Abstract
The aim of this study will provide a self-assembling peptide (RADA16-I) -derived hydrogel as a tool for investigation the malignant phenotype of human hepatocellular carcinoma cell. Characteristic analysis indicated that the peptide consists of a well-defined secondary structure and self-assembly property. Our results showed that these cells cultured in RADA16-I hydrogels showed a spindle-shaped phenotype with irregular and radial nuclei. Immunohistochemical results showed that the expression of fibronectin in hepatocellular carcinoma cells is positive cultured in RADA16-I hydrogels, and the expression levels of laminin are weakly positive. DNA contents cultured in RADA16-I hydrogel gradually increased up to Day 9. The expression levels of VEGFA, EGF and FGF2 in three hydrogels showed no statistically significant differences (P > 0.05), and the expression levels of IGF-1 in RADA16-I and collagen-I were significantly lower than those of in the Matrigel hydrogel (P ≤ 0.05). These findings suggested that the RADA16-I will help to provide a better physiological substrate for hepatocellular carcinoma cell culture, may serve as an ideal model for cancer biology research of tumorigenesis, growth, local invasion, and metastasis.
Keywords: Self-assembling peptide, hydrogel, three-dimensional culture, hepatocellular carcinoma cell, malignant phenotype
Introduction
In vivo cells growth in which the local microenvironment is three-dimensional, which involve cell production of extracellular matrix material, and their movement is often influenced by local chemical signal or molecule gradient. In order to best mimic natural cell growth, in vitro cultures attempt to closely model the extracellular support structure naturally occurring in tissues [1,2]. However, traditional cell culture method mostly uses traditional two-dimensional (2-D) in vitro tissue culture models. 2-D culture does not closely mimic in vivo environment and often overlooks important variables such as dimensionality and microenvironment signaling, which has an effect on cancer phenotype, aggressiveness, and drug resistance [3]. Biomedical researchers have become increasingly aware of the limitations of the conventional 2-D tissue cell cultures where most tissue cell studies including cancer and tumor cells have been carried out. As more understanding of its influences is revealed, it is increasingly common to see a shift in growth studies from 2-D surface cultures to three-dimensional (3-D) suspension cultures [4,5]. Such realistic environments should be the aim of any cell growth study, requiring new methods for culturing cells in vitro.
In vivo tissue growth involves cell production of extracellular matrix material, composed primarily of various proteins. Artificially produced extracellular matrices commonly used consist of mixtures of proteins such as collagen, fibrin, elastin, fibronectins (FN), and laminins (LN). Such components are understood to play a role in cell anchoring and separation, intercellular communication, and even nutrient feed and waste displacement [6-8]. However, there are many problems limit the use of these materials. Those materials might be toxic and non-biodegradable polymers of synthetic materials. Furthermore, such animal-derived materials can cause allergic reactions and carry dangerous pathogens including prions that cause a variety of neurodegenerative diseases in humans and animals. Other viruses might also be carried as pathogens in animal-derived materials [2,9]. Recent studies reveal that a class of self-assembling peptide might provide an ideal and simplified extracellular matrix material. Self-assembling peptides are a 100% chemically synthesized material. They may be synthesized by variable of amino acid sequence and electric dipole property based on the characterization of cultured cell, and further offer scaffold materials and extracellular matrix (ECM) structure for cell growth and differentiation. The application of self-assembling peptide in scaffolds and/or hydrogels has been proven in several cell culture systems for biocompatibility, biodegradability, the risk of biological contamination and the influence from the undefined factors [10-12].
Hydrogel is a superabsorbent material, primarily consisting of water; stable gel formation was consistently seen with as low as 0.5 wt% peptide derivative. An additional feature of the hydrogel makes it increasingly favorable for use within complex structures [13]. Previous studies with peptide nanotubes and peptide derivative hydrogels have shown response to various types of treatment, whether changing stability or degrading entirely [10-12,14]. Thus, peptide hydrogel scaffolds provide a platform that makes them ideal for nanomedical applications. However, a direct application of peptide hydrogel scaffold to recreate microenvironment for enrichment of human hepatoma cell has not been explored. In the present study, human hepatoma cell line were used to investigate the cell growth and malignant phenotype in self-assembling peptide RADA16-I hydrogel. The 3-D coculture model presented here suggests that SMMC7721 cells still retain tumor microenvironment phenotype l. Thus, these findings may help to provide a novel experimental method to study the biological characterizations of hepatoma cancer cells, and afford experimental and theoretical basis for standardization and engineering of hepatoma cell 3-D culture systems.
Experimental
Materials and reagents
RADA16-I was commercially synthesized from Shanghai Biotech Bioscience & Technology. collagen-I and Matrigel were purchased from BD Biosciences. PRMI-1640 and fetal bovine serum (FBS) were obtained from Gibco, USA. Trypsin-EDTA, calcein-AM, phalloidin, BrdU, BrdU antibody and DNA fluorescence quantitative detection Kit were from Sigma-Aldric (St. Louis, MO, USA). ELISA kits were purchased from Uscn, USA. Antibodies were obtained from Abcam, UK.
Atomic force microscopy
Peptide solutions were prepared at a concentration of 0.56% (w/v) with Mill-Q water (18 MΩ), and stored at 4°C until to use. Aliquot of 1 μl of the peptide solution was evenly deposited onto a freshly cleaved mica surface. Sample was left on the mica surface for about 30 sec. The surface was then rinsed about 10 times with 100 μl of Milli-Q water to remove unattached peptide. The samples were covered with Petri dishes to avoid contamination and then air-dried for AFM observation. Atomic force microscopy (AFM) was performed at room temperature using the tapping mode on a SPI4000 Probe Station and SPA-400 SPM Unit (Seiko Instruments Inc., Chiba, Japan).
Cells culture
SMMC7721 was obtained from Department of Microbiology, Zunyi Medical University (Introduced from ATCC). SMMC7721 cells were cultured in RADA16-I hydrogels shown as following. Cells were maintained in the RPMI 1640 medium consisting of 10% FBS, then cultured into 5% CO2 incubator. The cells were digested with 0.25% trypsin when the percentage of cell fusion is 80%, and then cell suspension was prepared, and cell contents was adjusted for further culture. To start the three-dimensional cell culture, the cells were washed and resuspended with 10% sucrose. The peptide solution was mixed thoroughly with the cell suspension at a radio of 9:1 (RADA16-I: peptide sequence; v/v), and the final concentration of RADA16-I reached to 0.5% (w/v) and final cell concentration 1 × 106/ml. Then, the cell-peptide mixtures were dropped into homemade mold, and incubated with RPMI 1640 media. The mold was removed after hydrogel formation. Finally, cells were cultured in 37°C incubator, and the media were changed every other day. As for cell cultured in collagen-I and Matrigel gels, cells were digested and centrifuged with trypsin, then the cell concentration in collagen-I and Matrigel was adjusted to 1.79 × 106 and 2 × 106/ml, respectively. Cultured in collagen-I hydrogel, collagen-I solution and cell suspension were thoroughly mixed at a radio of 0.79:1 under ice bath, and the final concentrations of collagen-I and cells were 1.5 mg/ml and 1 × 106/ml, respectively. Cultured in Matrigel hydrogel, Matrigel solution and cell suspension were thoroughly mixed at a radio of 1:1 under ice bath, and the final concentration of Matrigel and cells were 0.5% (W/V) and 1 × 106/ml, respectively. These mixtures were dripped quickly into the homemade molds, and incubated in 37°C incubator for 20 min. After gel formation, the molds were removed and RPMI 1640 medium was added. The medium was changed every other day.
Assay of phalloidin/DAPI staining
For Phalloidin/DAPI staining, cells were cultured in three hydrogels for 3 days, and rinsed with pre-warmed PBS buffer for three times, each for 5 min. Next, cells were fixed with 4% paraformaldehyde for 15 min, and washed with PBS. Then 10 μl of 8.3 μg/ml phalloidin were added to incubate with cell clumps for 20 min, and washed with PBS at room temperature. Finally, these cells were treated with 10 μl of 2.5 μg/ml DAPI for nuclei staining, and photographed by fluorescence microscope.
Assay of calcein-AM staining
SMMC7721 cells were cultured in three hydrogels for 3 days, and rinsed with pre-cooled PBS buffer for three times, each for 15 min. Next, 50 μl of 2 μM calcein was added, and cells were incubated in the dark for 45 min, rinsed for three times with PBS. Finally, cells were directly photographed under a fluorescence microscope.
Determination of DNA content
Gel clumps of cells were collected at Day 3, 6 and 9. The number of cells on the scaffold was determined by the fluorometric quantification of amount of cellular DNA. The cell-seeded scaffold was rinsed with PBS and recovered by Na Citrate buffer solution containing 50 mM Na Citrate and 100 mM NaCl and stored at -80°C until assay. Cells were placed in citrate buffer containing 5 mM sodium citrate and 100 mM sodium chloride, stored in -80°C. Cells were repeatedly frozen, thawed and shook until clumps completely dissolved. The DNA content was measured according to instructions of DNA fluorescence assay kit.
Assay of BrdU labelling
For BrdU labelling experiment, cells were collected at Day 3, 6 and 9, and an equal amount of 10 mM of BrdU reagent was added into the cell solution, and then incubated for 12 h. After washing, cells were treated with 4% paraformaldehyde at room temperature for 30 min, and washed thrice with PBS. Then, PBS containing 0.3% TritonX-100 was added for 30 min, and washed thrice with PBS. 60% HCl was added and incubated for 30 min at 37°C, and then washed thrice with PBS. PBS with 3% BSA, 0.2% TritonX-100 and 2% donkey serum were added and incubated for 60 min, and then washed thrice with PBS. Next, BrdU antibody and goat anti-mouse FITC successively added, and then 10 μl of 2.5 μg/ml DAPI was added at room temperature. Photographs were recorded under a fluorescence microscope, and FITC-labelled cells were counted in the DAPI labeled cells.
Assay of immunohistochemistry
For immunohistochemistry, gel clumps of cells were embedded with paraffin and operated according to standard procedures. After incubated with antibodies, and stained by hematoxylin, cells were washed with water and differentiated with 0.1% hydrochloric acid and ethanol. Finally, cells were washed by tap water, dehydrated with graded alcohol, sealed with gum, dyed and observed.
Assay of ELISA
After culturing at day 6, the cells were centrifugated at 2000 g for 20 min, and the supernatants were collected for further analysis. The VEGFA, EGF, FGF2 and IGF1 were analyzed according to kit instructions, and the expression levels w were detected at 450 nm using microplate reader.
Statistical analysis
SPSS 17.0 was used for statistical analysis. Results were presented as mean ± standard deviation (x ± SD), and One-Way ANOVA test was conducted between groups. Values 0.05 (*) and 0.01 (**) were assumed as levels of significance for the statistic tests carried out.
Results
Atomic force microscopy of RADA16-I
It has been previously reported that β-sheet structures of self-complementary peptides may be a requisite for self-assembly process into nanofibers [14,15]. The structure characteristic RADA16-I is reciprocally formed complementary ionic bonds in the hydrophilic interface, and has the charge distribution pattern of + - + -. This peptide may form a colorless and transparent aqueous solution under appropriate pH conditions [16]. The presence of nanofibers in RADA16-I peptides solutions was observed in Figure 1. As shown in Figure 1, these fibers have length of 1106 ± 186 nm, width of 17.1 ± 1.23 nm, height of 1.38 ± 0.56 nm. When the peptide solution and medium was mixed, salt ions in the culture medium may stimulate self-assembly of RADA16-I. These fibers immediately gather into nano-fiber and form mesh structure. Under the action of salt ions, nanoscale fibers assembled into a network, which makes RADA16-I may mix with the cells in aqueous solution. The AFM results not only confirmed the previous observation of hydrogel formation by visual inspection, but also their potential use as a more realistic local environment through the nanofiber scaffolds where the functional properties of cells can be observed and manipulated.
Figure 1.
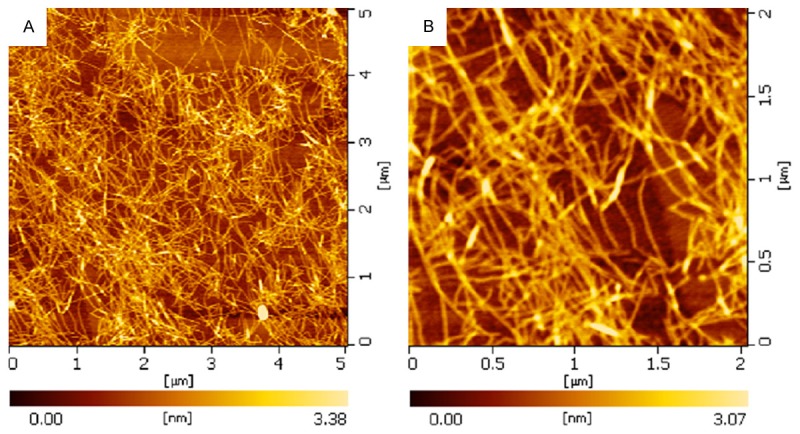
AFM images of nanostructures formed from RADA16-I peptides.
Cell morphology and aggregation states in different culture systems
As shown in Figure 1A, SMMC7721 cells cultured in 3-D system showed different characterizations, and grow suspendedly with slow proliferation rate. The cell clumps aggregation can even be observed at Day 3. Notably, cells cultured in RADA16-I and Matrigel hydrogels proliferated to large aggregates. When cultured in RADA16-I hydrogels, cell grew to be radial, multangular, and/or star like clusters. Cells cultured in Matrigel hydrogels were like grapes clusters or spheres. However, SMMC7721 cells cultured in collagen-I hydrogels showed no significant large aggregates, and only associated with significantly increased cell density. During these cultured process, cells cultured in RADA16-I hydrogels showed significantly cell elongation compared to those in the other hydrogels. However, cells cultured in Matrigel and collagen-I gels still showed round or spherical shapes. To further detect cell morphology of SMMC7721 cells in different cultured systems, we performed phalloidin/DAPI staining. Images showed the morphology of SMMC7721 cells (Figure 2B), blue represented the nuclei and red represented the F-actin of cytoskeletons. Cells cultured in RADA16-I hydrogels showed elongated cell bodies and polarized cell mass, while those cells cultured in Matrigel hydrogels had disordered cell arrangement, with various cell size and scattered sphere or grapes. Moreover, cells cultured in RADA16-I hydrogels showed diffused distribution, and remain round or oval shapes. These results revealed that the RADA16-I hydrogels may promote the growth and proliferation of SMMC7721 cell.
Figure 2.
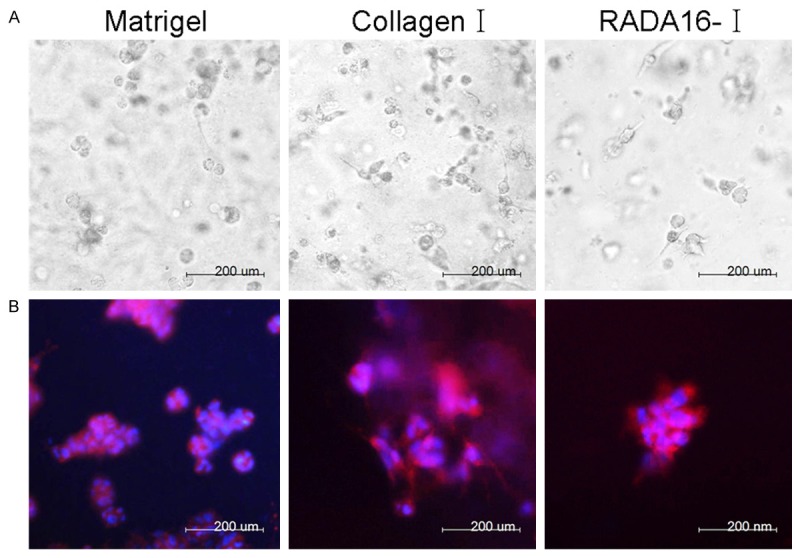
Different morphologies of SMMC7721 cells cultured in Matrigel, collagen-I and RADA16-I hydrogels. A. Light microscopy images of cells encapsulated in Matrigel, collagen-I and RADA16-I hydrogels for 3 day. B. F-actin (red) and nuclear (blue) fluorescence images of the morphology in Matrigel, collagen-I and RADA16-I for 3 day.
Cell viability of SMMC7721 cells in different culture systems
As shown in Figure 3, SMMC7721 cells grew well when they were cultured in RADA16-I, Matrigel and collagen-I hydrogels, suggesting that these scaffold materials have excellent biocompatibility to maintain the growth and proliferation of SMMC7721 cells. Moreover, those cells cultured in different hydrogels showed different cell morphologies and aggregation states. These results indicated that these scaffold materials may directly affect cell morphology in the nanofibers network microenvironment.
Figure 3.
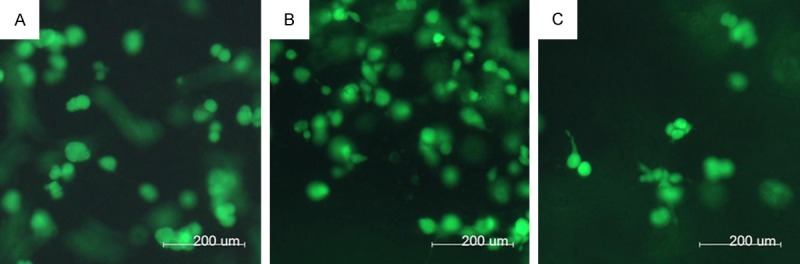
Encapsulation of SMMC7721 cells cultured in Matrigel, collagen-I and RADA16-I hydrogels. (A) Matrigel, (B) collagen-I and (C) RADA16-I hydrogels using calcein-AM staining for the living cells for 3 day.
Growth and proliferation of SMMC7721 cells in different culture systems
To confirm the cell proliferation status, we further applied BrdU labeling assays to these 3-D cultured cells. As shown in Figure 4A, our results suggested that SMMC7721 cells cultured in Matrigel, collagen-I and RADA16-I hydrogels showed well growth and proliferation during the culture process. The cell densities of SMMC7721 cells cultured in Matrigel, and collagen-I hydrogels gradually increased with the rising culture time up to Day 9. However, cell densities cultured in RADA16-I hydrogels gradually rapidly increased up to Day 6, and then maintain stable growth. As shown in Figure 4B, cell proliferation also analyzed by BrdU test, and results suggested that at least 50% of cell proliferation was inhibited when they were cultured in RADA16-I hydrogels. However, there are still more than 65% of the cells proliferation when they were cultured in Matrigel and collagen-I hydrogels.
Figure 4.
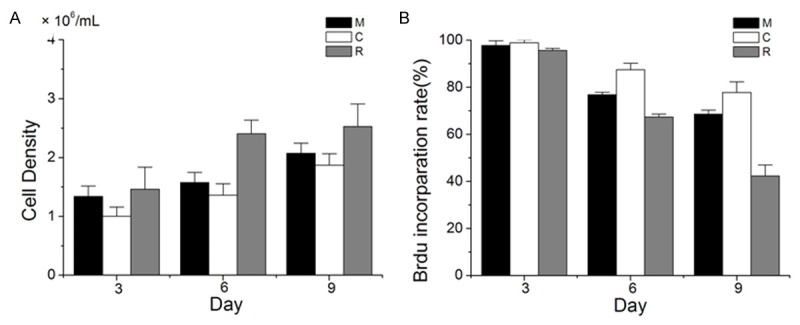
Growth and proliferation of SMMC7721 cells cultured in Matrigel, collagen-I and RADA16-I hydrogels. A. SMMC7721 cell density in different matrices calculated from DNA measurement at cultured Day 3, 6 and 9. B. Percentage of BrdU labeling cells cultured in Matrigel, collagen-I and RADA16-I hydrogels, expressed as the BrdU labeling index from three experiments (about 200 cells per experiment) at cultured Day 3, 6 and 9.
Different ECM protein expression of SMMC7721 cells in different culture systems
To further characterize SMMC7721 cell status in 3-D cultured systems, we investigated the expression levels of extracellular matrix (ECM) protein. By histochemical staining, we found that the expression levels of FN were observed in these three culture systems. Especially, the expression levels of FN were extremely highest in collagen-I hydrogels, which showed dark brown. The lowest expression levels of FN were observed in cultured in Matrigel hydrogels, and there are only small lighter brown precipitates (Figure 5A). As shown in Figure 5B, the expression levels of LN showed different expressions in the three cultured systems. The expression levels of LN were highest cultured in collagen-I hydrogels than those of cultured in Matrigel, and RADA16-I hydrogels.
Figure 5.
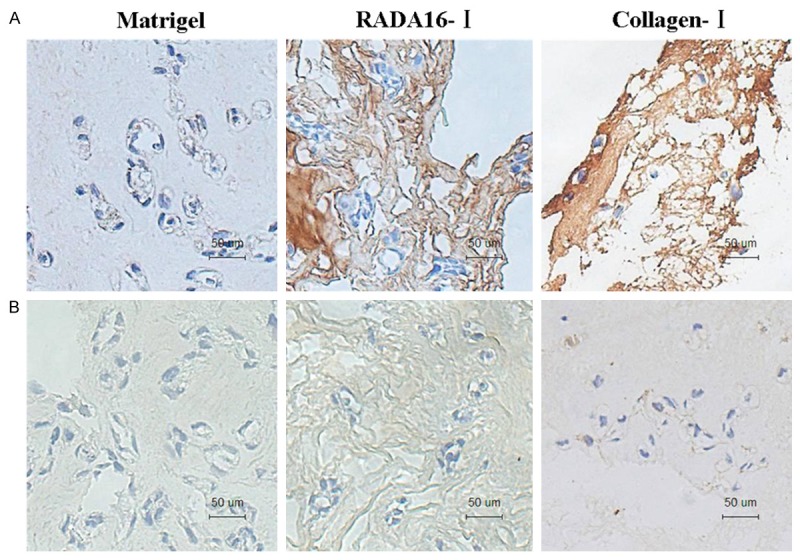
Distribution and expression pattern of Fibronectin (FN) (A) and Laminin (LN) (B) in SMMC7721 cells cultured in Matrigel, collagen-I and RADA16-I hydrogels.
Cell factors expressions of SMMC7721 cells in different culture systems
To examine the tumorigenesis state of SMMC7721 cells in 3-D cultured systems, we examined the expression levels of cell factors. As shown in Figure 6, our results showed that there were no significant differences in VEGFA, EGF and FGF2 levels of SMMC7721 cells in the three cultured systems (P > 0.05). At day 6, IGF1 levels of SMMC7721 cells cultured in RADA16-I and collagen-I systems were lower than that those of in Matrigel system, with significant differences (*P ≤ 0.05). However, FGF2 levels of SMMC7721 cells cultured in RADA16-I were higher than that those cultured Matrigel and collagen-I hydrogels. Based on the above results, these findings indicated that RADA16-I has similar function to maintain the expression of cell factors and biological behavior in SMMC7721 cells, and offer a 3-D microenvironment to support the growth and proliferation in vitro.
Figure 6.
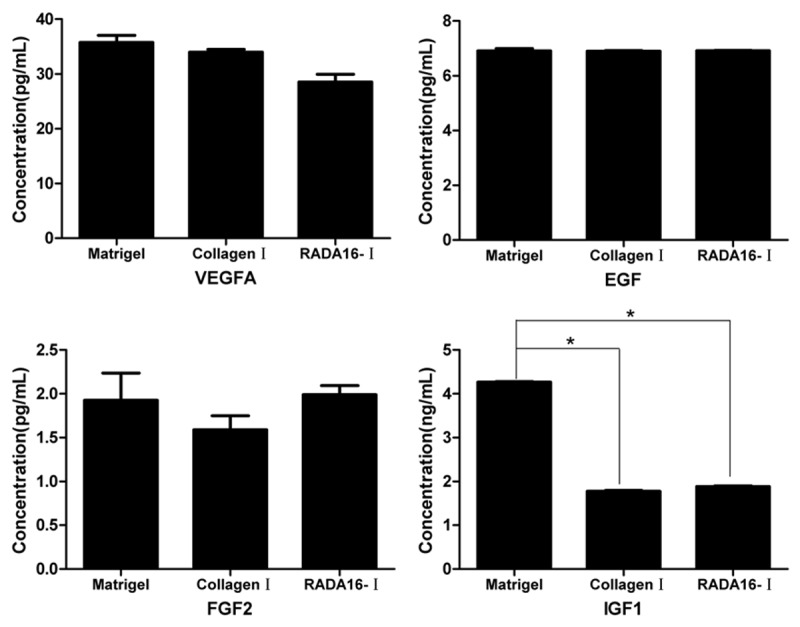
Expression levels of VEGFA, EGF, FGF2 and IGF1 in SMMC7721 cells cultured in Matrigel, RADA16-I and collagen-I systems (mean ± SD, ng/L).
Discussion
Tumor cells are embedded in a 3-D microenvironment in the tissue. However, the majority of reports on the mechanisms of tumorigenesis have utilized cancer cell cultures on rigid surfaces. Results from many studies have shown dramatic changes in morphology and function depending on the presence of extracellular matrix [1,2]. Thus, it is necessary to model the biological pathways and regulation of tumor cells in a true 3-D environment. In the present study, human hepatoma cell line were used to investigate the cell growth and malignant phenotype in self-assembling peptide RADA16-I hydrogel. Our results showed that self-assembling peptide RADA16-I scaffolds may enhance cellular proliferation and differentiation activities of SMMC7721 cells, and showed well biocompatibility. Similar to cells cultured in Matrigel and collagen-I hydrogels, these cells cultured in RADA16-I hydrogels showed and maintained cell morphology and phenotype during the 3-D culture. These results suggest that the RADA16-I peptide present similar properties as the natural materials used (collagen-I and Matrigel) in terms of mimicking biological basement membranes.
It has become more and more apparent that 3-D cell culture offers a more realistic local environment through the nanofiber scaffolds where the functional properties of cells can be observed and manipulated [17]. Recently, some synthetic polymer scaffolds, like polylactide (PL), polyglycolide (PG), and poly (D, L-lactide-co-glycolide) (PLG), as well as natural matrix scaffolds, such as collagen, Matrigel, and LN, have been widely used to culture tumor cells. However, there are some limitations to the actual application of these scaffolds in a cell culture. This is duo to that synthetic polymer scaffolds are toxic and nonbiodegradable, and animal-derived biomaterials might contain residual growth factors, undefined constituents or nonquantified substances that make it difficult to conduct well-controlled studies with these materials [9,18]. A series of designer self-assembling peptide nanofiber scaffolds now provides an ideal alternative system [10-12,14]. Previous studies have shown that the self-assembling peptide RADA16-I consists of natural amino acids that can undergo spontaneous assembly into nanofiber scaffolds of about 20 nm in diameter. This peptide may mimic the in vivo 3-D microenvironment and support the attachment of a variety of mammalian cells [10,16]. Our results that SMMC7721 cells grew well cultured in RADA16-I scaffolds compared to those in the Matrigel and collagen-I scaffolds, suggesting that hepatoma cells have excellent biocompatibility similar to these biomaterials. Moreover, SMMC7721 cells exhibited different morphology and aggregation state in different 3-D microenvironments during the culture processes, indicating that microenvironment might have a direct impact on the cell morphology and characterizations.
During the cell cultures, SMMC7721 cells showed a sustained and rapidly growth cultured in Matrigel and collagen-I hydrogels. However, a decline of proliferation rate cultured in RADA16-I hydrogels when grew rapidly after 6 day. This may be due to Matrigel and collagen-I derived from animal materials, which contained biological components such as growth factors, may promote cell growth and proliferation [9,19,20]. However, the RADA16-I peptide belongs full chemical synthetic biomaterial, and has advantages of controllable and single ingredient during the application process [10,16]. Thus, non-biological components of this material only provide a relative simple ingredient microenvironment, and will lead to decrease the rate of cell proliferation with the increasing culture time.
The development and progression of tumor cells is controlled by their interactions with neighboring host cells and a variety of microenvironmental factors including ECM molecules, growth factors and proteinases [6,21]. FN and LN play a critical role in the maintenance of normal cell morphology, cell adhesion, migration, hemostasis, thrombosis, differentiation and proliferation [22,23]. Growth factors play an important role in several intracellular processes, such as cellular growth and differentiation, angiogenesis and apoptosis, as well as in carcinogenesis, since they contribute significantly to the malignant transformation [21,24]. In the present study, SMMC7721 cells may maintain the expression and secretion of various related factors such as FN, LN, VEGFA, EGF, FGF2 and IGF1, when they were cultured in RADA16-I hydrogels. These expression levels are similar to the other two hydrogels. These results indicated that RADA16-I was suitable for 3-D culture of SMMC7721 cells in vitro, which will help to maintain the stability of biological functions in SMMC7721 cells. This may be due to the unique interaction between SMMC7721 cells and RADA16-I gels. Although many progresses have been made, the detailed mechanism between nano fiber matrixs and cells in microenvironment still need to study.
Conclusions
In summary, our present results suggested that self-assembling peptide RADA16-I scaffolds may enhance cellular proliferation and differentiation activities of SMMC7721 cells, and showed well biocompatibility. Similar to cells cultured in Matrigel and collagen-I hydrogels, these cells cultured in RADA16-I hydrogels showed and maintained cell morphology and phenotype during the 3-D culture. The present findings also indicated that cellular microenvironment is very important to maintain tumor cell phenotype. This is due to that microenvironment may directly affect cell survival, growth, proliferation, and even tumor metastasis, which will help to provide a new way of thinking for the future treatment of cancer. Thus, the self-assembling peptide RADA16-I and present findings may serve as a promising scaffold for the biological characterizations of hepatocellular carcinoma cells in 3-D culture systems. The ongoing studies are aimed at the activating and/or inhibiting of signal pathways on the cell proliferation, migration and malignant phenotypes of SMMC7721 cells cultured in RADA16-I hydrogels.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 81160290).
Disclosure of conflict of interest
None.
References
- 1.Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal. 2011;5:239–248. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu F, Burg KJ. Three-dimensional polymeric systems for cancer cell studies. Cytotechnology. 2007;54:135–143. doi: 10.1007/s10616-007-9065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y, Stolarska MA, Othmer HG. The role of the microenvironment in tumor growth and invasion. Prog Biophys Mol Biol. 2011;106:353–379. doi: 10.1016/j.pbiomolbio.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MS, Yeon JH, Park JK. A microfluidic platform for 3-dimensional cell culture and cellbased assays. Biomed Microdevices. 2007;9:25–34. doi: 10.1007/s10544-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 5.Song HH, Park KM, Gerecht S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv Drug Deliv Rev. 2014;79:19–29. doi: 10.1016/j.addr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79-80:3–18. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905–7912. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mi K, Wang G, Liu Z, Feng Z, Huang B, Zhao X. Influence of a self-assembling peptide, RADA16, compared with collagen I and Matrigel on the malignant phenotype of human breast-cancer cells in 3D cultures and in vivo. Macromol Biosci. 2009;9:437–443. doi: 10.1002/mabi.200800262. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Song H, Zhang L, Xu H, Zhao X. Self-assembly-peptide hydrogels as tissue-engineering scaffolds for three-dimensional culture of chondrocytes in vitro. Macromol Biosci. 2010;10:1164–1170. doi: 10.1002/mabi.200900450. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Zhang L, Zhao X. Hemostatic efficacy of biological self-assembling peptide nanofibers in a rat kidney model. Macromol Biosci. 2010;10:33–39. doi: 10.1002/mabi.200900129. [DOI] [PubMed] [Google Scholar]
- 13.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadatmousavi P, Soltani M, Nazarian R, Jafari M, Chen P. Self-assembling peptides: potential role in tumor targeting. Curr Pharm Biotechnol. 2011;12:1089–1100. doi: 10.2174/138920111796117409. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Zhang S. Designer self-assembling peptide materials. Macromol Biosci. 2007;7:13–22. doi: 10.1002/mabi.200600230. [DOI] [PubMed] [Google Scholar]
- 16.Yokoi H, Kinoshita T, Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci U S A. 2005;102:8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38:1139–1151. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 20.Koutsopoulos S, Zhang S. Long-term three-dimensional neural tissue cultures in functionalized self-assembling peptide hydrogels, matrigel and collagen I. Acta Biomater. 2013;9:5162–5169. doi: 10.1016/j.actbio.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaspar M, Zardi L, Neri D. Fibronectin as target for tumor therapy. Int J Cancer. 2006;118:1331–1339. doi: 10.1002/ijc.21677. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki K. Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006;97:91–98. doi: 10.1111/j.1349-7006.2006.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds AR, Kyprianou N. Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting. Br J Pharmacol. 2006;147:S144–152. doi: 10.1038/sj.bjp.0706635. [DOI] [PMC free article] [PubMed] [Google Scholar]


