Abstract
Objective: A meta-analysis was undertaken to provide an evidence-based basis of clinical trials comparing extralevator abdominoperineal excision with conventional abdominoperineal excision for low rectal tumor. Methods: We searched through the major medical databases such as PubMed, EMBASE, Medline, Science Citation Index, Web of Science for all published studies without any limit on language from January 2009 until January 2015. The following search terms were used: extralevator abdominoperineal excision or cylindrical abdominoperineal resection or conventional abdominoperineal excision or abdominoperineal excision or rectal cancer. Furthermore, Additional related studies were manually searched in the reference lists of all published reviews and retrieved articles. Results: In this meta-analysis, there are a total number of 1797 patients included: 1099 patients in the ELAPE group and 698 in the APE group, and there are not statistically differences between groups in CRM [RR=0.65, 95% CI (0.41, 1.04), P=0.07] and wound complications [RR=1.14, 95% CI (1.09, 1.66), P=0.45] between ELAPE and APE. However, ELAPE has a lower rate of intraoperation perforation [RR=0.44; 95% CI (0.33, 0.60); P<0.00001] and local recurrence [RR=0.45, 95% CI (0.27, 0.77), P=0.003] than APE in terms of short follow-up time.
Keywords: Rectal cancer, extralevator abdominoperineal excision, abdominoperineal excision, meta-analysis
Introduction
Surgery of the lower rectal cancer has improved the prognosis of rectal cancer, often in combination with neoadjuvant therapies. Conventional abdominoperineal Excision (APE) was firstly described by Miles [1] in 1908 and it had been a gold standard for the low rectal cancer. However, since the reintroduction of Miles’ original extended APE operation by the Swedish surgeon Torbjo ¨n Holm [2], ELAPE had gained popularity among colorectal surgeons and some studies [3,4] point out that ELAPE shares a better outcome in intra-operative perforation (IOP), circumferential resection margin (CRM) involvement, local recurrence (LR) rates, and complictions of wound (CW) than APE. The value of ELAPE still has remained questioned. One randomized control trials (RCT) [5] and several retrospective studies (RTs) [6-14] comparing Conventional APE with ELAPE have been conducted, Therefore, we performed this meta-analysis of the published data to compare the two kinds of surgeries to help surgeon make a better clinical choice.
Materials and methods
We searched through the major medical databases such as PubMed, EMBASE, Medline, Science Citation Index, Web of Science for all published studies without any limit on language from January 2009 until January 2015. The following search terms were used: extralevator abdominoperineal excision or cylindrical abdominoperineal resection or conventional abdominoperineal excision or abdominoperineal excision or rectal cancer. Furthermore, Additional related studies were manually searched in the reference lists of all published reviews and retrieved articles (Figure 1).
Figure 1.
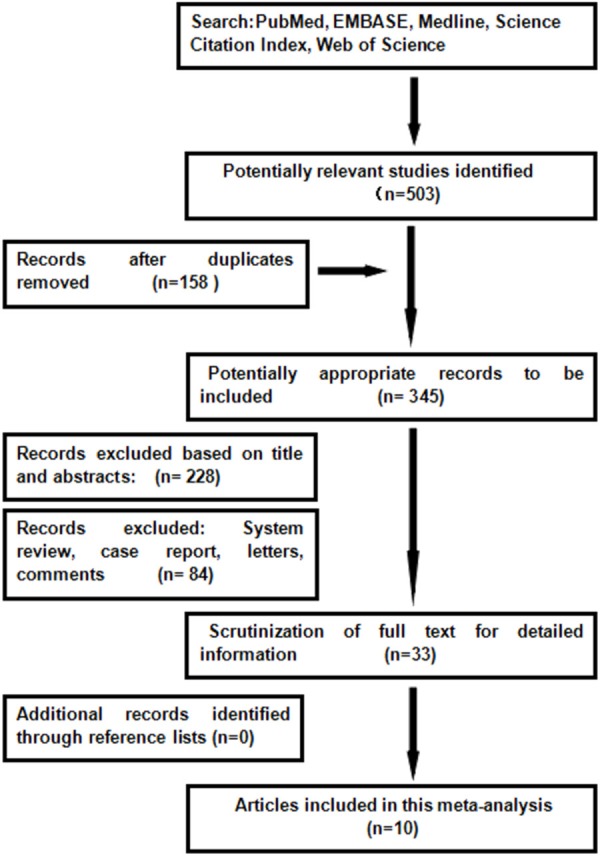
Preferred reporting items for meat-analysis flow of study selection.
Inclusion criteria
(1) The studies must be published as a full paper without any limitation in language; (2) The trials had to cover the original outcomes of patients of both ELAPE and APE; (3) The data of patients’ clinical and pathologic parameters (age, sex, tumor differentiation and so on) are reported; (4) Assessing at least one of our interested outcome: local recurrence (LR) rate, perforation, wound complication and positive CRM rate.
Exclusion criteria
(1) Studies that without full text articles or could not obtained; (2) No initial data or only assessing parameter of neither ELAPE or APE; (3) The study was not conducted on human; (4) Experimental trails, case report, letters, and comments were also excluded.
Data extraction and study quality assessment
Studies selection from the included trails were conducted independently by two authors, and any disagreement was resolved by consensus. The main extracted data included: first author, year of publication, institution, study design, inclusion and exclusion criteria, matching criteria, sample size (cases and controls or cohort size) and outcomes of interest. The Newcasle-Ottawa Scale (NOS) was applied to assess the quality of the studies, and a study with ≥7 awarded stars was considered as a high-quality study (Table 1).
Table 1.
Characteristics and quality assessment of the included studies
| Reference (year) | Country | Design | Period | No. of patients | Age (year) | Gender (F/M) | Neoadjuvant therapy (pre-operative) | Interested outcomes | Study quality | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Chemoradiotherapy | Radiotherapy | ||||||||||
|
| |||||||||||
| ELAPE/APEE | ELAPE/APE | ELAPE | APE | ELAPE/APE | ELAPE/APE | ||||||
| Barker. et al. 2012 | UK | N-RCT | 2004-2011 | 12/9 | 69/70 | 5/7 | 3/6 | 1/8 | 2/0 | 3, 4 | ******** |
| D. Asplund. et al. 2011 | Sweden | N-RCT | 2004-2009 | 79/79 | 67/68 | 35/44 | 24/55 | 15/5 | 60/66 | 1, 2, 3, 4 | ******* |
| Han. et al. 2012 | China | RCT | 2008-2010 | 35/32 | 63/68 | 15/20 | 11/22 | 10/9 | 1, 2, 3, 4 | ******* | |
| Mattias Prytz et al. 2014 | Sweden | N-RCT | 2007-2009 | 518/209 | 68/71 | 209/309 | 93/116 | 159/32 | 456/144 | 1, 2, 4 | ******** |
| N. P. West. et al. 2009 | UK | N-RCT | 1997-2008 | 176/124 | 66/68 | 54/116 | 37/87 | 84/48 | 130/90 | 1, 2, 4 | ***** |
| P. G. Vaughan-Shaw. et al. 2012 | UK | N-RCT | 2009-2011 | 16/20 | 71/72 | 9/7 | 7/13 | 9/7 | 7/9 | 1, 2, 3, 4 | ******** |
| S. K. perdawood et al. 2014 | Denmark | N-RCT | 2006-2012 | 68/39 | 68/69 | 23/45 | 12/27 | 58/19 | 1, 2, 3, 4 | ******** | |
| Stelzner. et al. 2011 | German | N-RCT | 1997-2010 | 28/46 | 66/64 | 9/19 | 9/37 | Data not available | 1, 2, 4 | ******* | |
| Zhangxin. et al. 2014 | China | N-RCT | 2011-2013 | 33/28 | 58/62 | 15/18 | 14/14 | Data not available | 2, 4 | ****** | |
| Martijnse. et al. 2011 | UK | N-RCT | 2000-2010 | 134/112 | 63/62 | 15/18 | All underwent | 1, 2, 3 | ***** | ||
1: CRM; 2: IOP; 3: LR; 4: Wound complication; RCT: Randomized Controlled Trial; N-RCT: Not Randomized Controlled Trial.
Statistical analysis
We used the Review Manager software (RevMan 5.3, Cochrane Collaboration) to carry out the meta-analysis. For analysis, RR was estimated to compare CRM, LR, IOP and CW between two groups, along with the 95% confidence intervals (95% CI). Moreover, The Cochrane chi-square test and inconsistency (I2) were used to evaluate the heterogeneity and if I2 is above 50%, which indicates a heterogeneity and a random effects model was used such as in CRM, LR, IOP and CW. Funnel plots were applied to assess publication bias, meanwhile sensitivity analysis was conducted by excluding the heterogeneity-causing studies.The pooled effects were determined by the Z-test, and a P value <0.05 was considered to be statistically significant.
Results
Description of eligible studies
Using the search strategy, we selected more than 500 abstracts which were published before January 2015. After carefully reviewing, we identified 10 eligible studies [5-14] for analyses from 2009 to 2014, and this resulted in a total number of 1797 patients: 1099 patients in the ELAPE group and 698 in the APE group. Of one trial was randomized controlled trial (RCT) [11], and rest of all were retrospective trails. In addition, four studies [6,7,11,13] were conducted in UK, two [9,12] in Sweden, two [5,8] in China, one [14] in German, and one [10] in Denmark. The quality of all the studies was satisfactory. The results showed that ELAPE had a lower rate of IOP and LR, but no significant difference between the two techniques of CRM and wound complication.In this meta-analysis, there are not statistically differences between groups in CRM [RR=0.65, 95% CI (0.41, 1.04), P=0.07] and wound complications [RR=1.14, 95% CI (1.09, 1.66), P=0.45] between ELAPE and APE. However, ELAPE has a lower rate of intraoperation [RR=0.45, 95% CI (0.27, 0.77), P=0.003] than APE in terms of short follow-up time (Figures 2, 3, 4 and 5).
Figure 2.
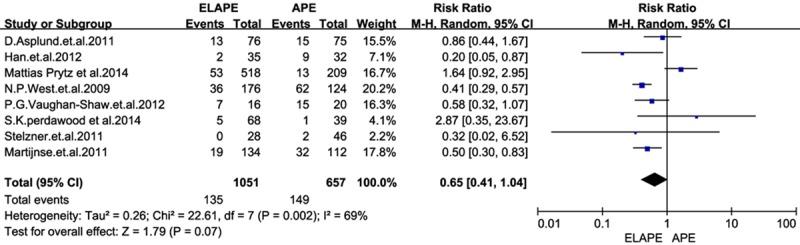
Forrest plot-analysis of circumferential resection margin.
Figure 3.
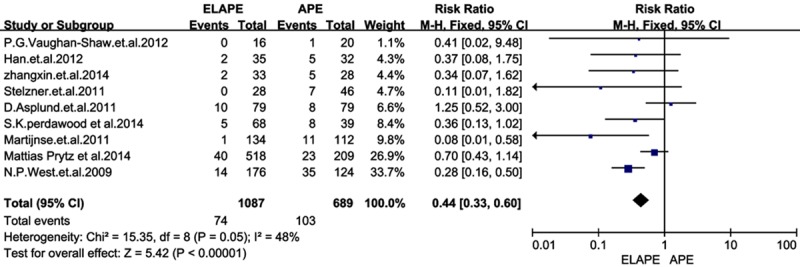
Forrest plot-analysis of intraoperation perforation.
Figure 4.
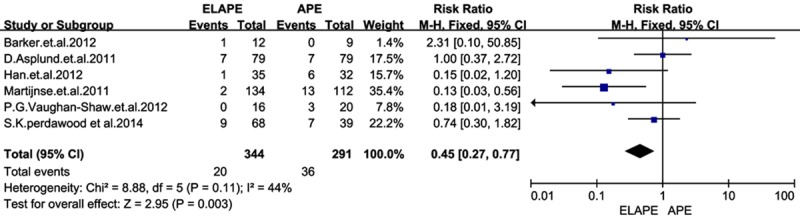
Forrest plot-analysis of local recurrence.
Figure 5.
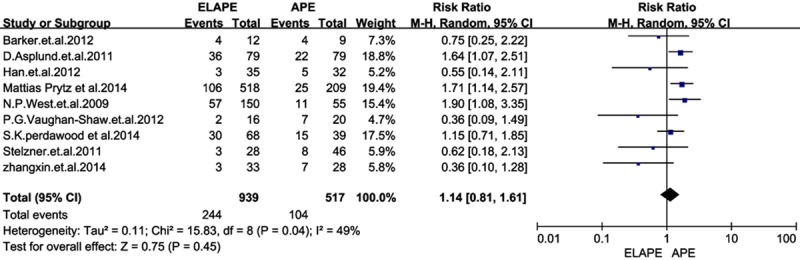
Forrest plot-analysis of Wound complication.
The sensitivity analysis was performed by omitting one study at a time, generating the pooled estimates and comparing with the original estimates. The results between studies was stable and heterogeneity was not significantly reduced by the sensitivity analysis in terms of CRM, CW and IOP,however it is significantly reduced in LR (I2=4%, P=0.12) when it was omitting of Martijnse [11] (Figure 6). And the funnel plots (Figure 7) showed there were no significant publication bias.
Figure 6.
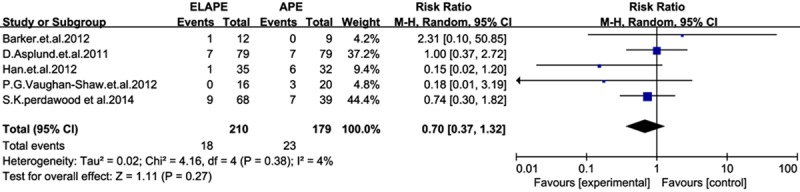
Sensitivity analysis of local recurrence.
Figure 7.
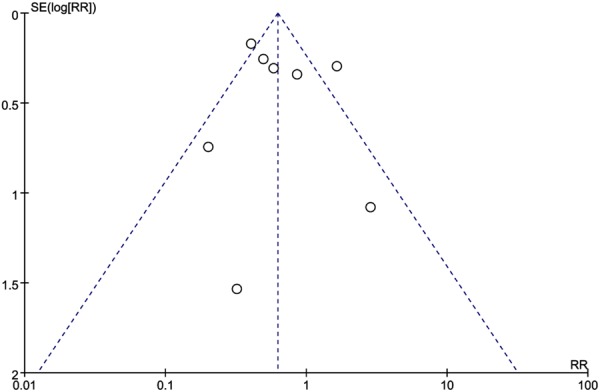
Funnel plots of studies to detect publication bias.
Discussion
For a century, conventional abdominoperineal excision (APE) has been a gold standard for the low rectal cancer. Regardless the approach, APE represented a uniquely challenging procedure where the surgeon is expected to reduce the CRM, LR and wound complication but shorter operation time. In this setting, the application of APE becomes even more questionable, thus ELAPE was reintroduced by Holm in 2009 [2]. Since then, some previous articles showed that ELAPE for low rectal cancer is associated with better oncologic results than APE in the short-term. Therefore, we aimed this meta-analysis to compare ELAPE with APE in our interested outcomes (local recurrence rate (LR), perforation, wound complication and positive CRM), and we found that there is no significant difference between the two techniques in CRM and wound complication. On the other hand, ELAPE is associated with a lower risk of IOP and LR.
As described by N.P.West et al [6], ELAPE was leading to a reduction in CRM involvement (from 49.6 to 20.3 percent; P<0.001) and IOP (from 28.2 to 8.2 percent; P<0.001), but with an increase in perineal wound complications (from 20 to 38.0 percent; P=0.019) compared with standard surgery. In contrast, we demonstrated that there were not statistically differences between groups in CRM and wound complications. In theory, ELAPE should have a lower rate of CRM than APE, but considering the involvement of CRM, there were kinds of influences on it such as the tumor staging, preoperative therapies and so on [15,16]. As the quality of the pathology report had improved, it became more structured with more consistent recording of CRM. Besides, preoperative adjuvant therapy [17,18] was more and more common among patients operated in both ELAPE and APE during the time, and this may reflect improved radiological tumour staging and standardized national guidelines, rather than a real difference between two groups in CRM. Admittedly, patients were with different clinical and pathologic parameters, all of which were hard to match, thus it might confer a possible bias to the heterogeneity. Moreover, reviewing the West’s comparative studies [6], they defined that ELPAE was with a lower rate of CRM positivity than APE while ELAPE were performed by 11 surgeons at nine different hospitals. In this regard, we should carefully judge the result of such a comparison. As with any other new technique, a learning curve is likely to exist in relation to ELAPE. The different surgeon with varied comprehension of EAPLE could make a difference in CRM. We have, thus, the reason to suspect that there might be any bias in reporting CRM to the European Extralevator Abdominoperineal Excision Study Group between the two types of procedures [6].
Moreover, wound complication rate was broadly considered as another surrogate of surgical competence [19]. Comparing the two procedures, both groups had a similar high rate of post-operation wound complication. Major perineal wound complications could include wound infection, dehiscence, and herniation in studies. From the anatomy of view [20], APE was in process with disappearing of structure of the mesorectum, which was hard to separate tissues in a distinct stratified anatomy and avoid bleeding under the direct vision. That might be a reason of the complication rate as high as 59.2% in APE [21]; meanwhile though, ELAPE evaded the malpractice of APE via changing the position and enlarge resection. Whereas leaving the levator muscles and ischiorecal fossa fat in EALPE, reconstruction of the pelvic defect with various flaps posed a difficult challenge for surgeons [22-25]. These could result in a higher rate of complication as 62.5%. There were pros and cons to both ELAPE and APE, we found no reason to believe that two groups differed in this respect.
In previous findings, intraoperative bowel perforation had a negative effect on local recurrence. We had shown in this meta-analysis, the local recurrence rate and interoperation perforation were significantly higher in APE than in ELAPE. The perforation rate was reported of 20.5% in APE in S.K. perdawood et al [10]. This high rate of IOP might attribute to the position of patients. The perineal part of ELAPE was usually done in prone jack-knife position while APE was done in supine position. Removal of the tissue combined with an easier view of the operation field could be reasons for the lower perforation rate in the ELAPE group. In addition, ELAPE involved mobilization of the mesorectum as far down as the origins of levator muscles and ischiorectal fossa fat attached to the specimen en bloc, and a more cylindrical specimen is created [26], thus ELAPE avoided forming a waist on the specimen, which may refrain from perforation. Bark et al. [13] have demonstrated that more tissue is removed in ELAPE than in APE, which could result in a lower rate of local recurrence. In the series reported by Han, Barker and Vaughan-Shaw [7,12,13], the results also supported this opinion as we expected. As for the heterogeneity showed in LR, the reason why Martijnse. [11] could be the responsibility may contribute to newadjuvant therapies, and local recurrence was associated with high pathologic T stage. Downstaging by neoadjuvant therapy is essential to achieve radical resection.As most APE in Martijnse’s cases [11] were performed during 2000-2006, with poor chemo-radiotherapy. We felt tha there was a increased risk for LR in APE in Martijnse’s studies, thus we call for more well-matched studies to carry out a conclusion.
When taking a closer look at each study, the RCT [5] show Patients who received ELAPE had the potential to reduce the risk of CRM (3.2% vs 7.8%, P=0.013) without increased complications. In our meta-analysis, we demonstrate there are not any differences between groups in CRM or wound complications. Therefore, we conducted a sensitivity analysis excluding the RCT, and the results were in accord with our overall analysis. Since there were too few RCTs to enable us to draw any definitive conclusions, we expected more sensitivity RCT to confirm the reliability of results.
The present meta-analysis carries few limitations that must be taken into account. The main limitation is that most of the studies were retrospective trails except for one RCT, and few had a small sample size. In addition, most patients were not matched in age, BMI, tumor stage, preoperative therapy, and other physical state. Nevertheless, the length of follow-up varied among studies and few even not mentioned, which could be a potential source of bias. All these factors may have contributed to the high heterogeneity between studies.
Conclusions
In this meta-analysis, there are not statistically differences between groups in CRM and wound complications between ELAPE and APE. However, ELAPE has a lower rate of intraoperation perforation and local recurrence than APE in terms of short follow-up time. Ultimately, the role of ELAPE and APE for low rectal cancer remains to be defined and, ideally, this novel approach should be compared with APE in a well-designed, large, prospective, randomized and matched study before gaining wider acceptance.
Disclosure of conflict of interest
None.
References
- 1.Holm T, Ljung A, Haggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94:232–8. doi: 10.1002/bjs.5489. [DOI] [PubMed] [Google Scholar]
- 2.Holm T. Controversies in abdominoperineal excision. Surg Oncol Clin N Am. 2014;23:93–111. doi: 10.1016/j.soc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Palmer G, Anderin C, Martling A, Holm T. Local control and survival after extralevator abdominoperineal excision for locally advanced or low rectal cancer. Colorectal Dis. 2014;16:527–32. doi: 10.1111/codi.12610. [DOI] [PubMed] [Google Scholar]
- 4.Kennelly RP, Rogers AC, Winter DC. Multicentre study of circumferential margin positivity and outcomes following abdominoperineal excision for rectal cancer. Br J Surg. 2013;100:160–6. doi: 10.1002/bjs.9001. [DOI] [PubMed] [Google Scholar]
- 5.Han JG, Wang ZJ, Wei GH, Gao ZG, Yang Y, Zhao BC. Randomized clinical trial of conventional versus cylindrical abdominoperineal resection for locally advanced lower rectal cancer. Am J Surg. 2012;204:274–282. doi: 10.1016/j.amjsurg.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 6.West NP, Anderin C, Smith KJ, Holm T, Quirke P. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg. 2010;97:588–99. doi: 10.1002/bjs.6916. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan-Shaw PG, Cheung T, Knight JS, Nichols PH, Pilkington SA, Mirnezami AH. A prospective case-control study of extralevator abdominoperineal excision (ELAPE) of the rectum versus conventional laparoscopic and open abdominoperineal excision: comparative analysis of short-term outcomes and quality of life. Tech Coloproctol. 2012;16:355–62. doi: 10.1007/s10151-012-0851-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Shen Z, Xie Q, Yin M, Yang X, Jiang K, Wang Y, Cao J, Ye Y, Wang S, Liang B. [Extralevator abdominoperineal excision versus traditional abdominoperineal excision in the treatment of low rectal cancer] . Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:1106–10. [PubMed] [Google Scholar]
- 9.Prytz M, Angenete E, Ekelund J, Haglind E. Extralevator abdominoperineal excision (ELAPE) for rectal cancer-short-term results from the Swedish Colorectal Cancer Registry. Selective use of ELAPE warranted. Int J Colorectal Dis. 2014;29:981–987. doi: 10.1007/s00384-014-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perdawood SK, Lund T. Extralevator versus standard abdominoperineal excision for rectal cancer. Tech Coloproctol. 2015;19:145–152. doi: 10.1007/s10151-014-1243-8. [DOI] [PubMed] [Google Scholar]
- 11.Martijnse IS, Dudink RL, West NP, Wasowicz D, Nieuwenhuijzen GA, van Lijnschoten I, Martijn H, Lemmens VE, van de Velde CJ. Focus on Extralevator Perineal Dissection in Supine Position for Low Rectal Cancer Has Led to Better Quality of Surgery and Oncologic Outcome. Ann Surg Oncol. 2012;19:786–793. doi: 10.1245/s10434-011-2004-9. [DOI] [PubMed] [Google Scholar]
- 12.Asplund D, Haglind E, Angenete E. Outcome of extralevator abdominoperineal excision compared with standard surgery: results from a single centre. Colorectal Dis. 2012;14:1191–1196. doi: 10.1111/j.1463-1318.2012.02930.x. [DOI] [PubMed] [Google Scholar]
- 13.Barker JA, Blackmore AE, Owen RP, Rate A. Prone cylindrical abdominoperineal resection with subsequent rectus abdominis myocutaneous flap reconstruction performed by a colorectal surgeon. Int J Colorectal Dis. 2013;28:801–806. doi: 10.1007/s00384-012-1586-4. [DOI] [PubMed] [Google Scholar]
- 14.Stelzner S, Hellmich G, Schubert C, Puffer E, Haroske G, Witzigmann H. Short-term outcome of extra-levator abdominoperineal excision for rectal cancer. Int J Colorectal Dis. 2011;26:919–925. doi: 10.1007/s00384-011-1157-0. [DOI] [PubMed] [Google Scholar]
- 15.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 16.Reid TD, Chan DS, Roberts SA, Crosby TD, Williams GT, Lewis WG. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer and the predictive role of endoluminal ultrasonography. Br J Cancer. 2012;107:1925–31. doi: 10.1038/bjc.2012.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Gu GL, Li ZW, Peng YF, Gu J. Abdominoperineal excision following preoperative radiotherapy for rectal cancer: unfavorable prognosis even with negative circumferential resection margin. World J Gastroenterol. 2014;20:9138–45. doi: 10.3748/wjg.v20.i27.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollett WG, Gibbs P, McLaughlin S, Eteuati J, Harold M, Marion K, Patel S, Jones I. Outcomes in the surgical treatment of low rectal cancer: does neoadjuvant treatment equalize results? ANZ J Surg. 2015;85:140–4. doi: 10.1111/ans.12786. [DOI] [PubMed] [Google Scholar]
- 19.Morris E, Quirke P, Thomas JD, Fairley L, Cottier B, Forman D. Unacceptable variation in abdominoperineal excision rates for rectal cancer: time to intervene? Gut. 2008;57:1690–7. doi: 10.1136/gut.2007.137877. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Shen Z, Wang S. [Three types of abdominoperineal excision procedures for the rectal cancer based on anatomic landmarks classification] . Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:1170–4. [PubMed] [Google Scholar]
- 21.West NP, Anderin C, Smith KJ, Holm T, Quirke P European Extralevator Abdominoperineal Excision Study Group. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg. 2010;97:588–99. doi: 10.1002/bjs.6916. [DOI] [PubMed] [Google Scholar]
- 22.Shihab OC, Heald RJ, Holm T, How PD, Brown G, Quirke P, Moran BJ. A pictorial description of extralevator abdominoperineal excision for low rectal cancer. Colorectal Dis. 2012;14:e655–60. doi: 10.1111/j.1463-1318.2012.03181.x. [DOI] [PubMed] [Google Scholar]
- 23.Fischer A, Tarantino I, Warschkow R, Lange J, Zerz A, Hetzer FH. Is sphincter preservation reasonable in all patients with rectal cancer? Int J Colorectal Dis. 2010;25:425–32. doi: 10.1007/s00384-010-0876-y. [DOI] [PubMed] [Google Scholar]
- 24.Nisar PJ, Scott HJ. Myocutaneous flap reconstruction of the pelvis after abdominoperineal excision. Colorectal Dis. 2009;11:806–16. doi: 10.1111/j.1463-1318.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 25.Barker T, Branagan G, Wright E, Crick A, McGuiness C, Chave H. Vertical rectus abdominis myocutaneous flap reconstruction of the perineal defect after abdominoperineal excision is associated with low morbidity. Colorectal Dis. 2013;15:1177–83. doi: 10.1111/codi.12286. [DOI] [PubMed] [Google Scholar]
- 26.Van Leersum N, Martijnse I, den Dulk M, Kolfschoten N, Le Cessie S, van de Velde C, Tollenaar R, Wouters M, Rutten HJ. Differences in circumferential resection margin involvement after abdominoperineal excision and low anterior resection no longer significant. Ann Surg. 2014;259:1150–5. doi: 10.1097/SLA.0000000000000225. [DOI] [PubMed] [Google Scholar]


