Abstract
IL33/ST2 axis activates airway eosinophils that exacerbate airway inflammation. The data was obtained from PubMed, EMBASE, Clinical trial, Cochrane Library, Web of science, CNKI and Wanfang database with time restrictions of 1 Jan, 2000 to 15 Feb, 2016. The meta-analysis was performed using Review Manager 5.2 software. After searching, total 15 documents were included into this meta-analysis, involving in 633 asthma patients and 379 healthy people. The meta-analysis results revealed that the serum IL33 or ST2 level was higher in asthma patients compared to that in healthy people. (P=0.02, 95% CI (7.57, 72.74); P<0.0001, 95% CI (31.27, 91.32)). Compared to healthy people, severe, moderate or mild asthma patients had much higher serum IL33 level. (P<0.00001, 95% CI (87.86, 188.09); P<0.00001, 95% CI (31.93, 72.29); P<0.00001, 95% CI (100.51, 153.08), respectively). The serum ST2 level in different asthma progress included severe or moderate was higher, (P<0.00001, 95% CI (50.76, 76.93); P<0.00001, 95% CI (1.02, 1.79), respectively) but nor mild. (P=0.30, 95% CI (-22.37, 72.61)). The meta-analysis result shown the sputum IL33 was not higher in moderate asthma patients than that in healthy people. (P=0.20, 95% CI (-1.99, 9.52)) The meta-analysis results shown that there were significantly difference between and among two asthma progress, (P<0.00001, 95% CI (14.02, 19.09), severe vs moderate; P<0.00001, 95% CI (0.52, 1.24), moderate vs mild) However, there was no significant differences between severe group and mild group. (P=0.08, 95% CI (-20.95, 336.50)). Serum IL33 and ST2 level is relevant to asthma disease. With asthma disease progress, IL33 and ST2 are increased significantly.
Keywords: Interleukin-33, ST2, asthma, meta-analysis
Introduction
Asthma is a complex inflammatory disease, characterized by airway hyperresponsiveness (AHR), airway inflammation, and reversible airway obstruction, affecting up to 300 million people worldwide [1]. The development of asthma is a multifactorial process which is associated with a variety of risk factors, including environmental factors and genetic factors [2]. Allergens, viral infections, environmental tobacco smokes and pollutants represent the most important exogenous risk factors [3]. As we known, the bronchial asthma is a disease that T helper 2 (Th2) cell plays important role. Th2 cells can produce a lot of cytokines, including interleukin (IL)-33 [4].
Interleukin-33 (IL-33) is a novel cytokine belonging to the IL-1 family which was found in 2005 [5]. It can bind to receptors ST2, which highly expressed on some cells, included mast cells and Th2 cells [6]. It is found in various cells included fibroblasts, bronchial and epithelial cells, endothelial cells, and some immune cells, including macrophages and dendritic cells [7]. IL-33 participates in many diseases with dual, proinflammatory or protective roles depending on the cellular and cytokine context, including asthma [8,9]. Moreover, the previous study also shown that ST2 is expressed in the adults and children patients with acute asthma [10]. However, the correlation between IL33 and its receptor ST2 and asthma is unknown. It is unknown whether IL33 or ST2 expression can be an indicator for asthma progress.
In this meta-analysis, we searched and analyzed the role of IL33 and ST2 in patients with asthma, which may be provide a foundation for clinical diagnosis and therapy.
Methods
A computerized literature search was conducted using Pubmed, MEDLINE, EMBASE and Chinese databases (including CNKI and WanFang database) from 1 Jan 2000 through 15 Feb 2016. The search strategy used medical subject heading (MeSH) terms and keywords “interleukin-33” or “IL-33”, “ST2”, “asthma”, ”endotrachial”, “sputum”, “lung” and “randomized controlled trials”. The patient had no other severe disease, including immune deficiencies. We also manually reviewed the reference lists to identify additional relevant studies. No language restrictions were imposed.
Inclusion and exclusion criteria
All studies should be fulfilled the following criteria: (1) the exposure of interest was interleukin-33 or the ST2; (2) the outcome of interest was asthma; (3) all included studies should be case-control study, cohort study, randomized controlled trials or cross-sectional study; (4) all included studies had no language limitation. The study that it was comment, duplicate data, abstract, review, and editorial or insufficient data should be excluded.
Data extraction
Two investigators independently performed the data extraction. When discrepancies were found, a third investigator would make the definitive decision for data extraction. The extracted information included: the first author’s last name, publication year, participant characteristics (age and sex), sample size, variables adjusted in the analysis.
Statistical analysis
Statistical analyses were performed with Review manager version 5.2 software (The Cochrane Collaboration). The heterogeneity of trail outcome among RCTs was assessed by χ2 test (P<0.1 was defined as a significant heterogeneity) or I 2 test. The publication bias was assessed by examining the funnel plot. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for all primary and secondary outcomes were calculated by Mantel-Haenszel fixed-effect model (FEM) if there was no statistical heterogeneity; otherwise, the DerSimonian-Laird random effects model (REM) was used.
Results
The general data
After searching, total 15 documents [11-25] were included into this meta-analysis (Figure 1), involving in 633 asthma patients and 379 healthy people. The characteristics of the included studies were shown in Table 1. No studies described randomized methods and allocation concealment. Three studies adopted double-blind, and the baseline of all researches was similar (Table 2).
Figure 1.
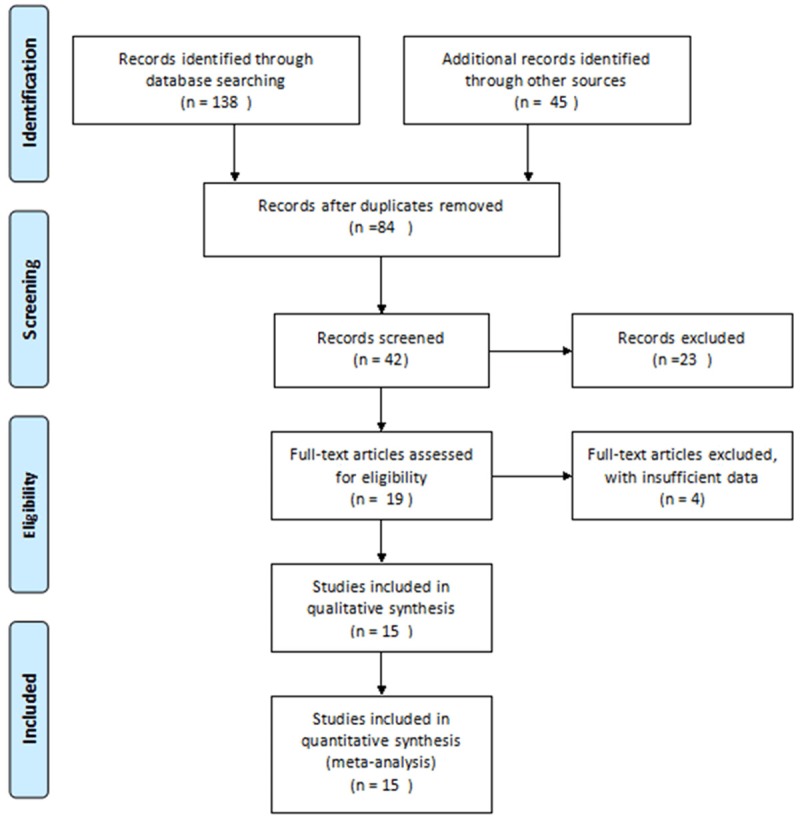
Flow chart of searching the relevant studies used in this meta-analysis.
Table 1.
The general data of included documents
| First Author | Publishing year | Methodological quality | Intervention | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Study design | Randomized method | Allocation concalment | Double-blind | Baseline | Outcomes | ||
| Azazi [11] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S; SA; MA |
| Liu [12] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; ST; SA; MDA |
| Zhang [13] | 2011 | RCT | Unclear | Unclear | No | Similar | IL33; S; SA; MDA |
| Yang [14] | 2011 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA |
| Prefontaine [15] | 2009 | RCT | Unclear | Unclear | No | Similar | IL33; S; SA; MDA |
| Prefontaine [16] | 2010 | RCT | Unclear | Unclear | No | Similar | IL33; S; SA; MDA; MA |
| Hamzaoui [17] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S; ST; MDA; MA |
| Liao [18] | 2014 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA |
| Li [19] | 2013(a) | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA; MA |
| Ma [20] | 2011 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA; MA |
| Gong [21] | 2002 | RCT | Unclear | Unclear | Yes | Similar | ST2; S |
| Zhang [22] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; S |
| Li [23] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; S |
| MiAeChu [24] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S |
| Gluck [25] | 2012 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S |
S: serum; ST: sputum; SA: severe asthma; MDA: moderate asthma; MA: mild asthma.
Table 2.
The general data of included documents
| First Author | Publishing year | Methohodogical quality | Intervention | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Study design | Randomized method | Allocation concalment | Double-blind | Baseline | Outcomes | ||
| Azazi [11] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S; SA; MA |
| Liu [12] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; ST; SA; MDA |
| Zhang [13] | 2011 | RCT | Unclear | Unclear | No | Similar | IL33; S; SA; MDA |
| Yang [14] | 2011 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA |
| Prefontaine [15] | 2009 | RCT | Unclear | Unclear | No | Similar | IL33; S; SA; MDA |
| Prefontaine [16] | 2010 | RCT | Unclear | Unclear | No | Similar | IL33; S; SA; MDA; MA |
| Hamzaoui [17] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S; ST; MDA; MA |
| Liao [18] | 2014 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA |
| Li [19] | 2013(a) | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA; MA |
| Ma [20] | 2011 | RCT | Unclear | Unclear | Yes | Similar | IL33; S; SA; MDA; MA |
| Gong [21] | 2002 | RCT | Unclear | Unclear | Yes | Similar | ST2; S |
| Zhang [22] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; S |
| Li [23] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; S |
| MiAeChu [24] | 2013 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S |
| Gluck [25] | 2012 | RCT | Unclear | Unclear | Yes | Similar | IL33; ST2; S |
S: serum; ST: sputum; SA: severe asthma; MDA: moderate asthma; MA: mild asthma.
Correlation between IL33 or ST2 and patients with asthma
Four documents [20,22,23,25] representing 222 asthma patients and two papers [21,25] involving in 335 cases evaluated the correlation with serum IL33 and ST2 level, respectively. As shown in Figure 2A and 2B, the meta-analysis results revealed that the serum IL33 or ST2 level was higher in asthma patients compared to that in healthy people (P=0.02, 95% CI (7.57, 72.74); P<0.0001, 95% CI (31.27, 91.32)).
Figure 2.
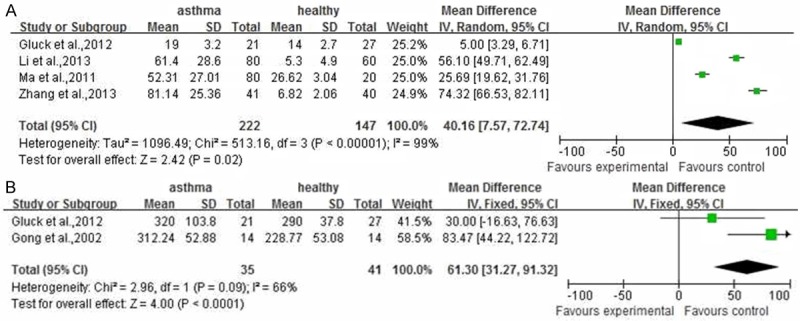
The meta-analysis of correlation between IL33 or ST2 and patients with asthma.
Subgroup analysis for IL33 or ST2 in patients with asthma
As described above result, there were significant difference between serum IL33 or ST2 level in asthma patients and healthy people. However, the correlation IL33 or ST2 level between different asthma progress and healthy people is unknown. As shown in Figure 3, compared to healthy people, the severe (involving in 10 documents [11,12-16,18-20,24]), moderate (involving in 10 documents [12-20,24]) or mild (involving in 5 documents [11,15,17,19,20]) asthma patients had more higher serum IL33 level. (P<0.00001, 95% CI (87.86, 188.09); P<0.00001, 95% CI (31.93, 72.29); P<0.00001, 95% CI (100.51, 153.08), respectively). Similarly to IL33 in asthma patients, the serum ST2 level in different asthma progress included severe (involving in 2 documents [11,24]), or moderate (involving in 2 documents [17,24]) was higher (Figure 4A and 4B) (P<0.00001, 95% CI (50.40, 76.57); P=0.03, 95% CI (3.41, 55.73)), but not mild involving in 2 documents [11,17]. (Figure 4C) (P=0.30, 95% CI (-22.37, 72.61) For sputum tissues from asthma patients or healthy people, only two documents [12,17] were included into here to reveal the correlation between IL33 level and moderate asthma progress. The meta-analysis result shown the sputum IL33 was not higher in moderate asthma patients than that in healthy people (Figure 5, P=0.20, 95% CI (-1.99, 9.52).
Figure 3.
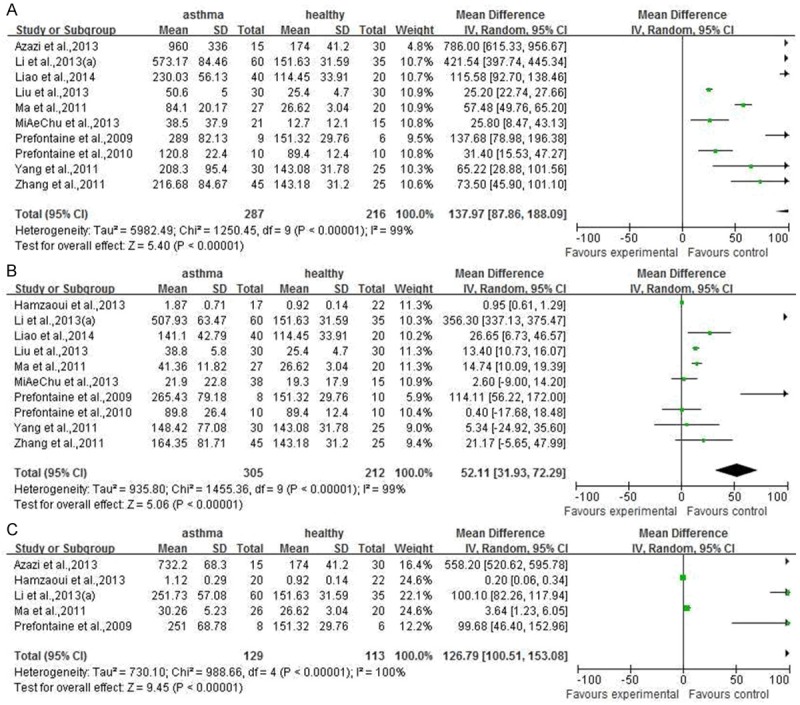
The meta-analysis of comparion between serum IL33 level in asthmatic with different disease progress and that in healthy people.
Figure 4.
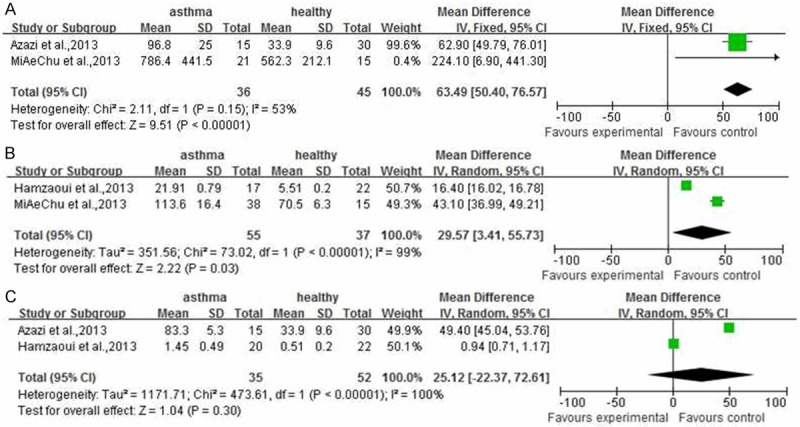
The meta-analysis of comparison between serum ST2 level in asthmatic with different disease progress and that in healthy people.
Figure 5.
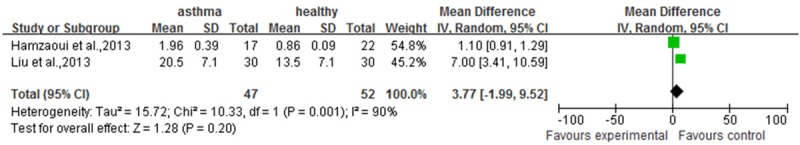
The meta-analysis of comparion between sputum IL33 level in moderate asthmatic and that in healthy people.
For further understanding the IL33 and ST2 changes in asthma progress, we evaluated the relations between and among severe, moderate and mild asthma patients for IL33 or ST2 level. As shown in Figure 6, the meta-analysis results shown that there were significantly difference between and among two asthma progress (Figure 6A, P<0.00001, 95% CI (24.02, 62.00), severe vs moderate; Figure 6C, P=0.0006, 95% CI (31.85, 116.92), moderate vs mild). However, there was no significant differences between severe group and mild group (Figure 6B, P=0.08, 95% CI (-20.95, 336.50)).
Figure 6.
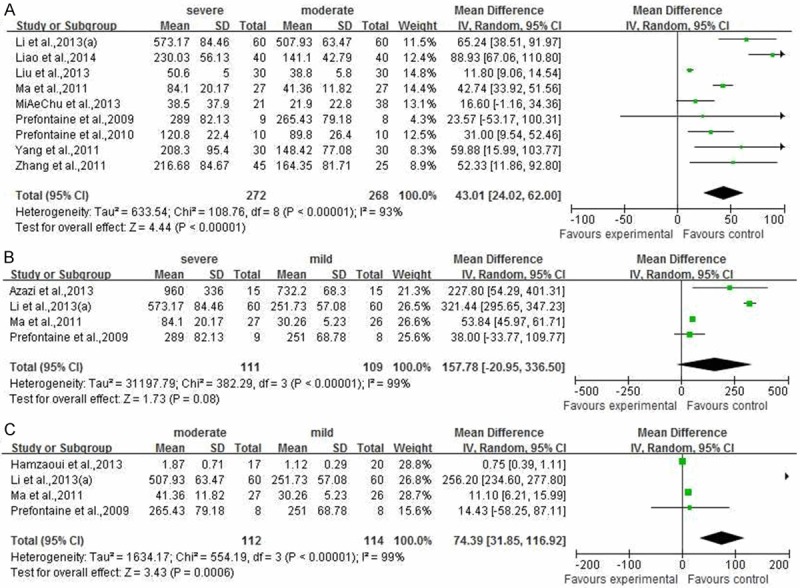
The meta-analysis of comparison among and between severe, moderate and mild asthmatic.
Discussion
In this meta-analysis, we reported that there was a stronger relation between IL33 and/or ST2 level of serum and/or sputum in asthma progress. Moreover, the IL33 and/or ST2 level in serum and/or sputum of asthma patients were higher than that in healthy people.
Interleukin-33 (IL-33) is a recently finding cytokine with lots of functions. IL-33 has been reported as a potent inducer of Th2 immune responses as well as “alarmin” cytokine released from necrotic cells [26-28]. IL33 binds to its receptor ST2 play role, including regulation of g inflammation and immunity [29]. IL33/ST2 axis interaction is thought to be involved in allergic inflammation promotion and maintenance by Th2 cells, basophils, mast cells and airway epithelium etc [30]. Therefore, IL33/ST2 axis aggravates air way inflammation by activates air way eosinophils [31]. The previous researches [17,32] revealed the IL33 and/or ST2 have previously been demonstrated to be elevated in young and adult asthmatic patients. However, as there were a series of factors influencing the IL33 and ST2 expression level in asthma patients, including year, sex, races, disease progress, which revealed difference research results, therefore, it is need to evaluate the relation between IL33 and ST2 and asthma patients by a systematic reviews.
As we known, IL33 is increased in airway epithelial cells and smooth muscle from asthmatics, and increasing IL33 maybe correlates with asthma disease severit [33]. And likewise, soluble ST2 (sST2) is elevated in the serum and sputum of asthma patients [17,33]. A study have reported that serum ST2 expression level is increased in patients with acute exacerbation of atopic asthma.15 Accumulated data suggesting that IL-33 is involved in lung inflammation, and support the concept that ST2 maybe a therapeutic target in patients with asthma. Endobronchial biopsies from adults with severe asthma mild and moderate were obtained. IL-33 was predominantly expressed by airway smooth muscle cells (ASMC) and endothelial and epithelial cells in patients with asthmatic lungs but was absent in control samples.
In this meta-analysis, the results shown there were significant differences between serum IL33 and ST2 level in asthma patients and healthy people (Figure 1). However, there is only a document involving in ST2 in sputum sample, so, the meta-analysis for sputum IL33 or ST2 level in patients with asthma was not shown in here. Two papers reported IL33 was also higher in the sputum sample from asthma patients, however, one of them only described the IL33 level in the sputum samples of severe and moderate asthma patients. Therefore, the meta-analysis results for IL33 and ST2 in sputum were not included into this paper. Even so, Liu et al. [12] and Hamzaoui et al. [17] reported IL33 and ST2 in sputum of patients with different asthma progress were higher than that in healthy.
As described in Figures 2 and 3, we known that different asthma progress included severe, moderate and mild asthma have higher IL33 and ST2 expression level than healthy people. For sputum sample, Hamzaoui et al. [17] reported IL33 and ST2 in sputum of patients with moderate and mild asthma progresses were higher than that in healthy. Similarly, with progress of asthma disease, the serum IL33 and ST2 expression level were increased significantly (Figure 5). However, there is also a paucity of evidence in the published literature to help interpret relation between the sputum IL33 and/or ST2 and patients with severe, moderate and mild. The evidence base was also limited by non-standardized and non-systematic reporting of outcomes.
In conclusion, although limited literatures have reported the relation between sputum IL33 and ST2 protein expression levels and asthma patients, our meta-analysis results shown serum IL33 and ST2 level in asthma patients are higher than that in healthy people. Moreover, with development of asthma disease, the serum IL33 and ST2 level is increased significantly. These findings may be useful for targeted therapy and prognosis for asthma patients.
Disclosure of conflict of interest
None.
References
- 1.Masoli M, Fabian D, Holt S. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, Chan-Yeung M, Becker AB, Dimich-Ward H, Ferguson AC, Manfreda J, Watson WT, Paré PD, Sandford AJ. Polymorphisms of the IL-4, TNF-alpha, and Fcepsilon RIbeta genes and the risk of allergic disorders in at-risk infants. Am J Respir Crit Care Med. 2000;161:1655–9. doi: 10.1164/ajrccm.161.5.9906086. [DOI] [PubMed] [Google Scholar]
- 3.Gold DR. Environmental tobacco smoke, indoor allergens, and childhood asthma. Environ Health Perspect. 2000;108:643–51. doi: 10.1289/ehp.00108s4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borish L, Steinke JW. Interleukin-33 in asthma: how big of a role does it play? Curr Allergy Asthma Rep. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces Thelper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Löhning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–5. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, Decker DC, de Araujo CA, Bryce PJ, Sperling AI. IL-33-dependent induction of allergic lung inflammation by FcγRIII signaling. J Clin Invest. 2013;123:2287–97. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–81. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 11.Azazi EA, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Serum levels of Interleukin-33 and its soluble receptor ST2 in asthmatic patients. Egyptian Journal of Chest Diseases and Tuberculosis. 2013 [Google Scholar]
- 12.Liu SP, Ma R, Li XA. The relationship between interleukin 33 promotes and lung inflammation in asthmatic children. Chin J Diffic and Compl Cas. 2013;12:422–4. [Google Scholar]
- 13.Zhang XL. Dalian Medical University. 2011. Study on the correlation between the levels of IL-18, IL-33 and other inflammatory factors and pulmonary function in different stages of asthma. Master Thesis. [Google Scholar]
- 14.Yang DX. Dalian Medical University. 2011. Effect of Glucocorticoid Treatment on the Levels of Serum IL-18 and IL-33 in Asthmatic Patients. Master Thesis. [Google Scholar]
- 15.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 16.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 17.Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013;50:803–9. doi: 10.3109/02770903.2013.816317. [DOI] [PubMed] [Google Scholar]
- 18.Liao XM. Changes of plasma interleukin 33 in asthmatic patients and its clinical significance. Journal of Clinical Pulmonary Medicine. 2014;19:33–5. [Google Scholar]
- 19.Li YM, Cheng XM. Clinical significance of IL-18 and IL-33 levels changes in the treatment of bronchial asthma with acute attack stage by glucocorticoids. Practical Pharmacy and Clinical Remedies. 2013;16:897–9. [Google Scholar]
- 20.Ma XY, Luo YL, Lai WY. IL-33 levels in peripheral blood plasma and sputum of patients with bronchial asthma and their correlation with symptoms of patients, pulmonary function, and eosinophil numbers. J Third Mil Med Univ. 2011;33:1526–9. [Google Scholar]
- 21.Gong ZJ, Li WY, Chen GH. A study on ST2 as a marker for Th2 cells and its relation to bronchial asthma. Shanghai Journal of Immunology. 2002;22:82–5. [Google Scholar]
- 22.Zhang HQ, Wu LF, Sun ZL. Serum levels of IL33 and IL5 in patients with allergic rhinitis and bronchial asthma. Journal of Zhejiang Medicine. 2013;35:1755–6. [Google Scholar]
- 23.Li QY, Huang XX, Zhang HY. Significance of changes of serum interleuin in patient with acute attack of bronchial asthma. China Medicine. 2013;8:1556–7. [Google Scholar]
- 24.Chu MA, Lee JH, Lee EJ. Increased serum soluble ST2 in asthmatic children and recurrent early wheezers. Allergy Asthma Respir Dis. 2013;1:314–20. [Google Scholar]
- 25.Glück J, Rymarczyk B, Rogala B. Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Inflamm Res. 2012;61:547–50. doi: 10.1007/s00011-012-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arshad MI, Piquet-Pellorce C, Samson M. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32:1200–10. doi: 10.1111/j.1478-3231.2012.02802.x. [DOI] [PubMed] [Google Scholar]
- 27.Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagälv A, Schlenner SM, Feyerabend T, Rodewald HR, Kjellén L, Hellman L, Åbrink M. Mast Cell Chymase Degrades the Alarmins Heat Shock Protein 70, Biglycan, HMGB1, and Interleukin-33 (IL-33) and Limits Dangerinduced Inflammation. J Biol Chem. 2014;289:237–50. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5:18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lécart S, Lecointe N, Subramaniam A, Alkan S, Ni D, Chen R, Boulay V, Pène J, Kuroiwa K, Tominaga S, Yssel H. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol. 2002;32:2979–87. doi: 10.1002/1521-4141(2002010)32:10<2979::AID-IMMU2979>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, Wenzel SE, Chon Y, Dunn M, Weng HH, Lin SL. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–96. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 31.Savenije OE, Kerkhof M, Reijmerink NE, Brunekreef B, de Jongste JC, Smit HA, Wijga AH, Postma DS, Koppelman GH. Interleukin-1 receptor-like 1 polymorphisms are associated with serum IL1RL1-a, eosinophils, and asthma in childhood. J Allergy Clin Immunol. 2011;127:750–6. doi: 10.1016/j.jaci.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Ali M, Zhang G, Thomas WR, McLean CJ, Bizzintino JA, Laing IA, Martin AC, Goldblatt J, Le Souëf PN, Hayden CM. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009;73:206–12. doi: 10.1111/j.1399-0039.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 33.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]


