Abstract
To study the effect of hyperbaric oxygen intervention on the microenvironment of nerve regeneration after spinal cord injury modeling and to explore the possible mechanism of nerve regeneration and functional recovery in rats with spinal cord injury. In 98 adult female SD rats, 90 successful models were obtained, which were divided into sham group, spinal cord injury group and hyperbaric oxygen group using randomized block method, 30/group. Spinal cord injury rat model was established in accordance with the modified Allen method. Motor function was assessed at the time points of before modeling, one day, three days, one week, two weeks, three weeks and four weeks after modeling respectively by BBB rating, inclined plane test and improved Tarlov score. At 3 days after modeling, apoptosis of neuronal cells in spinal cord injury region in experimental group was detected by TUNEL method; gene and protein expression of MMP9/2 in spinal cord injury and surrounding tissues was detected by RT-PCR and Western blot assay. At 4 weeks after modeling, histopathological morphological changes in spinal cord injury were observed by HE staining; fluorogold retrograde tracing was used to observe the regeneration and distribution of spinal cord nerve fibers and axon regeneration was observed by TEM. The three motor function scores in hyperbaric oxygen group at each time point after two weeks of treatment were significantly increased compared with spinal cord injury group (P < 0.05). At 3 d after modeling, apoptosis index in hyperbaric oxygen group were significantly lower than those in spinal cord injury group (P < 0.05). At 72 h after modeling, compared with spinal cord injury group, MMP9/2 gene and protein expression in hyperbaric oxygen group was significantly lower (P < 0.05). At four weeks after modeling, fluorogold positive nerve fibers were the most sham group, followed by hyperbaric oxygen group and spinal cord injury group in order; the differences among the groups were statistically significant (P < 0.05). Under TEM, newborn unmyelinated and myelinated nerve fibers could be observed in the middle cross-section in the sham group and hyperbaric oxygen group; unmyelinated and myelinated nerve fibers in hyperbaric oxygen group were more than those in spinal cord injury group. Hyperbaric oxygen therapy played a protective effect on spinal cord injury through reducing apoptosis of neuronal cells and expression of MMP9/2 gene and protein in rats with spinal cord injury.
Keywords: Spinal cord injury, HBO, MMP9/2, nerve regeneration, motor function, rat
Introduction
With the development of technology and modern society, the incidence of spinal cord injury is increasing, with features of acute onset, severe illness, high disability rate, poor prognosis and high mortality. How to effectively treat spinal cord injury in clinical, reduce disability and mortality, achieve the best functional rehabilitation and improve the prognosis of patients is still a major problem haunting neurosurgeons.
In recent years, clinical and research workers have paid more and more attention to the application of hyperbaric oxygen in the rehabilitation therapy of brain and spinal cord injuries [1,2]. Numerous clinical studies have confirmed that the microenvironment of spinal cord injury area could be significantly improved by hyperbaric oxygen; hyperbaric oxygen can protect the spinal cord cell and tissue structure, extend the regeneration period of nerve cells after injury, and promote the regeneration of nerve fibers, thus contributing to the nerve reparation; while the specific mechanism still needs further discussion. After the injury of central nervous system, MMP-2 and MMP-9 destructed the tight junctions of capillaries, basement membrane and blood-brain barrier by proteolysis, and then caused vasogenic edema in central nervous system; in this study, rats with spinal cord injury were treated with hyperbaric oxygen to observe its effect on MMP9/2 gene and protein expression and the improvement of motor function in spinal cord area.
Materials and methods
Animals, reagents and instruments
98 adult female SD rats (weighing 250-290 g) [License No. SCXK (Tianjin) 20,090,008] were purchased from animal laboratory of Tianjin Medical University. Reagents: β-actin rabbit anti-mouse polyclonal antibody and MMP9/2 rabbit anti-rat polyclonal antibody (Sigma, USA); goat anti-rabbit IgG antibody (Santa Cruz, USA). AMV reverse transcription kit (Shanghai Xin Yu Biotech Co., Ltd.); heat-resistant TaqDNA polymerase and RT-PCR kit (TaKaRa Company); M-MLV reverse transcriptase (Promega Corporation, USA); 100 bp DNA ladder (CusaBio Company); DAB (Beijing Seitz biotechnology Co., Ltd.), TUNEL apoptosis kit (BIOVISION company, USA), fluorescent gold (aminostilbamidine, methanesulfonate) (Invitrogen, USA). Special animal hyperbaric chamber was purchased from Shanghai 701 Yang Garden hyperbaric chamber Co., LTD.).
Establishment of SCI model and hyperbaric oxygen therapy
SD rats were intraperitoneally anesthetized by 10% chloral hydrate (350 mg/kg). The rats were fixed on the experimental platform in prone position; after skin preparation in lower back, the T8~9 spinous process was taken as the center to perform the incision layer by layer from the middle of the dorsal; T7~10 spinous process and lamina were fully revealed, and part of T8~9 spinous process and lamina were removed to expose the subdural maintaining the integrity, which was taken as the injury area. According to the improved Allen method [3,4], a 10 g object fell freely from a height of 2.5 cm to hit subdural and spinal cord tissue (swing spasms of rat tail and paralysis of the lower limbs indicated successful modeling). The wound was washed with hydrogen peroxide and incisions were sutured. After modeling, squeezing urine was performed 2-3 times a day until the restoration of the micturition reflex in rats. 3 failed rat models and four deaths after modeling were excluded, and the remaining 90 rats were included in the experiment. Rats were randomly divided into three groups: spinal cord injury group, hyperbaric oxygen group, sham group (Only spinal cord tissue was exposed, without Allen hit), 20 rats in each group. Hyperbaric oxygen Intervention: At four hours after the modeling, rats in hyperbaric oxygen group were placed in a hyperbaric oxygen chamber and washed with pure oxygen for 15 min; the pressure was uniformly pressurized to 0.2 Mpa at 0.01 Mpa/min, regulating for 30 min; pure oxygen was added intermittently to maintain oxygen concentration above 96.5%, followed by uniform decompression to atmospheric pressure for 10 min; in extravehicular, rats were fed routinely; treatment was conducted four times daily, continuing for 3 d.
Motor function assessment
The related motor functions of rats in three groups were assessed before surgery, at one day, three days, one week, two weeks, three weeks, and four weeks after modeling. Evaluation items [5,6] included improved Tar-lov score [6,7], BBB score [7,8] and the inclined plate test [9,10]. Evaluation time was unified on 8:00 am. Scoring was performed by two individuals using double-blind method, respectively before modeling, at one day three days, one week, two weeks, three weeks and four weeks after modeling; three groups of rats were measured, randomly testing 10 rats in each group; detection was performed eight times, and the mean value was calculated.
Apoptosis detection
After modeling for 72 h, six rats were collected in each group and anesthetized by chloral hydrate; after thoracotomy, aortic cannulation was performed in the left ventricle, fixed by 4% paraformaldehyde perfusion. Spinal cord injury area was taken as the center to collect 2.0 cm spinal cord tissue, which was soaked and fixed in paraformaldehyde. Paraffin sections were deparaffinized and TUNEL assay was performed according to the instructions provided in the kit of BIOVISION Company (USA). Cells with brownish yellow granules in the nucleus were TUNEL-positive cells; apoptotic cells in 10 photos were counted to calculate the apoptotic index; Apoptotic Index = positive cells/(positive cells + negative cells) × 100%.
RT-PCR detection
After modeling for 72 h, 50 mg spinal cord injury tissues of six rats in three groups were randomly collected. According to the instructions of Trizol reagent, extraction of total RNA in spinal cord tissue performed and content determination of total RNA was performed by UV spectrophotometer; using RT-PCR two-step kit (TaKaRa Company), reverse transcription of mRNA to cDNA was performed, and then PCR amplification of cDNA was conducted. Using the following primers [11], MMP9/2 and GAPDH cDNA was amplified: MMP-2 (414 bp) upstream 5’-TTTTTGTGCCCAAAGAAAGG-3’, downstream 5’-ATGTCAGACAACCCGAGTCC-3’; MMP9 (379 bp) primers sequence: Upstream 5’-GGTTTCTGTCCAGACCAAGG-3’, downstream 5’-TGCAAGGATTGTCATC TGG A-3’; GAPDH (300 bp) upstream 5’-GAGGACCAGGTTGTCTCC TG-3’, downstream 5’-GGATGGAATTGTGAGGGAGA-3’. Take the amplified product was separated by electrophoresis gel; optical density analysis of electrophoresis results was conducted using gel image analysis system; integral optical density ratio of MMP9/2 and GAPDH was used as the indicator of MMP9/2 mRNA expression.
Western blot assay
After RT-PCR, the rest sample was centrifuged with 1 500 r • min for 30 min; the supernatant (crude protein) was collected. Determination of protein concentration was performed by Bradford method. Then sample was treated with 5% stacking gel with 40 V constant voltage for 1 h, 10% separating gel with 60 V constant voltage for 3.5 h, wet-turn 14 V constant voltage for 14 h; sealing for 2 h on 37°C shaker and membrane washing (10 min × 3) were performed in order. MMP9/2 polyclonal antibody (1:500) was diluted by TBST to incubate the sample for 60 min at room temperature; after TBST membrane washing, rabbit anti-mouse antibody (1:2 000) was added (Alkaline phosphatase-labeled), incubating 60 min at room temperature, followed by TBST membrane washing (3 times) and TBS washing (10 min). Quantity one image analysis was performed. The gray values of target bands were divided by β-actin gray values for result analysis. The ratio of absorption area between MMP9/2 product and GAPDH product bands was taken as an indicator of MMP9/2 protein expression.
HE staining and fluorescence retrograde tracing
At 4 weeks after modeling, six rats were respectively sacrificed in sham group, spinal cord injury group and hyperbaric oxygen group; samples were collected for HE staining; After anesthesia by 10% chloral hydrate (350 mg/kg), thoracotomy was performed to fully expose rat heart; catheterization was performed through the ascending aorta, and after cutting the right atrial appendage, the tissue was washed with saline and fixed with 4% paraformaldehyde, and then the damaged area of spinal cord tissue was completely removed; 1 cm spinal cord injury tissue was dehydrated by gradient alcohol and wale frozen sections of spinal cord was prepared, with a thickness of 20 μm. After hematoxylin staining for 5 min, samples were rinsed with the tap water, differentiated in hydrochloric acid alcohol for 10 s, rinsed with tap water again for 10 min and stained with eosin for 7 min; after water washing, dehydration by gradient alcohol, transparent by xylene and sheet-sealing with neutral gum were performed. 5 rats were randomly selected in three groups and anesthetized; along the vastus lateralis gap, sciatic nerve was revealed and dampened by tissue forceps; using micro-syringe and under dark conditions, multi-point injection of 2% fluorescein gold was performed in the sciatic nerve dampened area, 0.4 μL in each side of sciatic nerve, with an injection speed of 0.1 μL/min. The needle was retained 5 min in each injection site, and 80,000 U penicillin powder was sprinkled inside the incision; incisions were sutured, normal feeding was performed after surgery. After 1 week, samples in spinal cord injury area were drawn for histological examination, which were soaked in 30% sucrose and saved overnight in a 4°C refrigerator; in the next day, they were embedded in OCT frozen section embedding agents at -18°C, and 20 μm thicken cross sections and coronal frozen sections were prepared; five slices were selected for each animal in each group to observe the distribution of fluorescent labeling cells under a fluorescence microscope.
TEM observation
At four weeks after modeling, six animals in each group were randomly selected, and heart perfusion by 2.5% glutaraldehyde was performed; samples were drawn and fixed with the same concentration of glutaraldehyde overnight; with the injury site as the central, 1 cm × 1 mm to spine cord was collected respectively in the proximal end and distal, which was fixed in osmium tetroxide at 4°C for 2 h; after rinsing, dehydration with gradient acetone, staining with uranyl acetate at 4°C for 4 h and 618 epoxy-emendation were performed before TEM observation.
Statistical analysis
Sample mean was expressed as x ± s. Experimental data were analyzed using SPSS 17.0 software; differences among multiple groups were compared using univariate analysis of variance (LSD method); differences between two groups were compared using t test.
Results
Motor function assessment results
There were no significant differences in BBB score, inclined plate test and improved Tarlov score before modeling for all rats (P > 0.05). After hyperbaric oxygen therapy, the three scores in hyperbaric oxygen group were significantly increased compared with spinal cord injury group at each time point (2-4 weeks after modeling), and the difference was statistically significant (P < 0.05). Three scores were also lower than those in the sham group at 2-4 weeks after modeling, and the difference was statistically significant (P < 0.05), shown in Table 1.
Table 1.
BBB score, inclined plate test and improved Tarlov score at each time point (x̅ ± s)
| Group | Cases | Before injury | After modeling | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 d | 3 d | 1 week | 2 weeks | 3 weeks | 4 weeks | |||
| BBB score | ||||||||
| Spinal cord injury group | 10 | 21.00±0.00 | 0.00±0.00 | 1.24±0.05 | 2.42±0.58 | 8.42±1.58# | 11.10±1.02# | 13.59±0.22# |
| Hyperbaric oxygen group | 10 | 21.00±0.00 | 0.00±0.00 | 2.43±0.09 | 3.93±1.04# | 10.31±1.38# | 12.60±1.72# | 15.32±0.16# |
| Sham group | 10 | 21.00±0.00 | 19.01±0.04 | 20.19±0.12 | 20.79±0.21# | 21.00±0.00# | 21.00±0.00# | 21.00±0.00 |
| Inclined plate test | ||||||||
| Spinal cord injury group | 10 | 42.50±1.48 | 15.64±1.47 | 16.49±1.28 | 21.80±1.70 | 23.02±1.83 | 26.24±2.02 | 28.21±1.25 |
| Hyperbaric oxygen group | 10 | 42.50±1.96 | 16.84±2.04 | 18.90±1.57# | 24.62±2.04# | 29.82±2.03 | 34.38±2.11# | 38.57±2.01# |
| Sham group | 10 | 42.50±2.21 | 40.44±2.06 | 41.27±2.16# | 42.52±2.20# | 42.51±2.06# | 42.48±1.81# | 42.50±1.74# |
| Improved Tarlov score | ||||||||
| Spinal cord injury group | 10 | 5.00±0.00 | 0.00±0.00 | 0.40±0.12 | 0.86±0.13# | 1.67±0.35 | 2.48±0.20# | 2.68±0.22# |
| Hyperbaric oxygen group | 10 | 5.00±0.00 | 0.00±0.00 | 0.76±0.13 | 1.61±0.18 | 2.82±0.22# | 3.60±0.15# | 3.92±0.24 |
| Sham group | 10 | 5.00±0.00 | 4.63±0.24 | 4.73±0.21 | 4.85±0.21 | 4.88±0.19# | 4.93±0.10# | 5.00±0.03 |
Compared with spinal cord injury group, there was a significant difference (P < 0.05).
TUNEL assay to detect apoptosis
Specific brown particles could be observed in the nucleus of apoptotic nerve cells; under the optical microscope, apoptotic cells scattered throughout the spinal cord injury area, and apoptotic positive cells can also be found at the edge of injury area. TUNEL assay showed that in hyperbaric oxygen group, the number of apoptotic cells with brown particles (13.64±3.68) was significantly less than that in spinal cord injury group (28.24±4.64) (P < 0.05); no apoptotic cells were found in sham group (00.00±0.00), shown in Figure 1.
Figure 1.
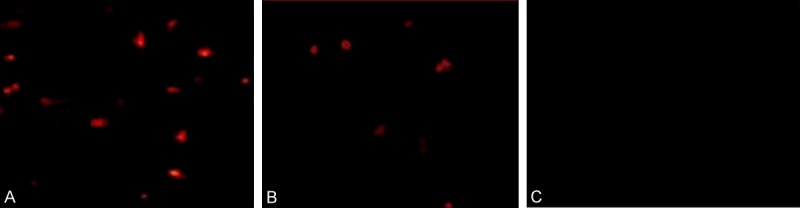
The results of TUNEL assay to detect apoptosis in each group. A: Apoptotic cells in spinal cord injury group (28.24±4.64); B: Apoptotic cells in hyperbaric oxygen group (13.64±3.68); C: No apoptotic cells in sham group (00.00±0.00).
MMP9/2 mRNA expression
The results (Figure 2; Table 2) showed that at 72 h after modeling, MMP9/2 mRNA expression in spinal cord injury area in hyperbaric oxygen group was higher than that in the sham group, and the difference was significant (P < 0.05); while MMP9/2 mRNA expression was significantly lower than that in spinal cord injury group; the difference was also statistically significant (P < 0.05).
Figure 2.
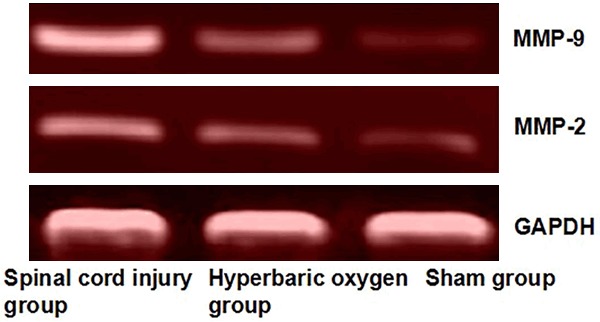
Electrophoresis of MMP9/2 mRNA in each group. MMP9/2 protein expression in spinal cord injury area in hyperbaric oxygen group was higher than that in the sham group, and the difference was significant (P < 0.05).
Table 2.
MMP9/2 gene and protein expression in spinal cord injury area in three groups (x̅ ± s)
| Group | n | MMP9/2 mRNA (MMP/GAPDH) | MMP9/2 protein (MMP/GAPDH) | ||
|---|---|---|---|---|---|
|
| |||||
| MMP-9 | MMP-2 | MMP-9 | MMP-2 | ||
| Spinal cord injury group | 6 | 0.82±0.05* | 1.35±0.16* | 0.91±0.17* | 1.42±0.22* |
| Hyperbaric oxygen group | 6 | 0.42±0.06*,Δ | 1.05±0.18*,Δ | 0.62±0.12*,Δ | 1.06±0.14*,Δ |
| Sham group | 6 | 0.15±0.02Δ | 0.45±0.05Δ | 0.28±0.06Δ | 0.70±0.16 |
Compared with spinal cord injury group, there was a significant difference (P < 0.05);
compared with sham group, there was a significant difference (P < 0.05).
MMP9/2 protein expression
The results (Figure 3; Table 2) showed that at 72 h after modeling, MMP9/2 protein expression in spinal cord injury area in hyperbaric oxygen group was higher than that in the sham group, and the difference was significant (P < 0.05); while MMP9/2 mRNA expression was significantly reduced compared with spinal cord injury group; the difference was also statistically significant (P < 0.05).
Figure 3.
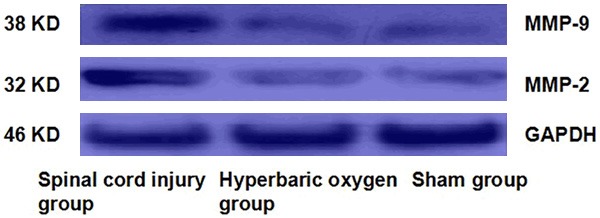
Western blotting of MMP9/2 expression in each group at 72 h after modeling.
Histopathological changes in the spinal cord
At four weeks after modeling, under the light microscope, the structure of spinal cord injury tissues in spinal cord injury group was loose, showing the formation of syringomyelia and a large number of neuronal necrosis. In hyperbaric oxygen group, the injury tissue was lightly loose, with small syringomyelia and part of neuronal necrosis. In sham group, organizational structure of spine cord was intact and clear, without syringomyelia and neuronal apoptosis, shown in Figure 4.
Figure 4.
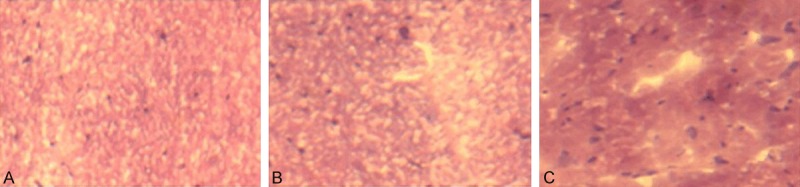
Histopathological changes in the spinal cord. A: Morphological structure of spine cord was intact and clear, without syringomyelia and neuronal apoptosis (× 40); B: In hyperbaric oxygen group, the injury tissue was lightly loose, with small syringomyelia and part of neuronal necrosis. C: The structure of spinal cord injury tissues in spinal cord injury group was loose, showing the formation of syringomyelia (× 40).
FG corticospinal tract retrograde tracing
There were respectively (8.6±2.4), (18.4±3.6) and (36.4±4.2) FG-labeled pyramidal cells and axons in the cephalic of spinal cord injury area in spinal cord injury group, hyperbaric oxygen group and sham group (Figure 5); Statistically significant differences had been found between hyperbaric oxygen group and spinal cord injury group (P < 0.05), sham group and spinal cord injury group (P < 0.01).
Figure 5.

Results of FG corticospinal tract retrograde tracing. A: Few FG-labeled pyramidal cells and axons in the cephalic of spinal cord injury area in spinal cord injury group, hyperbaric oxygen group and sham group (× 200); B: A few FG-labeled pyramidal cells and axons in the cephalic of spinal cord injury area in hyperbaric oxygen group (× 200); C: Many FG-labeled pyramidal cells and axons in the cephalic of spinal cord injury area in sham group, with gold fluorescence (× 200).
TEM
TEM results showed that at four weeks after injury, glial scar and a small amount of myelinated nerve fibers, macrophages degeneration and necrosis of myelinated nerve fibers were observed in spinal cord injury group shown in Figure 6A. In the injury area of the sham group, a large number of myelinated nerve fibers and non-myelinated nerve fibers, many axons and regenerated axonal myelin integrity could be observed, shown in Figure 6B. In the injury region of hyperbaric oxygen group, a few myelinated nerve fibers and non-myelinated nerve fibers can be observed, which were between those of spine cord injury group and the sham group, shown in Figure 6C.
Figure 6.
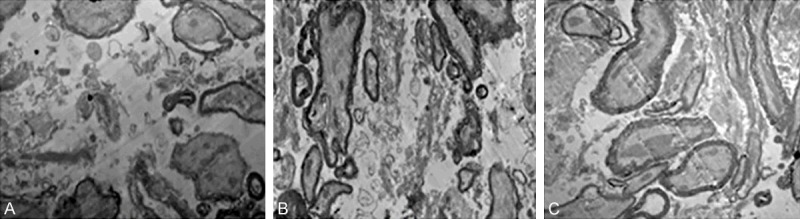
TEM results in each group. A: TEM in spinal cord injury group at 4 weeks after injury (× 8000); B: TEM in sham group at 4 weeks after injury (× 8000); C: TEM in hyperbaric oxygen group at 4 weeks after injury (× 8000).
Discussion
Spinal cord injury (SCI) is a serious disease which hazarded to health. With the increase in traffic accidents and high-altitude operations, SCI is increasing in clinical with high morbidity and poor prognosis. Instantaneous primary injury and subsequent secondary injury were involved in SCI. Secondary injury may result in serious spinal cord function damage. At the same time, secondary injury at this pathological process played an important role in prognosis of nerve damage [12-14]. After a series of neurochemical SCI secondary to primary trauma, microcirculation disorders caused formation of malignant pathology chain reaction were the main cause of permanent nerve damage. Apoptosis can be triggered by excitatory amino acids, free radicals, cytokines and inflammatory cytokines, which played an important role in the growth, development, differentiation, and regeneration process of nerve cell [15,16].
Neurological damage occurred after spinal cord modeling including two mechanisms: primary and secondary [17,18]. Secondary spinal cord injury was caused because a large number of free radicals were produced in damage zone after modeling, and then there was a series of oxidation reactions. Ultimately they can lead to damage in nerve tissue of spinal cord injury area, and result in a number of clinical neurological deficit symptoms and signs [19,20]. HBO has protective effect on spinal cord cells and tissue structures, extending the regeneration of nerve cells after injury and promoting nerve fiber regeneration. Hyperbaric oxygen therapy should be taken as soon as possible in spinal cord damage after modeling, which was benefit for reducing neuronal apoptosis, reducing the degree of disability of patients, and preventing and inhabiting the patient’s condition from further deteriorated. It has a good long-term, safe, reliable, effective obvious results. It also has the advantage of fewer complications. Studies have shown that excessive inflammatory response prompted oligodendrocyte apoptosis, further aggravated the spinal cord injury, and affected spinal cord damage after modeling neurological recovery [17-20]. This study showed that hyperbaric oxygen therapy after spinal cord modeling can effectively reduce the expression of neuronal edema-related genes and proteins in spinal cord injury area; MMP9/2 gene and protein expression can reduce the extent of edema, meanwhile, HBO after treatment can effectively reduce the apoptosis of neuronal cells in injury region. BBB score, inclined plate test and improved Tarlov score test showed that hyperbaric oxygen therapy can improve hindlimb motor function in rats. TEM observation showed that unmyelinated and myelinated nerve fibers in hyperbaric oxygen group were more than those in spinal cord injury group, which can effectively promote the recovery of rats with spinal cord injury.
In clinical, the main methods for the treatment of spinal cord injury were neurotrophic and physiotherapy rehabilitation methods to save the damaged area and necrotic neurons around the verge, restore spinal cord ischemia and hypoxia, and repair promote neuronal function [18-20]. Hyperbaric oxygen therapy is that the body is placed in high pressure environment by breathing pure oxygen equaled to environment, which can reduce secondary nerve injury and promote endogenous stem cell proliferation and differentiation [21]. Recent research showed that [22,23] hyperbaric oxygen therapy can improve the body’s blood pressure, increase tissue oxygen content of central nervous system and promote aerobic metabolism of the central nervous system tissue. Studies have shown [23,24] hyperbaric oxygen therapy can decrease the calcium content, inhibit lipid peroxidation, protect cell antioxidant capacity, reduce calcium ions flowing into the cell, protect never cell, and promote regeneration.
In short, hyperbaric oxygen can improve the microenvironment by the following methods in treatment of rats with spinal cord injury: hyperbaric oxygen can increase blood oxygen, oxygen content and oxygen diffusion. Rapidly improving hypoxic state, enhancing the activity of mitochondrial enzyme, promoting cell repair and normal metabolism [25] can significantly increase the generation of free radicals [26], thereby induced spinal cord ischemia and hypoxia tolerance. HBO can also inhibit the activation of microglial cells secrete certain cytokines, thereby adjusting the microglial cell mediated immune response [27]. In this study, rehabilitation therapy through hyperbaric oxygen provided a new theoretical basis for clinical intervention with spinal cord injury.
Disclosure of conflict of interest
None.
References
- 1.Niklas A, Brock D, Schober R, Schulz A, Schneider D. Continuous measurements of cerebral tissue oxygen pressure during hyperbaric oxygenation --HBO effects on brain edema and necrosis after severe braintrauma in rabbits. J Neurol Sci. 2004;219:77–82. doi: 10.1016/j.jns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Puttaswamy V, Bennett M, Frawley JE. Hyperbaric oxygenation treatment of acute paraplegia after resection of a thoracoabdominal aortic aneurysm. J Vascul Surg. 1999;30:1158–1161. doi: 10.1016/s0741-5214(99)70057-1. [DOI] [PubMed] [Google Scholar]
- 3.Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HM, Zhang Y. Medical Research Progress of nerve growth factor on neurons of animals. Progress in Veterinary Medicine. 2006;27:39–41. [Google Scholar]
- 5.Haku T, Okuda S, Kanematsu F, Oda T, Miyauchi A, Yamamoto T, Iwasaki M. Repair of cervical esophageal perforation using longus colli muscle flap: a case report of a patient with cervical spinal cord injury. Spine J. 2008;8:831–835. doi: 10.1016/j.spinee.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, Bunge MB. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- 7.Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. Grafts of Schwann cells engineered to express PSANCAM promote functional recovery after spinal cord injury. Brain. 2007;130:2159–2174. doi: 10.1093/brain/awm155. [DOI] [PubMed] [Google Scholar]
- 8.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Lowmolecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 9.Pallini R, Vitiani LR, Bez A, Casalbore P, Facchiano F, Di Giorgi Gerevini V, Falchetti ML, Fernandez E, Maira G, Peschle C, Parati E. Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery. 2005;57:1014–1025. doi: 10.1227/01.neu.0000180058.58372.4c. [DOI] [PubMed] [Google Scholar]
- 10.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen XF, Mo WB. Aescine sodium combined with umbilical cord mesenchymal stem cells transplantation for treatment of neurological function in rats with cerebral infarction. Chinese Journal of Tissue Engineering Research. 2011;15:5080–5084. [Google Scholar]
- 12.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Amar AP, Levy ML. Pathogenesis and phar macol ogical strategies formitigating secondary damage in acute s pinal cord injury. Neurosurgery. 1999;44:1027–1040. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 14.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 15.Ohta S, Iwashita Y, Takada H, Kuno S, Nakamura T. Neuroprection and enhanced recovery with edaravone after acute spinal cord injury in rats. Spine. 2005;30:1154–1158. doi: 10.1097/01.brs.0000162402.79482.fd. [DOI] [PubMed] [Google Scholar]
- 16.Banno M, Mizuno T, Kato H, Zhang G, Kawanokuchi J, Wang J, Kuno R, Jin S, Takeuchi H, Suzumura A. The radical scavenger edaravone prevents oxidative Neurotoxicity induced by peroxynitrite and activated microglia. Neuropharm. 2005;48:283–290. doi: 10.1016/j.neuropharm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Björklund E, Lindberg E, Rundgren M, Cronberg T, Friberg H, Englund E. Ischaemic brain damage after cardiac arrest and induced hypothermia-a systematic description of selective eosinophilic neuronal death. A neuropathologic study of 23 patients. Resuscitation. 2014;85:527–532. doi: 10.1016/j.resuscitation.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Ariake K, Ohtsuka H, Motoi F, Douchi D, Oikawa M, Rikiyama T, Fukase K, Katayose Y, Egawa S, Unno M. GCF2/LRRFIP1 promotes colorectal cancer metastasis and liver invasion through integrin-dependent RhoA activation. Cancer Lett. 2012;325:99–107. doi: 10.1016/j.canlet.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Verghese G, Verma R, Bhutani S. Hyperbaric oxygen therapy in the battlefield. Medical Journal Armed Forces India. 2013;69:94–96. doi: 10.1016/j.mjafi.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallini R, Vitiani LR, Bez A, Casalbore P, Facchiano F, Di Giorgi Gerevini V, Falchetti ML, Fernandez E, Maira G, Peschle C, Parati E. Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery. 2005;57:1014–1025. doi: 10.1227/01.neu.0000180058.58372.4c. [DOI] [PubMed] [Google Scholar]
- 21.Chuang SK. Limited Evidence to Demonstrate that the use of hyperbaric oxygen (HBO) therapy reduces the incidence of osteoradionecrosis in irradiated patients requiring tooth extraction. J Evid Based Dent Pract. 2011;11:129–131. doi: 10.1016/j.jebdp.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Jasaitis A, Ouellet H, Lambry JC. JUl trafast hemeligand recombination in truncated hemoglobin HbO from Mycobacterium tuberculosis: A ligand cage. Original Chemical Physics. 2012;396:10–16. [Google Scholar]
- 23.Ding JJ. Effect of hyperbaric oxygen therapy on acute phase serum levels in patients with cerebral infarction ACA. Chinese Journal of Practical Nervous Diseases. 2012;15:34–36. [Google Scholar]
- 24.Fuentes-Raspall R, Oliu-lsem G, Inoriza-Belzunce JM. Efficacy of hyperbaric oxygen therapy (HBO) for LATE pelvic radiation toxicity. Results of a prospective study. Radiotherapy and Oncology. 2011;98:23. [Google Scholar]
- 25.Calvert JW, Yin W, Patel M, Badr A, Mychaskiw G, Parent AD, Zhang JH. Hyperbaric oxygenation prevented brain injury induced by hypoxia- ischemia in a neonatal rat model. Brain Res. 2002;951:1–8. doi: 10.1016/s0006-8993(02)03094-9. [DOI] [PubMed] [Google Scholar]
- 26.Mink RB, Dutka AJ. Hyperbaric oxygen after global cerebral ischemia in rabbits does not promote brain lipid peroxidation. Crit Care Med. 1995;23:1398–1404. doi: 10.1097/00003246-199508000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Li CC, Sun XJ, Hang RC, Lian QL, Jiang CL, Tao HY. Effects of hyperbaric oxygen pretreatment on cytokines production in neonatal rat microglias in vitro. Academic Journal of Second Military Medical University. 2003;24:58–60. [Google Scholar]


