Abstract
Background: Arterial restenosis after percutaneous transluminal angioplasty (PTA) significantly reduces its therapeutic efficacy in treating lower extremity atherosclerotic occlusive diseases (LEAOD). Early external X-ray external radiation has demonstrated positive effects on restenosis; however, effective dosing and the mechanism(s) underlying its efficacy remain unknown. This study explored the effect of early external X-ray radiation on preventing post-PTA restenosis in an iliac intimal injury model. Methods: Twenty rabbits underwent iliac intimal injury via PTA and received five different radiation doses: 0 Gy (n=4), 3 Gy (n=4), 6 Gy (n=4), 9 Gy (n=4), and 12 Gy (n=4). Four rabbits were used as controls. All subjects were fed a high-fat diet prior to PTA and for an additional four-week period post-PTA and then sacrificed for immunohistochemical and Western blotting analysis. Results: Arterial stenosis was significantly improved post-PTA. Alpha smooth muscle actin (α-SMA) expression in the 0 Gy to 9 Gy groups was significantly increased post-PTA. Cytochrome C (Cyt C) expression was significantly increased post-PTA and was positively correlated with radiation intensity. Proliferating cell nuclear antigen (PCNA) expression was significantly increased post-PTA with the 0 Gy group showing significantly higher expression than the 3 Gy group. No significant differences were found in CD34 levels between the groups. Conclusions: Early external X-ray radiation at 6-24 Gy doses effectively restrained VSMC hyperplasia post-PTA, likely through inducing VSMC apoptosis via mitochondrial Cyt C release. However, this technique did not significantly affect the integrity of the vascular endothelium. Therefore, early external X-ray radiation shows promise in preventing post-PTA restenosis.
Keywords: Radiotherapy, restenosis, percutaneous transluminal angioplasty, PTA, intimal injury, vascular smooth muscle, VSMC
Introduction
Lower extremity atherosclerotic occlusive diseases (LEAOD) commonly lead to stenosis, embolism, or thrombus formation, further producing acute or chronic ischemia of the lower extremities. Due to an aging population in combination with poor lifestyle and dietary habits as well as the rising incidence of type II diabetes, the incidence rate of LEAOD has also significantly increased [1,2]. For example, 15.91% of Chinese persons over the age of 60 possess LEAOD [3].
LEAOD can be interventionally treated by percutaneous transluminal angioplasty (PTA), which uses an expanding balloon catheter to gradually expand the location of the stenosis or occlusion for recanalization. Even though PTA has become a promising treatment for LEAOD, a high rate of post-PTA restenosis significantly reduces its therapeutic efficacy even in cases of stent placement. Therefore, preventing post-PTA restenosis is a pressing clinical issue.
To this end, early external X-ray external radiation has demonstrated positive effects on restenosis post-PTA; however, the effective intensities of X-ray radiation and the mechanism(s) underlying its efficacy remain unknown. Therefore, this study aimed to explore the effect of early external X-ray radiation on preventing post-PTA restenosis in an iliac intimal injury model.
Materials and methods
Ethics statement
This study was conducted in accordance with the recommendations of the Weatherall report entitled “Guide for the Care and Use of Laboratory Animals”. The Committee on Ethics of Animal Experimentation at Chongqing Medical University (Chongqing, China) approved the protocols of this study prior to its implementation (approval No. 20121253).
Animal subjects
A total of 24 adult New Zealand white rabbits (2.5±0.5 kg) provided by the Laboratory Animal Center of Chongqing Medical University were fed with a high-fat diet (100 g/day, 94.5% ordinary fodder, 5% lard, and 0.5% cholesterol). These 24 rabbits were randomly divided into one control group (n=4) and five PTA groups with each PTA group receiving a different intensity radiation on the first and second day post-balloon dilation injury: 0 Gy (n=4), 3 Gy (n=4), 6 Gy (n=4), 9 Gy (n=4), and 12 Gy (n=4). All subjects received a high-fat diet for four more weeks post-operation and were then sacrificed by euthanasia.
Iliac intimal injury model
Subject was administered general anesthesia, then the left femoral artery was exposed carefully, and a Fogarty “3F” type balloon catheter was catheterized into the left femoral artery. The catheter was inserted though the femoral artery for a distance of 8 cm to approach the iliac artery, then 0.1 ml saline was injected into the balloon to expand the iliac artery for at least one minute. Afterwards, the saline was aspirated out, and the catheter was pulled back by a distance of 0.5 cm. Then, 0.1 ml saline was injected to expand the balloon again for one minute before being aspirated out, and the catheter was then pulled back by an additional 0.5 cm. This balloon procedure was repeated once again until the balloon catheter was inserted by a distance of 7 cm. Then, the balloon catheter was fully retracted, and the surgical incision site was sutured and dressed. Finally, 400,000 U penicillin and 1000 U heparin were subcutaneously injected to reduce the risk of post-operative infection and thrombogenesis, respectively.
X-ray external radiation
On postoperative days one and two, all PTA group subjects were intravenously anesthetized and supinely fixed prior to radiation. A 4 cm×8 cm rectangular exposure field (long axis in the same direction with the lower extremity) received 3 Gy (n=4), 6 Gy (n=4), 9 Gy (n=4), or 12 Gy (n=4) external X-ray radiation (based on the PTA group) under a Varian Clinac 600C (Varian Inc., USA). No movement was observed during the radiation process.
Immunohistochemical analysis
After sacrifice on week four post-operation, paraffin sections of iliac artery tissues were immunohistochemically stained with primary rat monoclonal anti-α-SMA (1:100, ab7388, Abcam, USA, Wuhan Boster Co., China), primary rat monoclonal anti-CD34 (1:100, ab7388, Abcam, USA, Wuhan Boster Co., China), andprimary goat multi-clonal anti-Cyt C (1:100, ab7388, Abcam, USA). Horseradish peroxidase (HRP)-conjugated anti-goat IgG antibody (EarthOx Co., China) was used as the secondary antibody. Four high power fields (×400) were randomly selected from each slice, and the average optical density (AOD) data was calculated using Image Pro Plus and presented as means ± SDs.
Western blot analysis
Tissue samples from iliac arterial tissues were obtained as described above. Equal amounts of protein from each tissue sample were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The blotted membranes were incubated with primary antibodies to Cyt C (1:500, ab7388, Abcam, USA) or β-actin (1:1000, Sigma, USA) as an internal control. Corresponding horseradish peroxidase-conjugated antibodies were used as secondary antibodies. Subsequently, specific bands were visualized using an ECL detection kit (Beyotime Institute of Biotech, China). Each band was quantified using Quantity One software with the level of Cyt C expression normalized to that of β-actin and expressed as a fold-increase.
Statistical analysis
All data management and statistical analysis were performed using Stata 12.0 (StataCorp LP, USA). One-way analysis of variance (ANOVA) was used to distinguish among all groups, while Bonferroni’s post hoc test for multiple comparisons was performed to test differences between two groups. P-values of less than 0.05 were deemed significant for all analyses.
Results
X-ray radiation alleviated post-injury luminal stenosis
The H&E-stained specimens of the rabbit iliac artery from the control group presented clear demarcations between the iliac artery intima, media, and adventitia (Figure 1A and 1B). The intima was smooth with flat endothelial cells. The tunica media was composed of vascular smooth muscle cells (VSMCs) and circular elastic fibers in a wave-like distribution with internal and external elastic lamina at its external and internal boundaries. The tunica adventitia was composed of thin connective tissue and blood vessels. The whole vascular wall displayed a uniform thickness.
Figure 1.
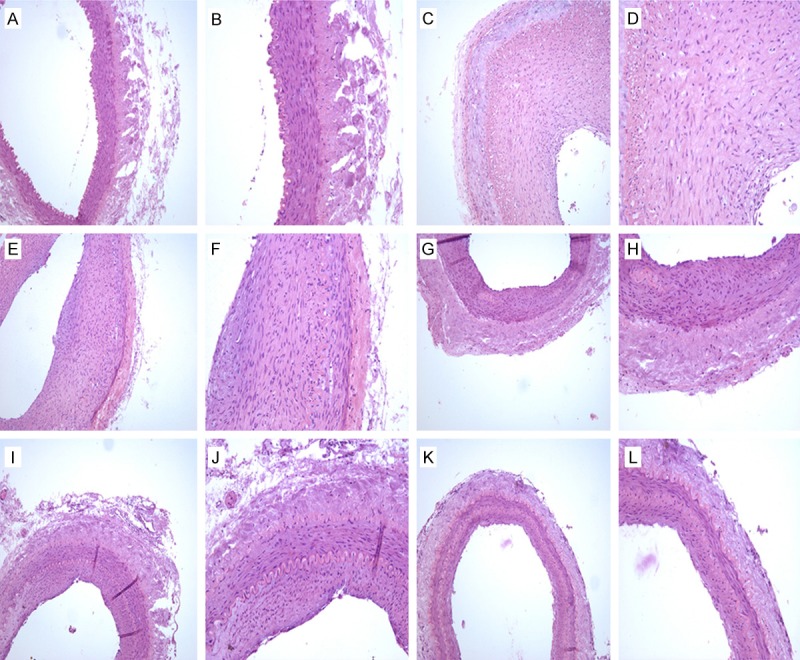
H&E staining of iliac arteries. A. Control group (HE, 100×); B. Control group (HE, 200×); C. 0 Gy PTA group (HE, 100×); D. 0 Gy PTA group (HE, 200×); E. 3 Gy PTA group (HE, 100×); F. 3 Gy PTA group (HE, 200×); G. 6 Gy PTA group (HE, 100×); H. 6 Gy PTA group (HE, 200×); I. 9 Gy PTA group (HE, 100×); J. 9 Gy PTA group (HE, 200×); K. 12 Gy PTA group (HE, 100×); L. 12 Gy PTA group (HE, 200×).
The specimens from the PTA group that did not receive any radiation post-balloon intimal injury (0 Gy) displayed an obviously thickened intima, which lead to luminal stenosis. The uniformity in the vessel wall’s thickness disappeared, and there were large amounts of hyperplastic VSMCs in the media with a highly irregular arrangement (Figure 1C and 1D). The specimens from the PTA groups that received various levels of radiation post-balloon intimal injury also displayed a thickened intima, a stenosed lumen, and some hyperplastic VSMCs in the intima; however, all irradiated PTA groups showed significant alleviation of injury as compared with the non-irradiated PTA group (Figure 1E-L).
X-ray radiation inhibited post-injury VSMC hyperplasia
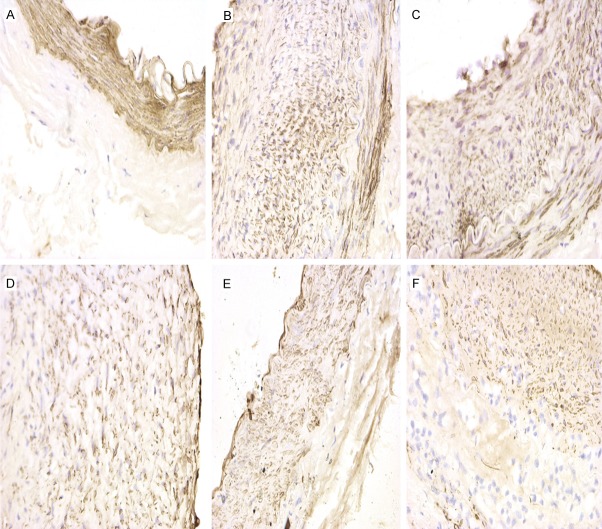
α-SMA is a SMC-specific antigen and can be used to reflect the effect of X-ray radiation on VSMCs. αSMA+ cells, located in the intima and media, displayed a tan cytoplasm. α-SMA expression was significantly increased in all PTA groups as compared to the control group (F=10.01, P<0.05, Table 1). Interestingly, α-SMA expression in the 0 Gy to 9 Gy PTA groups was significantly increased as compared with the control group (P<0.05); however, the 12 Gy PTA group displayed no significant increase in α-SMA expression as compared with the control group (Bonferrroni test, P=1.000), indicating that X-ray radiation can inhibit VSMC hyperplasia postintimal injury (Table 1; Figures 2, 3).
Table 1.
Average optical density (AOD) of protein expression
| Protein | Group | n | Mean | SD | F | P |
|---|---|---|---|---|---|---|
| α-SMA | 0 Gy | 16 | 164.61 | 12.50 | 10.01 | 0.000 |
| 3 Gy | 16 | 150.45 | 13.98 | |||
| 6 Gy | 16 | 150.79 | 15.58 | |||
| 9 Gy | 16 | 150.86 | 15.55 | |||
| 12 Gy | 16 | 134.03 | 19.18 | |||
| Control | 16 | 129.76 | 18.89 | |||
| CD34 | 0 Gy | 16 | 135.68 | 17.27 | 0.83 | 0.5301 |
| 3 Gy | 16 | 136.11 | 11.08 | |||
| 6 Gy | 16 | 131.31 | 16.39 | |||
| 9 Gy | 16 | 136.18 | 17.13 | |||
| 12 Gy | 16 | 132.93 | 10.11 | |||
| Control | 16 | 127.23 | 19.04 | |||
| Cyt C | 0 Gy | 16 | 127.23 | 19.04 | 27.61 | 0.000 |
| 3 Gy | 16 | 135.22 | 13.37 | |||
| 6 Gy | 16 | 157.14 | 23.55 | |||
| 9 Gy | 16 | 165.45 | 18.51 | |||
| 12 Gy | 16 | 172.13 | 15.51 | |||
| Control | 16 | 113.98 | 13.47 | |||
| PCNA | 0 Gy | 16 | 121.72 | 21.29 | 11.89 | 0.000 |
| 3 Gy | 16 | 116.4 | 17.76 | |||
| 6 Gy | 16 | 99.264 | 12.69 | |||
| 9 Gy | 16 | 98.336 | 15.41 | |||
| 12 Gy | 16 | 96.514 | 15.63 | |||
| Control | 16 | 83.96 | 12.43 |
Figure 2.
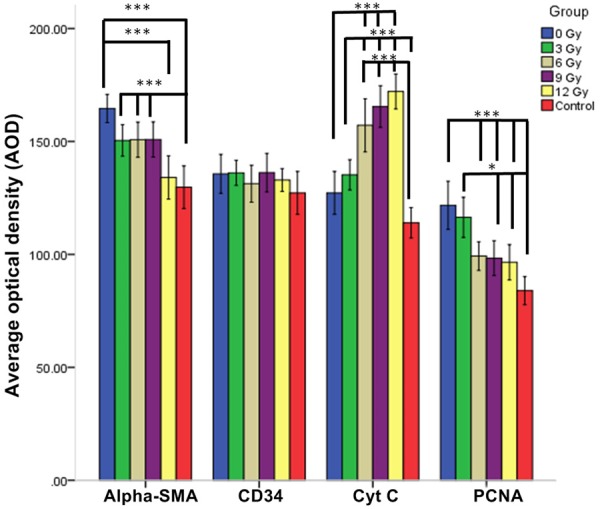
α-SMA, CD34, Cyt C, and PCNA expression levels by immunohistochemistry. AOD: Average Optical Density. ***P<0.001; *P<0.05.
Figure 3.
α-SMA expression by immunohistochemistry. A. Control group (×400); B. 0 Gy PTA group (×400); C. 3 Gy PTA group (×400); D. 6 Gy PTA group (×400); E. 9 Gy PTA group (×400); F. 12 Gy PTA group (×400).
X-ray radiation did not impact endothelial cells post-injury
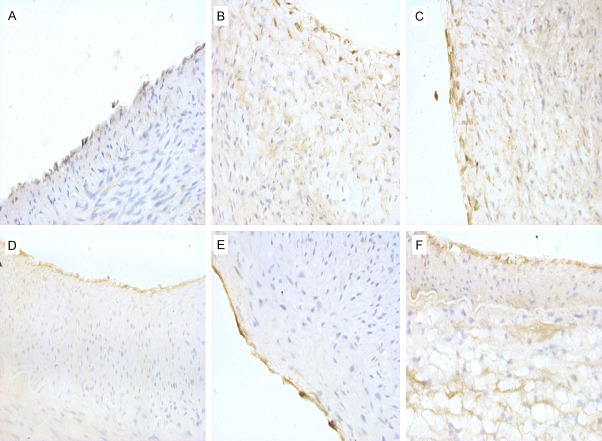
CD34 molecule, a highly glycosylated type I transmembrane glycoprotein, is mainly expressed in the endothelial cells of the vascular intima. CD34+ cells displayed a tan cytoplasm by immunohistochemistry staining. No significant differences were found in CD34 levels between the groups (F=0.83, P=0.5301; Table 1; Figure 4), and the integrity of the endothelium was well-preserved in all groups (Figure 4), signifying that 3-12 Gy X-radiation does not significantly affect the number of endothelial cells or the integrity of the endothelium.
Figure 4.
CD34 expression by immunohistochemistry. A. Control group (×400); B. 0 Gy PTA group (×400); C. 3 Gy PTA group (×400); D. 6 Gy PTA group (×400); E. 9 Gy PTA group (×400); F. 12 Gy PTA group (×400).
X-ray radiation increases mitochondrial Cyt C release post-injury
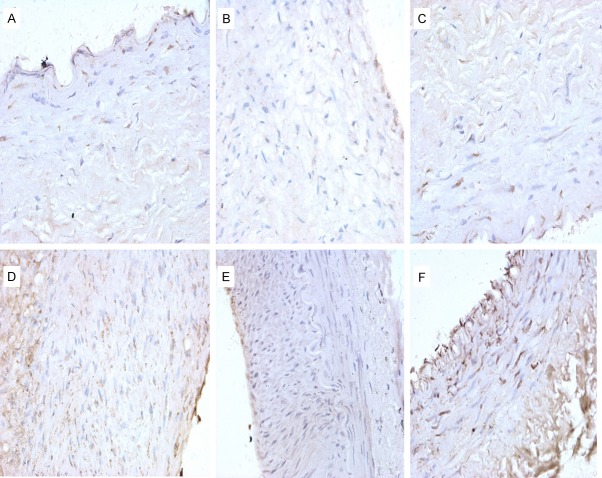
Cytochrome C (Cyt C) is expressed in the mitochondria. Cyt C+ cells, which displayed a tan cytoplasm by immunohistochemistry staining, were more predominantly scattered in the intima and media but were also observed in the adventitia. The expression of Cyt C in the PTA groups was significantly increased as compared to the control group (F=27.61, P<0.001) (Table 1; Figures 2, 5).
Figure 5.
Cyt C expression by immunohistochemistry. A. Control group (×400); B. 0 Gy PTA group (×400); C. 3 Gy PTA group (×400); D. 6 Gy PTA group (×400); E. 9 Gy PTA group (×400); F. 12 Gy PTA group (×400).
The Western blot of Cyt C expression validated the immunohistochemistry results, as Cyt-C expression in the PTA groups were significantly increased as compared to the control group (F=22.95, P<0.001). Moreover, the fold-change in Cyt C protein expression was positively correlated with the intensity of radiation, indicating that X-ray radiation increases the mitochondrial release of Cyt C post-intimal injury (Table 2; Figure 6).
Table 2.
Cyt C expression by western blotting
| Group | n | Relative Cyt C expression | |
|---|---|---|---|
|
| |||
| Mean | SD | ||
| 0 Gy | 4 | 0.311 | 0.060 |
| 3 Gy | 4 | 0.500 | 0.118 |
| 6 Gy | 4 | 0.796 | 0.093 |
| 9 Gy | 4 | 0.803 | 0.197 |
| 12 Gy | 4 | 0.908 | 0.130 |
| Control | 4 | 0.247 | 0.021 |
One-way ANOVA: F=22.95, P<0.001. Bonferroni’s post hoc test for multiple comparisons: control vs 6 Gy, control vs 9 Gy, and control vs 12 Gy, P<0.0001; 0 Gy vs 6 Gy, 0 Gy vs 9 Gy, and 0 Gy vs 12 Gy, P<0.0001; 3 Gy vs 6 Gy, 3 Gy vs 9 Gy, and 3 Gy vs 12 Gy, P<0.05.
Figure 6.
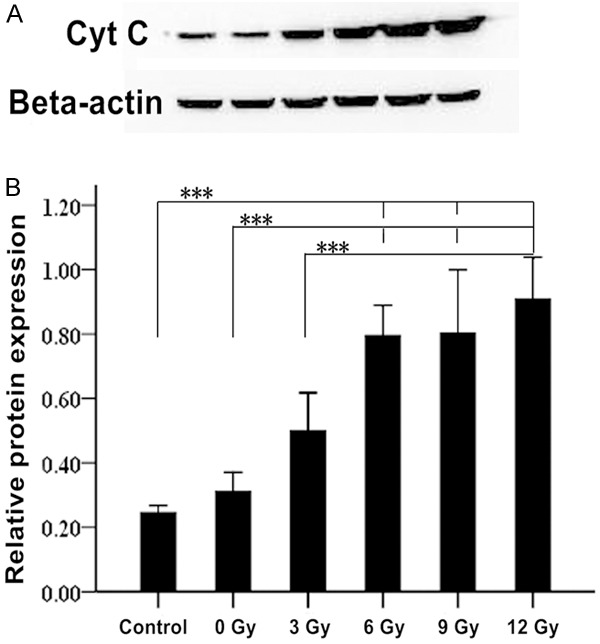
Cyt C expression by western blotting.
With regard to the expression of proliferating cell nuclear antigen (PCNA), the PTA groups displayed significantly higher PCNA expression compared to the control group (F=11.89, P<0.001). Moreover, the 0 Gy PTA group showed a significantly higher expression than the 3 Gy PTA group; however, no significant differences were observed among the remaining three PTA groups (6 Gy, 9 Gy, and 12 Gy).
Discussion
Percutaneous transluminal angioplasty (PTA) has become the main option in the treatment of LEAOD due to its minimal invasiveness, reliable repeatability, and rapid recovery times. Fortunately, this method has significantly reduced the amputation rate [4]. However, the incidence of post-PTA restenosis approaches 30% [5], which has a negative impact on its therapeutic efficacy. Therefore, there has been an increased focus on measures to prevent post-PTA restenosis.
Post-PTA restenosis occurs as a result of intimal hyperplasia and vascular remodeling following the initial PTA-induced injury. Expansion of the PTA balloon damages the intima and causes endothelial cells injury and sloughing, which exposes the basilar membrane. As a result, platelets aggregate and a series of growth factors and cytokines are released, including platelet-derived growth factor (PDGF), insulin-like growth factors-1 (IGF-1), basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). These factors stimulate the proliferation of VSMCs, which are generally non-proliferative. These proliferative VSMCs then secrete a variety of vasoactive substances, growth factors, and extracellular matrix proteins to enhance their proliferation and migration. These VSMCs can also activate the cells around them to proliferate by paracrine action, which also causes cell migration from the media to the intima. Finally, these migratory proliferative cells lead to a thickened vascular wall and stenosis in a process termed remodeling, which eventually lowers the compliance of the vessel [6,7].
In recent years, radiation therapy has been applied to prevent post-PTA restenosis on account of its promising ability to reduce cell proliferation by inducing cell necrosis and apoptosis [8]. During the process of restenosis, proliferative VSMCs have been shown to be highly sensitive to radiation, allowing selective inhibition of intimal hyperplasia [9]. Internal radiation therapy for restenosis has been applied to endovascular procedures through implanting radioactive stents or using radioactive liquids to fill the balloon. Although these techniques have achieved definite effects on preventing restenosis, they have also led to certain complications and side effects. For example, radioactive stents frequently produce edge effects [10,11], and radioactive liquids can leak upon balloon rupture. Due to these complications of internal radiation therapy, external radiation therapy has been introduced as an alternative. However, external radiation can cause radiation injury to the heart, lungs, gastrointestinal tract, and urogenital system. Nevertheless, if lower intensities of radiation are used to avoid undesired radiation injury to internal organ systems, this technique may provide a superior solution for preventing post-PTA restenosis in LEAOD patients.
External radiation has been shown to inhibit the migration and proliferation of VSMCs in the tunica media, which is one of the most important processes underlying restenosis [12]. Zhu et al. found external X-ray radiation (2-20 Gy) was able to inhibit rat VSMC hyperplasia in vitro with 10-20 Gy displaying a more obvious effect [13]. Moreover, Verin et al. gave injured carotid and iliac arteries external β-ray radiation (18 Gy) that significantly decreased the average VSMC density through inducing VSMC apoptosis [14]. In addition, Heckenkamp et al. found that γ-irradiation reversed the secretory phenotype of cultured VSMCs to a contractile type while significantly decreasing their vertical migration [15]. Consistent with these previous findings, we found that external X-ray radiation at 6-24 Gy doses directly reduced VSMC hyperplasia as evidenced by decreased α-SMA protein expression. Notably, the 24 Gy dose reduced intimal hyperplasia to such a degree that there was no significant difference observed with the control group.
In this study, both immunohistochemistry and Western blotting revealed that early external X-ray radiation at 18-24 Gy doses significantly increased mitochondrial Cyt C release, which is the primary mechanism underlying apoptosis induced by ionizing radiation [16]. In a dose-dependent manner, X-ray radiation produces mitochondrial matrix swelling and decreases in the potential of the outer mitochondrial membrane, thereby raising mitochondrial membrane permeability and releasing Cyt C and other signal molecules into the cytoplasm [17-19]. In the cytoplasm, Cyt C combines with apoptosis protease activating factor-1 (Apaf-1) and caspase-9 to form a complex known as apoptosome, which causes chromosomal condensation, DNA breakage, and apoptosis [20-22]. Using a vascular stenosis model, Friesen et al. found that external β-ray radiation induced VSMC apoptosis via this mitochondrial pathway [23]. Based on these findings, we can surmise that external X-ray radiation prevents restenosis through inducing VSMC apoptosis via mitochondrial Cyt C release.
Endothelial cells form the physico-chemical barrier between the blood and the vessel wall, thereby preventing platelet aggregation, thrombogenesis, and VSMC proliferation [24]. Bi et al. found that low-dose radiation delayed the endothelialization of the injured vessel, a process which produces restenosis [25]. Our immunohistochemical results revealed no significant difference in the expression of the endothelial cell marker CD34 between all groups receiving external X-ray radiation, indicating that early external X-ray radiation at 6-24 Gy doses does not affect endothelial integrity.
In conclusion, early external X-ray radiation at 6-24 Gy doses effectively restrained vascular VSMC hyperplasia post-PTA, possibly through inducing VSMC apoptosis via mitochondrial Cyt C release. However, this technique did not significantly affect the integrity of the vascular endothelium. Therefore, early external X-ray radiation shows promise in preventing post-PTA restenosis and requires further preclinical and clinical study.
Acknowledgements
This work was supported by the Medical Scientific Research Project of Chongqing (Grant No. 2010-2-037).
Disclosure of conflict of interest
None.
References
- 1.Pasternak RC, Criqui MH, Benjamin EJ, Fowkes FG, Isselbacher EM, McCullough PA, Wolf PA, Zheng ZJ American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605–2612. doi: 10.1161/01.CIR.0000128518.26834.93. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Li XY, He Y, Ni B. [A cross-sectional study of peripheral arterial occlusive disease in Wanshoulu area, Beijing] . Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:221–224. [PubMed] [Google Scholar]
- 4.Faglia E, Mantero M, Caminiti M, Caravaggi C, De Giglio R, Pritelli C, Clerici G, Fratino P, De Cata P, Dalla Paola L, Mariani G, Poli M, Settembrini PG, Sciangula L, Morabito A, Graziani L. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: clinical results of a multicentric study of 221 consecutive diabetic subjects. J Intern Med. 2002;252:225–232. doi: 10.1046/j.1365-2796.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 5.van der Loo B, Krieger E, Katavic J, Spring S, Rousson V, Amann-Vesti B, Koppensteiner R. Carotid intima-media thickness, carotid wall shear stress and restenosis after femoro-popliteal percutaneous transluminal angioplasty (PTA) Eur J Vasc Endovasc Surg. 2005;30:469–474. doi: 10.1016/j.ejvs.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Lijnen HR. Role of the fibrinolytic and matrix metalloproteinase systems in arterial neointima formation after vascular injury. Verh K Acad Geneeskd Belg. 2001;63:605–622. [PubMed] [Google Scholar]
- 7.Yang Z, Eton D, Zheng F, Livingstone AS, Yu H. Effect of tissue plasminogen activator on vascular smooth muscle cells. J Vasc Surg. 2005;42:532–538. doi: 10.1016/j.jvs.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Crocker I, Robinson KA. Rationale for coronary artery radiation therapy. Semin Radiat Oncol. 2002;12:3–16. doi: 10.1053/s1053-4296(02)80021-2. [DOI] [PubMed] [Google Scholar]
- 9.Gierga DP, Shefer RE. Characterization of a soft X-ray source for intravascular radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:847–856. doi: 10.1016/s0360-3016(00)01510-8. [DOI] [PubMed] [Google Scholar]
- 10.Voisard R, Hob J, Baur R, Herter T, Hannekum A, Hombach V. Edge restenosis: impact of low dose irradiation on cell proliferation and ICAM-1 expression. BMC Cardiovasc Disord. 2006;6:32. doi: 10.1186/1471-2261-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiemer M, Konig A, Rieber J, Sohn HY, Leibig M, Theisen K, Klauss V, Langer C, Lindner O, Horstkotte D, Schiele TM. Sirolimus-eluting stent implantation versus beta-irradiation for the treatment of in-stent restenotic lesions: clinical and ultrasound results from a randomised trial. EuroIntervention. 2011;6:687–694. doi: 10.4244/EIJV6I6A117. [DOI] [PubMed] [Google Scholar]
- 12.Kotzerke J, Hanke H, Hoher M. Endovascular brachytherapy for the prevention of restenosis after angioplasty. Eur J Nucl Med. 2000;27:223–236. doi: 10.1007/s002590050032. [DOI] [PubMed] [Google Scholar]
- 13.Zhu LH SW. Effects of X-ray radiation on cultured vascular smooth muscle cells. Chi J Radiat Oncol. 2001;10:126–130. [Google Scholar]
- 14.Verin V, Popowski Y, Bochaton-Piallat ML, Belenger J, Urban P, Neuville P, Redard M, Costa M, Celetta G, Gabbiani G. Intraarterial beta irradiation induces smooth muscle cell apoptosis and reduces medial cellularity in a hypercholesterolemic rabbit restenosis model. Int J Radiat Oncol Biol Phys. 2000;46:661–670. doi: 10.1016/s0360-3016(99)00426-5. [DOI] [PubMed] [Google Scholar]
- 15.Heckenkamp J, Nigri GR, Waterman PR, Overhaus M, Kossodo SC, Lamuraglia GM. Gamma-irradiation modulates vascular smooth muscle cell and extracellular matrix function: Implications for neointimal development. J Vasc Surg. 2004;39:1097–1103. doi: 10.1016/j.jvs.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y, Nishioka A, Kobayashi T, Kariya S, Hamasato S, Saibara T, Nakayama K, Seguchi H, Yoshida S. Mitochondrial cytochrome c release in radiation-induced apoptosis of human peripheral T cells. Int J Mol Med. 2002;10:263–268. [PubMed] [Google Scholar]
- 17.Zhou XF YY. Study progress of mitochondria or cytochreme C-mediated apotosis pathway. Med Recapitulate. 2007;13:16–19. [Google Scholar]
- 18.Chen J YZ, Xiao MX. The inhibition effect of P-glycoprotein on cytochrome c release and caspase-3 activation by X-ray irradiation 2006. Chin J Radiol Med Protect. 2006;6:118–120. [Google Scholar]
- 19.Wang ZC ZH, Gong SL. Changes of Cyt c and caspase-3 expressions in mouse testis induced by low dose irradiation. Chin J Radiol Med Protect. 2006;26:151–154. [Google Scholar]
- 20.Chereau D, Zou H, Spada AP, Wu JC. A nucleotide binding site in caspase-9 regulates apoptosome activation. Biochemistry. 2005;44:4971–4976. doi: 10.1021/bi047360+. [DOI] [PubMed] [Google Scholar]
- 21.Gao W, Pu Y, Luo KQ, Chang DC. Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J Cell Sci. 2001;114:2855–2862. doi: 10.1242/jcs.114.15.2855. [DOI] [PubMed] [Google Scholar]
- 22.Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Dev Biol. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- 23.Friesen C, Lubatschofski A, Glatting G, Debatin KM, Reske SN. Activation of intrinsic apoptotic pathway by Re-188 irradiation and paclitaxel in coronary artery smooth muscle cells. Q J Nucl Med Mol Imaging. 2008;52:289–295. [PubMed] [Google Scholar]
- 24.Ferrario CM. Use of angiotensin II receptor blockers in animal models of atherosclerosis. Am J Hypertens. 2002;15:9S–13S. doi: 10.1016/s0895-7061(01)02274-9. [DOI] [PubMed] [Google Scholar]
- 25.Bi YY TY, Gao GY. Experimental study of radioactive nitinol intravascular stents. Chin J Pract Intern Med. 2005;25:245–246. [Google Scholar]