedetomidine, cromolyn, compoud48/80 in vitro. After 24 h, expression of FcεR at the MCs surface was down-regulated (Figure 7), and there was no difference between the cromolyn group and DEX group. So, dexmedetomidine could alleviate the MCs activity to block them degranulation.
Figure 7.
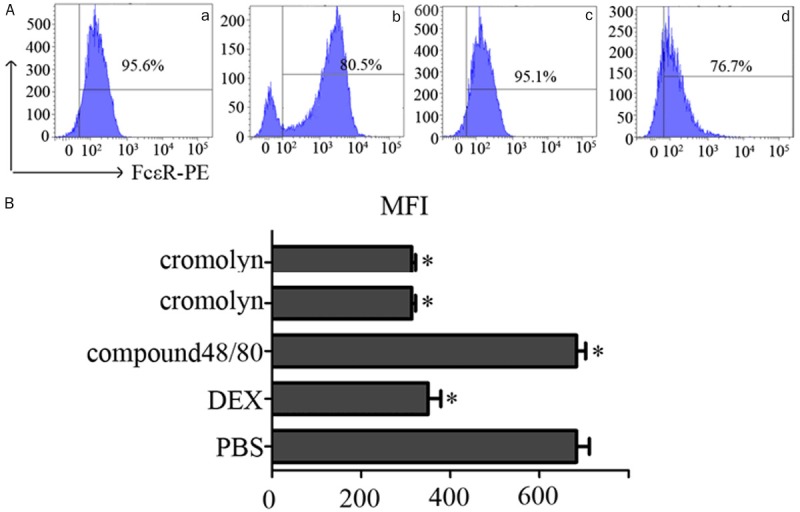
Expression of FcεR on the surface of MCs. MCs pretreated with PBS DEX cromolyn compound 48/80. A. FcεR expression on MCs decreased with DEX (b) and cromolyn (d). PBS (a) and compound 48/80 (c) had the opposite results. B. Expression of the FcεR on the MCs surface shown as mean fluorescence intensity (MFI) measured by flow cytometry after incubation for 24 h (with isotope control subtracted). Data are presented as the percentage MFI of MCs alone which was set as 100%. Data are means ± SEM of three independent experiments. Statistical significance was calculated using the Student’s t-test. *P<0.05.
The degranulation of MCs pretreated with dexmedetomidine was decreased
MCs pretreated with PBS, DEX, cromolyn, compound 48/80 after 24 h. Using anti-DNP-IgE to stimulate MCs degranultion, we found the MCs pretreated with DEX, cromolyn they degranulation were decreased, which compared with the group of PBS and compound 48/80 (*P<0.05) (Figure 8). So, the dexmedetomidine could stabilize the MCs membrane.
Figure 8.
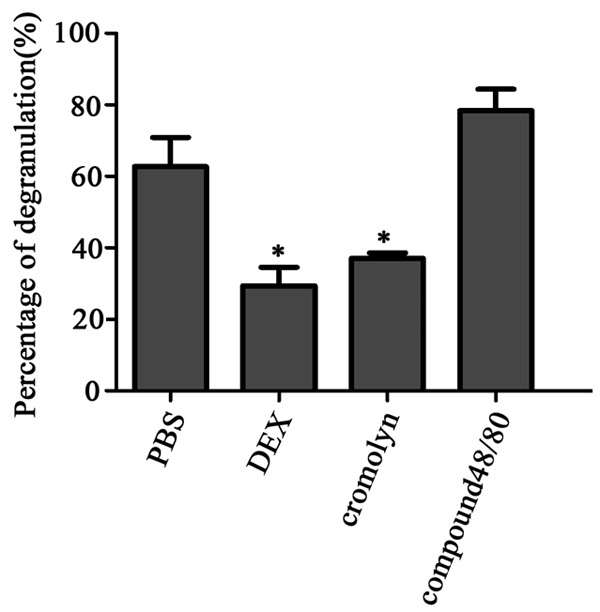
MCs degranulation induced by anti –DNPIgE mAb and DNP-HAS was evaluated by 4-Nitrophenyl N-acetyl-β-D-glucosaminide after pretreatment. Compared with the control group, pretreatment with PBS, compound 48/80 obviously elicited MCs degranulation, whereas the DEX and cromolyn groups decreased the MCs degranulation, there were not significantly different. Data are shown as the mean ± SD of three independent experiments performed in triplicate. *P<0.05.
Discussion
Ischemiaereperfusion (I/R) injury is inevitable in many surgery, vascular and musculoskeletal traumas, diseases, and free tissue transfers and during time-consuming reconstructive and transplantation surgeries [21]. The mechanism of organ damage after I/R injury has been studied extensively and consists of complex interactions of multiple pathways. The major contributors to I/R-induced injury included production of reactive oxygen species (ROS), release of proinflammatory cytokines that exacerbate inflammation and tissue damage and activation of immune cells to promote cell death by apoptosisor necrosis [22]. Some studies found that the dexmedetomidine could alleviate the I/R injury in rat skeletal muscle [23]. Experimental studies have shown that dexmedetomidine exerts an antioxidant effect in several organs, including brain, kidney, and heart [24]. In addition, dexmedetomidine has been shown to reduce the expression of I/R injury markers during upper extremity surgery using a tourniquet in human studies [25]. A previous study also showed that severe damage in the cerebral injury was accompanied by a increase in proinflammtion cytokines, which was restored after dexmedetomidine administration [26], and as α-2a adrenergic agonist could increase phosphorylation of protein kinase B and CREB and subsequent up-regulation of antiapoptotic factor brain-derived neurotrophic factor to protective mechanism in the spinal cord following ischemia [27,28]. But the specific mechaniam of the role of dexmedetomidine in I/R injury is not clear. MCs have long been thought to only possess effecter cell functions, given that they store an armada of pro-inflammatory cytokines and chemokines in their granules. MCs have been shown to promote I/R injury in many organs, mainly through their degranulation [29,30]. However, the role of MCs in spinal I/R injury is less clear. MCs have bidirectional role, they can release much of proinflammation cytokines to induce I/R injury through degranulation, and they also can release anti-inflammation cytokine to alleviate the I/R injury in the resting state [31]. So, we pretreated the rats with dexmedetomidine, cromolyn (the stabilizer of MCs membrane), compound 48/80 (inducer of MCs degranulation)to observe the mechanism of dexmedetomidine in spinal I/R injury. In our study, we found that pretreated the rats with dexmedetomidine has higher neurologic function at all time points after I/R injury. We collected the serum of rats then detected the pro-inflammation cytokines TNF-α, INF-γ and IL-1β levels and anti-inflammation cytokinses TGF-β, IL-10 and IL-6 levels, found that the pro-inflammation cytokines of dexmedetomidine group was decreased whereas the anti-inflammation cytokinses was increased. At the same time the protect protein of AKT, CREB and mRNA BDNF were increased. They had the same results with cromolyn group, and opposite with the compound 48/80 group. So, we thought dexmedetomidine might stabilize the MCs membrane to alleviate the spinal I/R injury in vivo. To observe the indirect role of dexmedetomidine on MCs. We pretreated MCs with dexmedetomidine in vitro, and found that the activity surface molecular of MCs was down-regulation, and MCs degranulation was decreased. We thus demonstrate a possible mechanism by which dexmedetomidine alleviates spinal cord I/R injury through blocking the MCs degranulation.
Acknowledgements
This study is supported by Zhejiang provincial Medical and Health Science Foundation (2015KYB249) and Zhejiang provincial Student Innovation and Xinmiao Talents Foundation (2015R413040).
Disclosure of conflict of interest
None.
References
- 1.Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118:808–17. doi: 10.1161/CIRCULATIONAHA.108.769695. [DOI] [PubMed] [Google Scholar]
- 2.Amaro S, Chamorro Á. Translational stroke research of the combination of thrombolysis and antioxidant therapy. Stroke. 2011;42:1495–1499. doi: 10.1161/STROKEAHA.111.615039. [DOI] [PubMed] [Google Scholar]
- 3.Chen YF, Tsai HY, Wu KJ, Siao LR, Wood WG. Pipoxolan ameliorates cerebral ischemia via inhibition of neuronal apoptosis and intimal hyperplasia through attenuation of VSMC migration and modulation of matrix metalloproteinase 2/9 and Ras/MEK/ERK signaling pathways. PLoS One. 2013;8:e75654. doi: 10.1371/journal.pone.0075654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Han B, Ma X, Qi S. The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia-reperfusion in rats. Brain Res. 2010;1356:11–23. doi: 10.1016/j.brainres.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Demuro JP, Botros D, Nedeau E. Use of dexmedetomidine for postoperative analgesia in spine patients. J Neurosurg Sci. 2013;57:171–174. [PubMed] [Google Scholar]
- 6.Reardon DP, Anger KE, Adams CD. Role of dexmedetomidine in adults in the intensive care unit: an update. Am J Health Syst Pharm. 2013;70:767–777. doi: 10.2146/ajhp120211. [DOI] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 8.Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: Impact on anaesthetic-induced neuroapoptosis in the rat developingbrain. Acta Anaesthesiologica Scandinavica. 2010;54:710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 9.Ayoglu H, Gul S, Hanci V. The effects of dexmedetomidine dosage on cerebral vasospasm in a rat subarachnoid haemorrhage model. J Clin Neurosci. 2010;17:770–773. doi: 10.1016/j.jocn.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response elementbinding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–8. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiologica Scandinavica. 2011;55:1272–1278. doi: 10.1111/j.1399-6576.2011.02526.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanci V, Erol B, Bektas S. Effect of dexmedetomidine on testicular torsion/detorsion damage in rats. Urologia Internationalis. 2010;84:105–111. doi: 10.1159/000273476. [DOI] [PubMed] [Google Scholar]
- 13.Okita Y. Fighting spinal cord complication during surgery for thoracoabdominal aortic disease. Gen Thorac Cardiovasc Surg. 2011;59:79–90. doi: 10.1007/s11748-010-0668-x. [DOI] [PubMed] [Google Scholar]
- 14.Jaggi AS, Singh M, Sharma A. Cardioprotective effects of mast cell modulators in ischemiareperfusion-induced injury in rats. Methods Find Exp Clin Pharmacol. 2007;29:593–600. doi: 10.1358/mf.2007.29.9.1161005. [DOI] [PubMed] [Google Scholar]
- 15.Biran V, Cochois V, Karroubi A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008;18:1–9. doi: 10.1111/j.1750-3639.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldford SA, Haidl ID, Howatt MA. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J Immunol. 2010;185:7067–76. doi: 10.4049/jimmunol.1001137. [DOI] [PubMed] [Google Scholar]
- 17.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007;217:141–54. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 19.Kageyama Yahara N, Suehiro Y, Yamamoto T, adowaki KM. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 2008;377:321–325. doi: 10.1016/j.bbrc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Smith PD, Puskas F, Meng X, Cho D, Cleveland JC, Weyant MJ. Ischemic dose-response in the spinal cord: both immediate and delayed paraplegia. J Surg Res. 2011;7:1–7. doi: 10.1016/j.jss.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Wang WZ, Baynosa RC, Zamboni WA. Therapeutic interventions against reperfusion injury in skeletal muscle. J Surg Res. 2011;17:175–82. doi: 10.1016/j.jss.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: Established mechanisms and recent advancements. Surg Clin North Am. 2001;90:665–77. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemiaereperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Gu J, Sun P, Zhao H. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yagmurdur H, Ozcan N, Dokumaci F. Dexmedetomidine reduces the ischemia-reperfusion injury markers during upper extremity surgery with tourniquet. J Hand Surg Am. 2008;33:941–7. doi: 10.1016/j.jhsa.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Grosu I, Lavand’homme P. Continuous regional anesthesia and inflammation, a new target. Minerva Anestesiol. 2015;81:1001–9. [PubMed] [Google Scholar]
- 27.Bell MT, Puskas F, Bennett DT, Herson PS, Quillinan N, Fullerton DA, Reece TB. Dexmedetomidine, an a-2a adrenergic agonist, promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion. J Thorac Cardiovasc Surg. 2014;147:500–6. doi: 10.1016/j.jtcvs.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Kim KH. Safe Sedation and Hypnosis using Dexmedetomidine for Minimally Invasive Spine Surgery in a Prone Position. Korean J Pain. 2014;27:313–320. doi: 10.3344/kjp.2014.27.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abonia JP, Friend DS, Austen WG Jr. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol. 2005;174:7285–91. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin FS, Shen SQ, Chen ZB, Yan RC. Betaestradiol attenuates reduced-size hepatic ischemia/reperfusion injury by inhibition apoptosis via mitochondrial pathway in rats. Shock. 2012;37:183–90. doi: 10.1097/SHK.0b013e31823f1918. [DOI] [PubMed] [Google Scholar]
- 31.Schemann M, Kugler EM, Buhner S. The mast cell degranulator compound 48/80 directly activates neurons. PLoS One. 2012;7:e52104. doi: 10.1371/journal.pone.0052104. [DOI] [PMC free article] [PubMed] [Google Scholar]


