Abstract
Objective: This study is to investigate the effect and mechanism of berberine on vascular endothelial cell injury. Methods: The isolated aortic endothelial cells were divided into negative control group, spontaneous hypertension group, and berberine group (1.25, 2.5, and 5 μmol/L berberine). CCK-8 assay was performed to detect cell proliferation. Annexin V-FITC flow cytometry and Hochest33342/PI staining were used to measure cell apoptosis. Expression of TLR4, Myd88, and NF-κB was detected with Western blotting analysis. Level of IL-6 and TNF-α was measured with ELISA. Results: Compared with spontaneous hypertension group, cell proliferation in berberine group was significantly improved (P < 0.05). Flow cytometry showed that cell apoptosis was reduced in berberine group in a dose-dependent manner and there was statistically significant difference between spontaneous hypertension group and berberine group (P < 0.05). This result was further confirmed by Hochest33342/PI staining. Expression levels of TLR4, Myd88 and NF-κB were increased in spontaneous hypertension group. However, their expression levels were significantly reduced in berberine group than those in spontaneous hypertension group (P < 0.05). Similarly, levels of IL-6 and TNF-α were increased in spontaneous hypertension group and decreased in berberine group. And, the difference was significant (P < 0.05). Importantly, there were significant differences between negative control group and spontaneous hypertension group in cell proliferation, apoptosis, and expression of TLR4, Myd88, NF-κB, IL-6 and TNF-α. Conclusion: Berberine plays a protective role in vascular endothelial cell injury through inhibiting apoptosis and expression of TLR4, Myd88, NF-κB, IL-6 and TNF-α.
Keywords: Berberine, spontaneous hypertension, vascular endothelial cell injury, TLR4, Myd88, NF-κB, IL-6, TNF-α
Introduction
Vascular endothelial cells could produce many vasoactive substances, such as nitric oxide, prostacyclin2, and endothelin-1, through autocrine, endocrine, or paracrine secretion [1,2]. Vascular endothelial cells play important roles in regulating vascular tension, inhibiting thrombosis, repressing proliferation of smooth muscle cells and inhibiting inflammation of the vessel wall [3]. When vascular endothelial cells are stimulated by factors such as oxidative stress, renin-angiotensin system, oxidized low density lipoprotein, and homocysteine, the production of vasodilator factors is decreased whereas the production of vasoconstrictor factors is increased [4-7]. This could break the homeostasis of vasoconstriction and vasodilation, resulting in a series of cardiovascular events [8]. There is endothelial damage in almost all patients with essential hypertension [9]. It is believed that endothelial damage is secondary to hypertension [10,11]. And, endothelial damage is the initiating event of atherosclerosis associated with hypertension [12]. Therefore, drug intervention against endothelial cell dysfunction has become a new trend in the field of cardiovascular disease research [13].
The myeloid differentiation factor 88 (MyD88) dependent toll-like receptor 4 (TLR4) signaling pathway expresses in human embryonic kidney cells (HEK293), cardiac cells, and microvascular endothelial cells [14]. It may play a role in endothelial damage induced by hypertension [15,16]. It could activate interleukin-1 receptor associated kinase (IRAK-1), nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), leading to production of large amounts of inflammatory cytokines (such as COX-2, IL-1, and IL-6) and resulting in endothelial cell injury [17].
Berberine is the active ingredient extracted from the rhizome of Ranunculaceae Coptis [18]. Studies have found that berberine has clinical application in the treatment of cancer, diabetes, cardiovascular disease, high cholesterol, inflammation, and bacterial and viral infections [19-22].
In this study, the protective effect of berberine on vascular endothelial cell injury was investigated. Whether this protective effect is related with MyD88 dependent TLR4 signaling pathway was also studied.
Materials and methods
Animals
SD rats and rats with spontaneous hypertension were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). They were kept in standard conditions with free access to food and water. All animal experiments were conducted according to the ethical guidelines of Harbin Medical University.
Isolation and culture of aortic endothelial cells
Aortic endothelial cells were isolated from SD rats and rats with spontaneous hypertension as previously described [23,24]. Briefly, rats were anesthetized with ether and the thoracic aortas were isolated under sterile condition. After washing with PBS and removing vascular adventitia, vascular intima was exposed. The vascular intima was digested with 2.0 g/L of type I collagenase (Beyotime Institute of Biotechnology, Shanghai, China) for 1 h and then the vascular intima were cultured with DMEM/F12 medium (General Electric Company, South Logan, Utah, USA) in flasks coated with collagen. After culturing for 3 to 4 days, endothelial cells were released from the intima tissue. Endothelial cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (General Electric Company, South Logan, Utah, USA) and 0.1% penicillin-streptomycin (Beyotime Institute of Biotechnology, Shanghai, China) in an incubator at 37°C with 5% CO2.
Immunohistochemistry
Immunohistochemistry was performed to identify aortic endothelial cells. As previously described [25], the endothelial cell specific antigen von Willebrand factor (vWF) was detected. The isolated endothelial cells were cultured on glass slides and then incubated with 0.3% H2O2-methanol solution at room temperature for 10 min. After washing with PBS, the slides were blocked with 10% normal goat serum (General Electric Company, South Logan, Utah, USA) for 1 h. Then the primary antibody of rabbit anti-rat vWF (Cell Signaling Technology, Danvers, MA, USA) was added and incubated at 4°C overnight. After washing with PBS for 3 times, the secondary antibody of HRP-labeled goat anti-rabbit antibody (Cell Signaling Technology, Danvers, MA, USA) was added and incubated in the dark at room temperature for 1 h. Finally, the slides were developed with DAB reagent. After dehydration and transparency, the slides were mounted with neutral gum and observed under a fluorescent microscope (Biological Microscope XSP-8C, SHANGHAI ZOUSUN OPTICAL INSTRUMENT CO., LTD., Shanghai, China).
Cell grouping
The cells were divided into negative control group (aorta endothelial cells isolated from SD rats), spontaneous hypertension group (aorta endothelial cells isolated from rats with spontaneous hypertension), berberine group (aorta endothelial cells isolated from rats with spontaneous hypertension + berberine (1.25, 2.5, and 5 μmol/L)). Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h.
CCK-8 assay
The isolated endothelial cells at logarithmic growth phase were seeded in 96-well plates at a concentration of 1 × 105/L. After culturing for 24 h, CCK-8 was added to each well and cultured for 1 h. The OD value was measured at 450 nm using a microplate reader (SpectraMax M3, Molecular Devices, Sunnyvale, CA, USA). The cell viability was represented by OD450 values.
Flow cytometry analysis after Annexin V-FITC staining
Cells were collected, washed twice with cold PBS, and suspended in Annexin V buffer (cell concentration 1 × 106/ml). Then 5 μl Annexin V-FITC and 5 μl PI (Beyotime Institute of Biotechnology, Shanghai, China) were added to 100 μl cell suspension. After gently mixing, cells were incubated in the dark at room temperature for 15 min. Flow cytometry analysis was performed within 1 h to detect cell apoptosis.
Hochest33342 and PI double staining
Cells were collected and suspended in 1 ml culture medium. Then 10 μL Hochest 33342 stock solution (100 mg/L, dissolved in distilled water) and incubated for 15 min. After centrifugation, the cells were re-suspended in 1 ml PBS and 5 μL PI stock solution (1 g/L, dissolved in distilled water) was added. Cells were observed under a fluorescent microscope (XSP-17CE, Shanghai Changfang Optical Instrument Co., LTD, Shanghai, China).
Western blotting
Total proteins were extracted and separated by SDS-PAGE. Then proteins were transferred onto nitrocellulose membrane. After blocking with non-fat milk, the membrane was incubated with primary antibodies of rabbit anti-rat polyclonal TLR-4 antibody (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-rat polyclonal Myd88 antibody (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-rat polyclonal NF-κB antibody (Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-rat polyclonal β-actin antibody (Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. After washing, the membrane was then incubated with goat anti-rabbit monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA) at room temperature for 1 h. Finally, the membrane was developed by enhanced chemiluminescence plus reagent. The developed film was scanned using the AlphaImager gel imaging systems (AlphaImager, Santa Clara, California, USA). And the Western blot images were analyzed using Image-Pro Plus analysis software (Media Cybernetics, Inc., Rockville, MD, USA). β-actin was used as an internal control.
ELISA
The assay was carried out according to the manual provided by the ELISA kit (Rat IL-6 ELISA kit and Rat TNF-α ELISA kit, Uscn Life Science Inc., Wuhan China). Briefly, cell culture supernatant was added to the pre-coated microplate and incubated at 37°C for 30 min. After washing for 5 times, detection antibody was added to the wells. After incubation at room temperature for 1 h, the microplate was washed again. Then HRP conjugated antibody was added and incubated at room temperature for 30 min. After washing for 5 times, substrate solution was added and incubated at room temperature for 15 min. Finally, stop solution was added to stop color development and the plate was read at 450 nm using a microplate reader (Spectra-Max M3, Molecular Devices, Sunnyvale, CA, USA). The standard curve was generated by 2-fold serial dilutions of the standard samples. The content of IL-6 and TNF-α was calculated according to the standard curve.
Statistical analysis
Data was processed using SPSS 19.0 statistical software. All data were presented as mean ± standard deviation. One-Way ANOVA was performed to compare differences among different groups. Mean values between groups were analyzed by SNK method. P < 0.05 was considered statistically significant.
Results
Identification of aortic endothelial cells
To identify the isolated cells, the isolated cells were first observed under microscope. As shown in Figure 1A, cells were flat showing short spindle or polygonal morphology and were arranged in monolayer, like “paving stone”. This morphology is characteristic of endothelial cells. Then, immunohistochemistry was performed to detect the expression of the endothelial cell specific antigen vWF. As shown in Figure 1B, there was positive vWF expression in the isolated cells. Therefore, the isolated cells were identified as aortic endothelial cells.
Figure 1.

Identification of aortic endothelial cells. Aortic endothelial cells were isolated from SD rats and rats with spontaneous hypertension as previously described. A. Cell morphology was observed under microscope. Magnification: × 400. B. Immunohistochemistry was performed to detect vWF expression in isolated cells. Magnification: × 400. Cells with brown staining were defined as positive.
Effect of berberine treatment on proliferation of aortic endothelial cells
To determine the effect of berberine on proliferation of aortic endothelial cells, CCK-8 assay was performed. Cells in negative control group were aorta endothelial cells isolated from SD rats. Cells in spontaneous hypertension group and berberine group were aorta endothelial cells isolated from rats with spontaneous hypertension. Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h. As shown in Figure 2, cell proliferation in spontaneous hypertension group was significantly decreased than that in negative control group (P < 0.05). However, after treatment with berberine, cell proliferation was increased. Compared with spontaneous hypertension group, cell proliferation in berberine group was significantly increased (P < 0.05). Thus, proliferation of aortic endothelial cells isolated from rats with spontaneous hypertension is inhibited and this inhibition is alleviated by berberine treatment.
Figure 2.
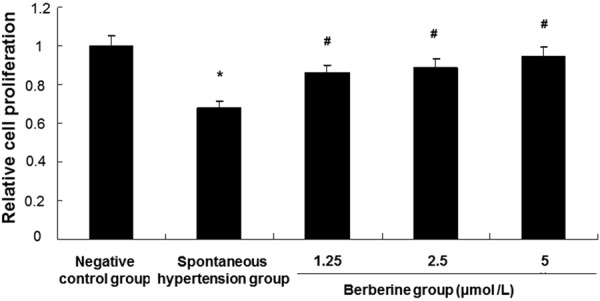
Analysis of aortic endothelial cell proliferation. The isolated cells were divided into negative control group (aorta endothelial cells isolated from SD rats), spontaneous hypertension group (aorta endothelial cells isolated from rats with spontaneous hypertension), berberine group (aorta endothelial cells isolated from rats with spontaneous hypertension + berberine). Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h. Cell proliferation was detected with CCK-8 assay. Compared with negative control group, *P < 0.05. Compared with spontaneous hypertension group, #P < 0.05.
Effect of berberine treatment on apoptosis of aortic endothelial cells by flow cytometry
To investigate the effect of berberine on apoptosis of aortic endothelial cells, flow cytometry analysis was conducted. Cell grouping was described in Materials and methods. Representative flow cytometry results were shown in Figure 3A and quantitative flow cytometry results were shown in Figure 3B. The apoptosis rate in negative control group was 21.02 ± 0.05% whereas in spontaneous hypertension group was 60.69 ± 5.92%. Compared with negative control group, the apoptosis rate in spontaneous hypertension group was significantly higher (P < 0.05). The apoptosis rate in berberine group treated with 1.25, 2.5, and 5 μmol/L berberine was 37.51 ± 0.19%, 31.44 ± 0.25%, and 25.63 ± 0.21%, significantly lower than that in spontaneous hypertension group (P < 0.05). And, this decrease in apoptosis rate was dependent on the dose of berberine. These results indicate that apoptosis of aortic endothelial cells isolated from rats with spontaneous hypertension is increased and this increased apoptosis is inhibited by berberine treatment.
Figure 3.

Analysis of apoptosis of aortic endothelial cells by flow cytometry. The isolated cells were divided into negative control group (aorta endothelial cells isolated from SD rats), spontaneous hypertension group (aorta endothelial cells isolated from rats with spontaneous hypertension), berberine group (aorta endothelial cells isolated from rats with spontaneous hypertension + berberine). Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h. Cell apoptosis was analyzed by flow cytometry after Annexin V staining. A. Representative flow cytometry results. Numbers in the upper right quadrant indicate the percentage of apoptotic cells. B. Quantitative flow cytometry results. Compared with negative control group, *P < 0.05. Compared with spontaneous hypertension group, #P < 0.05.
Effect of berberine treatment on apoptosis of aortic endothelial cells by Hochest33342/PI staining
To further verify the inhibitory effect of berberine on apoptosis of hypertensive aortic endothelial cells, Hochest33342/PI staining was performed. Similarly, as shown in Figure 4, in the negative control group, cells were stained blue and apoptotic cells were barely observed. In the spontaneous hypertension group, some cells were stained red, indicating that there were apoptotic cells. However, the number of apoptotic cells was reduced in the berberine group in a dose-dependent manner. This data further suggests that berberine treatment inhibits apoptosis of aortic endothelial cells isolated from rats with spontaneous hypertension.
Figure 4.

Analysis of apoptosis of aortic endothelial cells by Hochest33342/PI staining. The isolated cells were divided into negative control group (aorta endothelial cells isolated from SD rats), spontaneous hypertension group (aorta endothelial cells isolated from rats with spontaneous hypertension), berberine group (aorta endothelial cells isolated from rats with spontaneous hypertension + berberine). Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h. Cell apoptosis was analyzed by Hochest33342/PI staining.
Effect of berberine on expression of TLR4, Myd88 and NF-κB
To detect the expression of TLR4, Myd88 and NF-κB in aortic endothelial cells after berberine treatment, Western blotting analysis was performed. As shown in Figure 5, the expression levels of TLR4 (Figure 5A), Myd88 (Figure 5B) and NF-κB (Figure 5C) were relatively low in the negative control group. However, their expression levels were increased in the spontaneous hypertension group, significantly higher than those in the negative control group (P < 0.05). After treatment with berberine, the expression levels of TLR4, Myd88 and NF-κB in the berberine group were further decreased. Statistically, the expression levels of TLR4, Myd88 and NF-κB in the berberine group were significantly lower than those in the spontaneous hypertension group (P < 0.05). Thus, these findings showed that berberine treatment inhibited the expression of TLR4, Myd88 and NF-κB in aortic endothelial cells isolated from rats with spontaneous hypertension.
Figure 5.

Analysis of TLR4, Myd88 and NF-κB expression. The isolated cells were divided into negative control group (aorta endothelial cells isolated from SD rats), spontaneous hypertension group (aorta endothelial cells isolated from rats with spontaneous hypertension), berberine group (aorta endothelial cells isolated from rats with spontaneous hypertension + berberine). Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h. Expression of TLR4 (A), Myd88 (B) and NF-κB (C) was detected with Western blotting analysis. Representative Western blotting results were shown in the upper panel and quantitative Western blotting results were shown in the lower panel. Compared with negative control group, *P < 0.05. Compared with spontaneous hypertension group, #P < 0.05.
Effect of berberine on expression of IL-6 and TNF-α
ELISA was carried out to measure the levels of IL-6 and TNF-α in culture supernatant of aortic endothelial cells. The levels of IL-6 (Figure 6A) and TNF-α (Figure 6B) in the spontaneous hypertension group were significantly higher than those in the negative control group (P < 0.05). However, after berberine treatment, the levels of IL-6 and TNF-α decreased. Statistically, the berberine group had significantly lower levels of IL-6 and TNF-α than the spontaneous hypertension group (P < 0.05). Therefore, the levels of IL-6 and TNF-α in culture supernatant of aortic endothelial cells isolated from rats with spontaneous hypertension are decreased by berberine.
Figure 6.
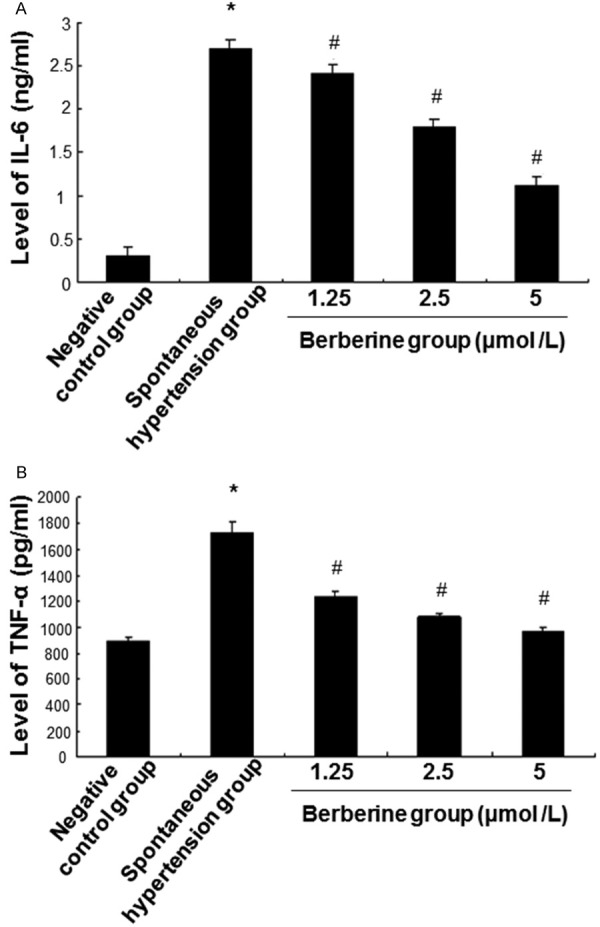
Analysis of IL-6 and TNF-α expression. The isolated cells were divided into negative control group (aorta endothelial cells isolated from SD rats), spontaneous hypertension group (aorta endothelial cells isolated from rats with spontaneous hypertension), berberine group (aorta endothelial cells isolated from rats with spontaneous hypertension + berberine). Cells in berberine group were treated with berberine (1.25, 2.5, and 5 μmol/L) for 24 h. Expression of IL-6 (A) and TNF-α (B) was detected with ELISA. Compared with negative control group, *P < 0.05. Compared with spontaneous hypertension group, #P < 0.05.
Discussion
There is vascular endothelial cell injury during the development of hypertension [26]. However, the mechanism underlying hypertensive vascular endothelial cell injury is not clear. It is generally considered that vascular endothelial cell injury is secondary to hypertension [27]. Vascular endothelial cell injury is accompanied with inflammation and apoptosis [28,29]. Meanwhile, TLR4 pathway is an important pathway involved in inflammation [30]. As a traditional Chinese medicine, berberine is widely used in the treatment of heart failure, arrhythmia, high cholesterol, and inflammatory diseases [31]. The results of this study showed that berberine could improve the proliferation of cells isolated from rats with spontaneous hypertension. Compared with spontaneous hypertension group, the difference was statistically significant. Thus, berberine has certain protective effect on hypertensive vascular endothelial injury.
To further reveal the underlying mechanism of the protective effect of berberine on vascular endothelial cells, we detected cell apoptosis after berberine treatment. Flow cytometry analysis showed the apoptosis rate in hypertension group was significantly higher than that in negative control group. However, after treatment with berberine, the apoptosis rate was significantly reduced in a dose dependent manner. Similarly, Hochest33342/PI staining showed that many cells in hypertension group were stained red. And, the number of cell with red staining in berberine group was obviously lesser. These results suggest that apoptosis of aortic endothelial cells isolated from rats with spontaneous hypertension is increased and this increased apoptosis is inhibited by berberine treatment.
Hypertensive vascular endothelial cell injury is related with TLR4 signaling pathway [32,33]. TLR4 signaling pathway is involved in inflammation and immune response through promoting the synthesis and release of cytokines [34]. The results of this study showed that expression levels of TLR4, Myd88 and NF-κB were increased in hypertension group. After the intervention with berberine, expression levels of TLR4, Myd88 and NF-κB was significantly reduced.
TNF-α and IL-6 play important roles in endothelial damage, inflammation, smooth muscle proliferation and vascular remodeling in hypertension patients [35,36]. IL-6 is a glycoprotein with a molecular weight of 26 kD and is produced by a variety of tissues [37]. It can induce activation of B cells to produce immunoglobulins, promote T cell growth, and induce the generation of hepatic acute phase protein [38]. TNF-α, which is mainly produced by mononuclear macrophages, is a kind of peptide with a molecular weight of 17 kD [35]. It is an important inflammatory mediator and immunomodulatory factor that is involved in many pathophysiological processes, such as immune response, tissue repair, and cell apoptosis [39,40]. In this study, levels of IL-6 and TNF-α in spontaneous hypertension group were significantly higher than those in negative control group. However, after treatment with berberine, IL-6 and TNF-α levels were significantly decreased, compared with spontaneous hypertension group.
In conclusion, our findings indicate that berberine may protect vascular endothelial cells isolated from hypertensive rats from injury. And, this protective effect may be acted through regulating apoptosis and the expression of TLR4, Myd88, NF-κB, IL-6 and TNF-α.
Acknowledgements
We thank Lanfeng Wang from Department of CCU, the First Affiliated Hospital, Harbin Medical University for his valuable help during the preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Qin Q, Chen M, Yi B, You X, Yang P, Sun J. Orphan nuclear receptor Nur77 is a novel negative regulator of endothelin-1 expression in vascular endothelial cells. J Mol Cell Cardiol. 2014;77:20–28. doi: 10.1016/j.yjmcc.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brzezinska AK, Merkus D, Chilian WM. Metabolic communication from cardiac myocytes to vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2005;288:H2232–2237. doi: 10.1152/ajpheart.00202.2004. [DOI] [PubMed] [Google Scholar]
- 3.Hill JW, Nemoto EM. Transient middle cerebral artery occlusion with complete reperfusion in spontaneously hypertensive rats. MethodsX. 2014;1:283–291. doi: 10.1016/j.mex.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F, Qian LH, Deng B, Liu ZM, Zhao Y, Le YY. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci Ther. 2013;19:675–681. doi: 10.1111/cns.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rakugi H, Nakamura Y, Ohishi M, Okamura A, Yanagitani Y, Higaki J, Ogihara T. Vascular endothelial cells and renin-angiotensin system. Rinsho Byori. 1998;46:1135–1141. [PubMed] [Google Scholar]
- 6.Wang YQ, Dai M, Zhong JC, Yin DK. Paeonol inhibits oxidized low density lipoprotein-induced monocyte adhesion to vascular endothelial cells by inhibiting the mitogen activated protein kinase pathway. Biol Pharm Bull. 2012;35:767–772. doi: 10.1248/bpb.35.767. [DOI] [PubMed] [Google Scholar]
- 7.Chaussalet M, Lamy E, Foucault-Bertaud A, Genovesio C, Sabatier F, Dignat-George F, Charpiot P. Homocysteine modulates the proteolytic potential of human vascular endothelial cells. Biochem Biophys Res Commun. 2004;316:170–176. doi: 10.1016/j.bbrc.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Ma X, Lan Y, Li L, Wang Y, Jin C, Mao X. Effect of alcohol extract from Coreopsis tinctoria Nutt. on spontaneous hypertension rats by metabolomic methods. Chinese Pharmacological Bulletin. 2014;30:1311–1315. [Google Scholar]
- 9.Chung NA, Beevers DG, Lip G. Effects of losartan versus hydrochlorothiazide on indices of endothelial damage/dysfunction, angiogenesis and tissue factor in essential hypertension. Blood Press. 2004;13:183–189. doi: 10.1080/08037050410033312. [DOI] [PubMed] [Google Scholar]
- 10.Tse WY, Maxwell SR, Thomason H, Blann A, Thorpe GH, Waite M, Holder R. Antioxidant status in controlled and uncontrolled hypertension and its relationship to endothelial damage. J Hum Hypertens. 1994;8:843–849. [PubMed] [Google Scholar]
- 11.Spescha RD, Glanzmann M, Simic B, Witassek F, Keller S, Akhmedov A, Tanner FC, Luscher TF, Camici GG. Adaptor protein p66(Shc) mediates hypertension-associated, cyclic stretchdependent, endothelial damage. Hypertension. 2014;64:347–353. doi: 10.1161/HYPERTENSIONAHA.113.02129. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis. 2005;180:113–118. doi: 10.1016/j.atherosclerosis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Xiao M, Men LN, Xu MG, Wang GB, Lv HT, Liu C. Berberine protects endothelial progenitor cell from damage of TNF-alpha via the PI3K/AKT/eNOS signaling pathway. Eur J Pharmacol. 2014;743:11–16. doi: 10.1016/j.ejphar.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Li Y, Jin J, Zhang X, Lopes-Virella MF, Huang Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler Thromb Vasc Biol. 2012;32:1696–1706. doi: 10.1161/ATVBAHA.112.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause DL, Muller N. Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer’s disease. Int J Alzheimers Dis. 2010;2010:4061–4070. doi: 10.4061/2010/732806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lush CW, Cepinskas G, Kvietys PR. LPS tolerance in human endothelial cells: reduced PMN adhesion, E-selectin expression, and NFkappaB mobilization. Am J Physiol Heart Circ Physiol. 2000;278:H853–861. doi: 10.1152/ajpheart.2000.278.3.H853. [DOI] [PubMed] [Google Scholar]
- 17.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun. 2011;79:3576–3587. doi: 10.1128/IAI.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Tang H, Deng R, Wang N, Zhang Y, Wang Y, Liu Y, Li F, Wang X, Zhou L. Berberine Suppresses Adipocyte Differentiation via Decreasing CREB Transcriptional Activity. PLoS One. 2015;10:e0125667. doi: 10.1371/journal.pone.0125667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan W, Li N, Tan R, Zhong Z, Suo Z, Yang X, Wang Y, Hu X. Berberine interfered with breast cancer cells metabolism, balancing energy homeostasis. Anticancer Agents Med Chem. 2014;15:66–78. doi: 10.2174/1871520614666140910120518. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Hung TH, Lee CY, Wang LF, Wu CH, Ke CH, Chen SF. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS One. 2014;9:e115694. doi: 10.1371/journal.pone.0115694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Pierro F, Villanova N, Agostini F, Marzocchi R, Soverini V, Marchesini G. Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes Metab Syndr Obes. 2012;5:213–217. doi: 10.2147/DMSO.S33718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang QL, Lai ML, Zhong YF, Wang AM, Su JK, Zhang MQ. Antinociceptive effect of berberine on visceral hypersensitivity in rats. World J Gastroenterol. 2013;19:4582–4589. doi: 10.3748/wjg.v19.i28.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, Qu Y, Li J. Establishment of a simple and efficient method for isolating and culturing vascular endothelial cells of rat thoracic aorta. Basic & Clinical Medicine. 2011;31:1278–1282. [Google Scholar]
- 24.Pan L, Dai M, Wang W. A new method for culturing endothelial cells from the rat aorta. Chinese Pharmacological Bulletin. 2007;23:109–113. [Google Scholar]
- 25.Wang C, Li J, Lv X, Zhang M, Song Y, Chen L, Liu Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620:131–137. doi: 10.1016/j.ejphar.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Garland CJ. Compromised vascular endothelial cell SK (Ca) activity: a fundamental aspect of hypertension? Br J Pharmacol. 2010;160:833–835. doi: 10.1111/j.1476-5381.2010.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 28.Anggrahini DW, Emoto N, Nakayama K, Widyantoro B, Adiarto S, Iwasa N, Nonaka H, Rikitake Y, Kisanuki YY, Yanagisawa M, Hirata K. Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovasc Res. 2009;82:143–151. doi: 10.1093/cvr/cvp026. [DOI] [PubMed] [Google Scholar]
- 29.Fang K, Chen Z, Liu M, Peng J, Wu P. Apoptosis and calcification of vascular endothelial cell under hyperhomocysteinemia. Med Oncol. 2015;32:403. doi: 10.1007/s12032-014-0403-z. [DOI] [PubMed] [Google Scholar]
- 30.Janssens S, Burns K, Vercammen E, Tschopp J, Beyaert R. MyD88S, a splice variant of MyD88, differentially modulates NF-kappaBand AP-1-dependent gene expression. FEBS Lett. 2003;548:103–107. doi: 10.1016/s0014-5793(03)00747-6. [DOI] [PubMed] [Google Scholar]
- 31.Lin S, Tsai SC, Lee CC, Wang BW, Liou JY, Shyu KG. Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Mol Pharmacol. 2004;66:612–619. [PubMed] [Google Scholar]
- 32.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 33.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 34.Abreu MT, Arditi M. Innate immunity and toll-like receptors: clinical implications of basic science research. J Pediatr. 2004;144:421–429. doi: 10.1016/j.jpeds.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Korkosz M, Gasowski J, Surdacki A, Leszczynski P, Pawlak-Bus K, Jeka S, Siedlar M, Grodzicki T. Disparate effects of anti-TNF-alpha therapies on measures of disease activity and mediators of endothelial damage in ankylosing spondylitis. Pharmacol Rep. 2013;65:891–897. doi: 10.1016/s1734-1140(13)71070-3. [DOI] [PubMed] [Google Scholar]
- 36.Kornej J, Dinov B, Blann AD, Rolf S, Arya A, Schmidl J, Husser D, Hindricks G, Bollmann A, Lip GY. Effects of radiofrequency catheter ablation of atrial fibrillation on soluble P-selectin, von Willebrand factor and IL-6 in the peripheral and cardiac circulation. PLoS One. 2014;9:e111760. doi: 10.1371/journal.pone.0111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andreozzi GM, Martini R, Cordova R, D’Eri A, Salmistraro G, Mussap M, Plebani M. Circulating levels of cytokines (IL-6 and IL-1beta) in patients with intermittent claudication, at rest, after maximal exercise treadmill test and during restore phase. Could they be progression markers of the disease? Int Angiol. 2007;26:245–252. [PubMed] [Google Scholar]
- 38.Marini V, Moretti E, Bermejo D, Basso B. Vaccination with Trypanosoma rangeli modulates the profiles of immunoglobulins and IL-6 at local and systemic levels in the early phase of Trypanosoma cruzi experimental infection. Mem Inst Oswaldo Cruz. 2011;106:32–37. doi: 10.1590/s0074-02762011000100005. [DOI] [PubMed] [Google Scholar]
- 39.Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA. 2003;290:3000–3002. doi: 10.1001/jama.290.22.3000. [DOI] [PubMed] [Google Scholar]
- 40.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]


