Abstract
Objective: Operation on the infrarenal aorta could cause ischemic-reperfusion (IR) injury in local tissues and remote organs (e.g. the lung). We aim to explore the method of reducing lung ischemia-reperfusion damage after lower limb IR with post conditioning (LIPC). Methods: Bilateral lower limb ischemia was performed in Sprague-Dawley (SD) rats, and then animals were divided into 4 groups: IR-Sham-operated, IR, post conditioned-IR (LIPC) and bilateral lower limb ischemia (LIR). The serum free radical, histological changes, Wet/Dry (W/D) ratio, levels of TNF-α, IL-6, cytokines and chemokines were tested and compared. Results: Post-conditioning could ameliorate histological injuries in the lung when compared to IR group. The serum free radical is significantly lower in LIPC group than IR groups. W/D ratio in LIPC groups is significantly lower. LIPC also could reduce the expression of cytokines and chemokines. Conclusion: post conditioning could reduce long-term damages of the lung after lower limb ischemic-reperfusion injury.
Keywords: Ischaemia-reperfusion injury, post-conditioning, TNF-α, lung
Introduction
Pulmonary ischemia-reperfusion (IR) injury occurs after various trauma and clinical procedures such as lung transplantation, cardiopulmonary bypass, pulmonary thrombo-endarterectomy [1,2]. IR injury of the lung causes significant morbidity and mortality with the characterized of neutrophil extravasation, interstitial edema, disruption of epithelial integrity and leakage of protein into the alveolar space, which are associated with severe alterations in gas exchange [3]. In the past decades, compelling studies have demonstrated that ischemic and pharmacological pre-conditioning have beneficial effects in reducing the extent of lung injury [4-7]. However, the clinical applicability of pre-conditioning has been limited, that is, it can be performed only when the occurrence of ischemic event is predictable.
Recent studies demonstrated that brief intermittent cycles of ischemia alternated with reperfusion applying after the prolonged ischemic event attenuated myocardial injury [8-10]. The novel approach for myocardial protection has been termed as ‘ischemic post-conditioning’ (IPC). Subsequently, beneficial effects of IPC were shown in a wide range of organs, including the heart, brain, spinal cord, liver, kidney and skeletal muscle [11-14]. As a potential alternative method, post-conditioning has been demonstrated to attenuate various types of organ injuries experimentally and clinically, however, it is remains unknown whether post-conditioning could confer protective effects against IR injury in the lung.
In this study, we used a simulated limb ischemia-reperfusion injury rat model to determine whether ischemic post-conditioning could prevent or reduce lung ischemia-reperfusion injury caused by clipping pulmonary artery. Markers that reflect changes in the barrier function and mechanism of damage in lung tissues were examined, and the degree of damage to the lung tissues was classified using Chiu’s pathology grading system.
Materials and methods
Animals
The experimental procedures and protocols used in this investigation were approved by the Animal Use Committee of Beijing Anzhen hospital. Specific pathogen-free Sprague-Dawley (SD) rats (male or female) were purchased from Weitong Lihua animal Ltd (Beijing, China), the rats (weighing between 190 and 230 g) were housed under constant temperature (23±1°C) with 12-h light/dark cycles.
Surgical and experimental protocol
After animals were anaesthetized with 7% chloral hydrate (5 ml kg-1, i.p.), a 14-gauge angiocatheter was inserted into the trachea through a midline neck incision. The animals were then connected to a volume-controlled ventilator (DW-2000, JiapengKeji Ltd, Shanghai, China) with room air at the breath rate of 40 min-1, tidal volume of 12 ml kg-1 and positive end-expiratory pressure of 2 cm H2O. The left femoral vein was catheterized and a crystalloid to colloidal fluid mixture (3:1) was infused intravenously. The right femoral artery was catheterized for continuous monitoring of mean arterial pressure (MAP) and blood sampling. A heating pad was applied during anesthesia in order to keep the body temperature at the range of 36.5°C and 37.5°C.
The animals were randomly assigned to 4 groups (8 rats/group). Under aseptic conditions, an in situ unilateral lung warm ischemia model was used. In brief, a left antero lateral thoracotomy in the fifth inter costal space was made. The left lung was mobilized, the pulmonary hilum was dissected and perivascular and peri-bronchial tissues were removed. Then, all animals received 500 Ukg-1 of heparin intravenously in saline (total volume 500 μL). In group 1 (sham), animals received a sham thoracotomy, the lungs were not rendered ischemic, meanwhile, rats were subjected to the limb IR interventions described following. In group 2 (IR group), 5 min after heparin administration, the left pulmonary artery, bronchus and pulmonary vein were occluded with an on-crushing microvascular clamp to maintain the lung in a partially in flatted state. Lungs were kept moist with periodic applications of warm, sterile saline. The incision was covered to minimize evaporative losses. The period of ischemia was kept for 40 min, after which the clamp was removed and the lung re-perfused for up to 105 min. For the limb IR, animals underwent lower limb ischemia for 180 min followed by reperfusion for 240 min. In brief, median laparotomy was performed and the retroperitoneal space was opened. The rats underwent 180 min of bilateral lower limb ischemia using an infrarenal cross clamping of the abdominal aorta with an atraumatic microvascular clip. There after, the wound of laparotomy was closed and 4 h of reperfusion was performed with the maintenance of general anesthesia. Group 3 (LIPC), Animals in the Post-conditioning (Post C) group were subjected to ischemia-reperfusion and post-conditioning, which include 6 cycles of 10-seconds aortic de-clamping (reperfusion) and 10 seconds -re-occlusion at the onset to freperfusion. Group 3 received only bilateral lower limb ischemia (LIR).
The microcirculation of the lower limb (femoral biceps muscle) was monitored using a laser Doppler flowmetry (LDF) throughout the whole experiment.
Assessment of the lower limb microcirculation
The lower limb microcirculation was measured by a laser Doppler monitor (Moor Instruments DRT4; Millwey, Axminster, UK), 2 mW laser power at, λ = 632.8 nm and a DP1T surface probe. Flux, as defined for the LDF, is in direct proportion to the total number of the moving red blood cells in the measured volume (1 mm3) and the mean speed of the red blood cells. In each animal, the 5-mm wide LDF surface probe was placed directly at an identical spot on the surface of the femoral biceps muscle through a 10 mm-long incision on the skin and the fascia lata. LDF measurements (flux) were taken at relevant time points at 6-s intervals. On-line computer monitoring and processing were applied. The measurements taken from a tiny segment of muscle was extrapolated to describe the relative characteristics of the microcirculation of the whole lower limb.
Mathematical transformations were utilized as previously described [15]. For numeric characterization of the curves, two parameters were calculated: reperfusion area (RA) as the integral of the reperfusion segment of the graphs (proportional to the average blood-flow during reperfusion), and the plateau maximum (PM) as the arithmetical mean of the last plateau-shaped 10 min of the reperfusion-slope (representing a relatively stable set blood-flow at the end of the reperfusion time). To compare the curves of the individual animals accurately, each measured flux and parameter was calculated as a percentage on an arbitrary relative scale, the baseline flux before the aortic clamping was considered 100% and the ischemic flow was referred to as 0% (‘biological zero’: bz). (i.e. View the MathML source).
Preparation of specimens
After the experiments completed (60 min after reperfusion), blood was taken from carotid artery. A median sternotomy was performed; the left main bronchus and right lower lobe bronchus were clamped. The trachea was cannulated, and the right upper and middle lobes were lavaged 3 times with 2 mL of saline containing 0.07 mmol L-1 EDTA. The collected blood and bronchoalveolar lavage (BAL) fluid were centrifuged at 3000 rpm for 15 min, and the supernatant was stored at -80 degree for subsequent measurement of protein content. The right lower lung was divided into 2 parts for histology examination and pulmonary edema assessment, respectively. The left upper and lower lungs were used for biochemical detection.
Arterial blood gas analysis
Arterial blood sample for blood gas analysis was taken at 20 min of mechanical ventilation (baseline) and 105 min after reperfusion (postoperative). PaO2 and PaCO2 were analyzed using blood gas analyzer.
Lung wet-to-dry weight ratio
At the end of the experiments, the left lower lobe of the lung was dissected and dried at a constant temperature of 80°C for 24 h to obtain a dehydrated consistency. The ratio of wet weight to dry weight (W/D) was calculated to assess tissue edema, as described previously [2,16].
Lung histopathological analysis
At the end of the experiments, the left upper lobe of lung was fixed in 10% buffered formalin; 4-μm sections were prepared from paraffin-embedded tissues. The level of histological tissue injury was assessed by haematoxylin-eosin (H&E) staining using light microscopy. For each animal, three random tissue sections (eight fields per section) were examined. The severity of lung injury was graded by an investigator who was initially blinded to research groups, four-point scale was used according to combined assessments of amount of alveolar congestion, hemorrhage, infiltration or aggregation of neutrophils in the airspace or vessel wall, and thickness of alveolar wall/hyaline membrane formation [17]. The following criteria were considered: 0 = no damage, l = mild damage, 2 = moderate damage and 3 = severe damage.
Determination of plasma tumor necrosis factor alpha (TNF-α) and interlukin-6 (IL-6)
Serum levels of TNF-α and IL-6 were determined using commercially available ELISA kits (R&D, Minneapolis, MN) according to manufacturer’s procedure [18]. The results were expressed as pg/mL.
Western blot
The pulverized left lung tissue and cells were homogenized and sonicated in a tissue lysis buffer containing Tris-HCl (20 mM, pH 7.4), NaCl (150 mM), ethylenediaminetetraacetate (l mM), ethylene glycol tetraacetic acid (l mM), β-glycerolphosphate (1 mM), sodium pyrophosphate (2.5 mM), Triton X-100 (1%), phenylmethylsulfonyl fluoride (1 mM), dithiothreitol (1 mM), leupeptin (1 mg/mL), aprotinin (1 mg/mL), and pepstatin (1 mg/mL). The homogenate was centrifuged at 1000 g for 10 min at 4°C to collect the supernatant as a total protein preparation. Equal amounts of protein were combined with 5 × sodium dodecyl sulfate loading buffer and boiled for 5 min, then separated using 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis, and subsequently transferred to polyvinyl dinediflouride membrane for immunoblot analysis. The membranes were blocked in 5% nonfat milk for 2 h at room temperature and then incubated overnight at 4°C with primary antibodies against monocyte chemoattractant protein-1 (MCP-1), p38, Jun N-terminal kinase (JNK), phosphorylated JNK (p-JNK), nuclear factor kappa-B (NF-κB) and phosphor-NF-κB (p-NF-κB) (1:1000, Cell Signaling Technology, Beverly, MA). After being washed with Tris-buffered saline Tween 20, the membranes were incubated with proper secondary horseradish peroxidase-conjugated antibodies (1:5000-1:10,000, Cell Signaling Technology) and developed with enhanced chemiluminescence reagent (GE Healthcare, Rahway, NJ). The membranes were subsequently re-blotted for β-actin (1:2000; Cell Signaling Technology), and the results were normalized to β-actin to correct for loading.
Measurement of ROS
A highly sensitive (nmol/L) chemiluminescence assay to detect reactive oxygen species (ROS) was used to measure the total scavenger capacity in the plasma (Lumat LB 9051 luminometer, Lumat, Berthold, Windbad, Germany), which was determined by chemiluminescence in a H2O2 luminol micro-peroxidase system. Unstable free radicals, originating from H2O2 in the luminol micro-peroxidase system, were catalyzed through transformation of luminol into aminophthalic acid via a Fenton-type reaction. The chemiluminescence light intensity, was defined as relative light units (RLU), is in direct proportion to the concentration of the oxygen free radicals in the sample. Luminol, microperoxidase and hydrogen peroxide were obtained from SIGMA (St. Louis, MO), other chemical reagents were purchased from Reanal Chemical Co. (Budapest, Hungary).
Statistical analysis
Parametric data were expressed as means ± S.D. Statistical comparisons within groups were analyzed using paired Student’s t-test. Comparisons for multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni’s multiple t-test. Lung injury score was presented as median (range) and analyzed by Kruskal-Wallis rank test. P < 0.05 was considered as statistically significant.
Results
Haemodynamics and blood gas
The haemodynamics of all animals were stable during the experimental procedure. The effect of LIPC on lung function as measured by PaO2 is shown in Figure 1A. No group differences in the values of PaO2 were observed at baseline (P > 0.05). No substantial changes in PaO2 were observed in the sham group and the limb-IR (P > 0.05). At 105 min of reperfusion, PaO2 decreased significantly in the lung-IR and the LIPC groups (P < 0.05 or P < 0.001 vs. respective baseline values). However, the LIPC group had significantly higher PaO2 compared with the lung-IR (P < 0.01) group. PaCO2 level (Figure 1B) at 105 min of reperfusion was significantly higher in the IR (P < 0.01) and the LIPC (P < 0.01) groups when compared with the sham group, while post conditioning significantly reduced the PaCO2 level (P < 0.05).
Figure 1.
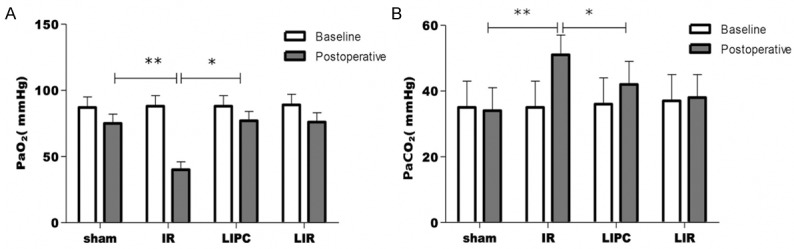
Arterial blood sample was taken at 20 min of mechanical ventilation (baseline) and 105 min after reperfusion (postoperative). The effect of LIPC on lung function is shown as follows: PaO2 (A) and PaCO2 (B). Sham, IR, LIPC, and LIR indicate sham control, ischemic post-conditioning, limb IR with post-conditioning, and limb ischemic-reperfusion, respectively. Data are mean ± S.D., n = 8 per group. **P < 0.05 or P < 0.01, IR versus baseline; *P < 0.05 or P < 0.01, LIPC versus IR.
Lung wet-to-dry weight ratio
The effects of post-conditioning IR on the lung wet-to-dry weight ratio are illustrated in Figure 2. Lungs exposed to IR (group IR) had significantly higher lung wet-to-dry weight ratio compared with the sham group (P < 0.001). LIPC significantly prevented the increasing in wet-to-dry weight ratio in response to exposure to IR (P < 0.001 LIPC vs. IR group). On the other hand, limb IR did not cause significant change of wet-to-dry ratio.
Figure 2.
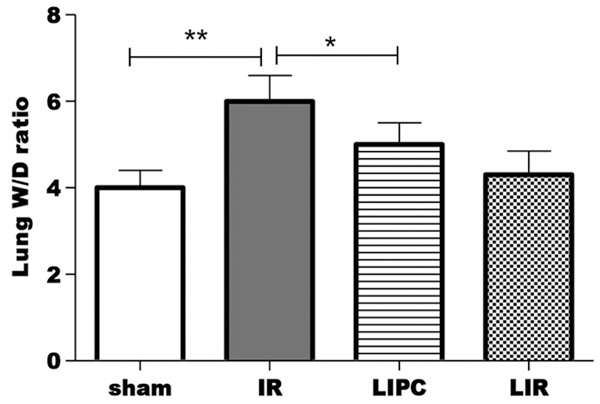
Effects of limb IR with post-conditioning on the lung wet-to-dry (W/D) weight ratio after 120 min of reperfusion. Sham, IR, LIPC, and LIR indicate sham control, ischemic post-conditioning, limb IR with post conditioning, and limb ischemic-reperfusion, respectively. Data are mean ± S.D., n = 8 per group. **P < 0.01, IR versus Sham; *P < 0.01, LIPC versus IR.
Lung histopathological changes
The histopathological changes in the left upper lobe of lung tissues at the end of reperfusion were assessed by standard H&E staining. Representative images of lung sections from each group are shown in Figure 3. No histological alteration was observed in the lung sections from sham-operated rats (Figure 3A). The IR group showed acute lung injury characterized in areas of necrosis, neutrophilic inflammation, intra-alveolar and interstitial edema (Figure 3B). The LIPC group revealed markedly reduced neutrophilic inflammation and interstitial edema with preservation of alveoli when compared with the IR group (Figure 3C). The Limb IR did not cause significant ling injury (Figure 3D).
Figure 3.
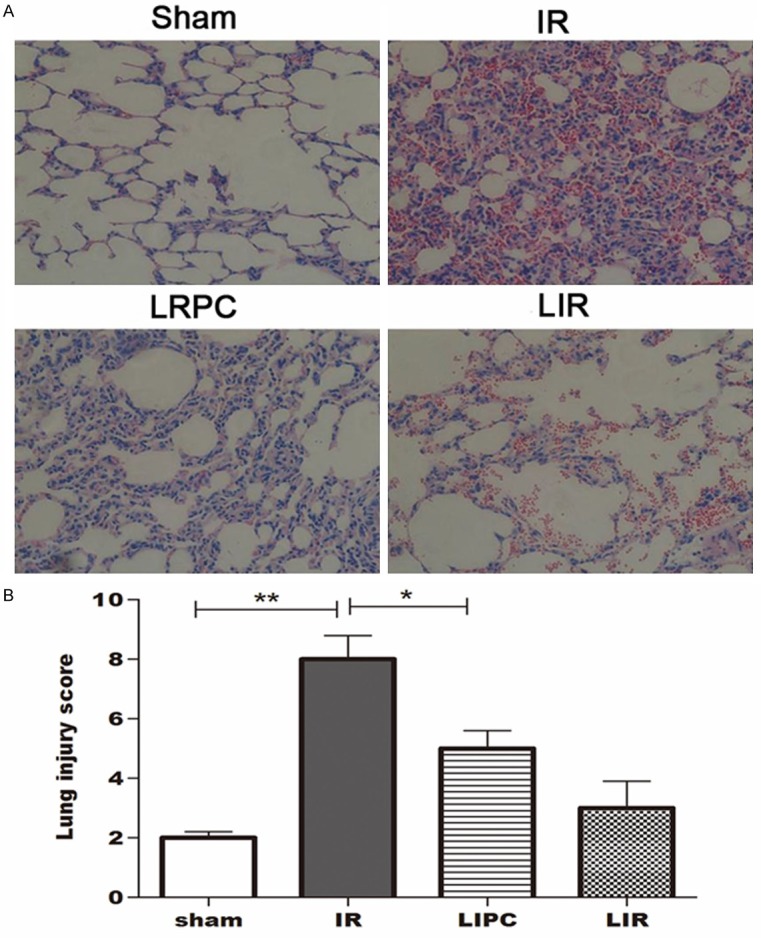
Histopathology of lung tissue in different groups (haematoxylin-eosin (H&E) staining, 200 ×).
Changes of plasma TNF-α and IL-6 levels
As shown in Figure 4, the level of plasma TNF-α in the IR group was significantly higher than sham group (P < 0.05). LIPC markedly reduced the level of TNF-α (P < 0.05, versus injury). Likewise, the level of IL-6 in the injury group was significantly higher than the sham group (P < 0.01, Figure 4). LIPC markedly reduced the level of IL-6 (P < 0.05, versus injury).
Figure 4.
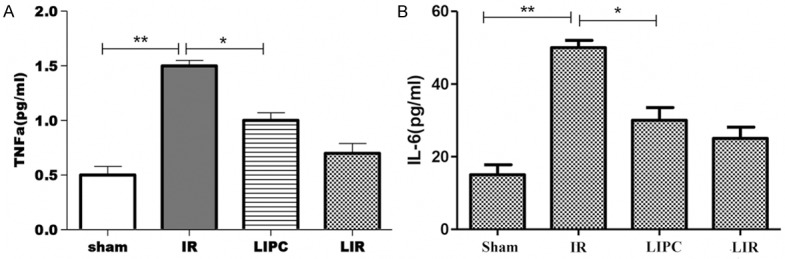
Plasma levels of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) (A), and interleukin-6 (IL-6) (B) after 105 min of reperfusion. Sham, IR, LIPC, and LIR indicate sham control, ischemic post-conditioning, limb IR with post-conditioning, and limb ischemic-reperfusion, respectively. Data are mean ± S.D., n = 8 per group. **P < 0.05 or P < 0.01, IR versus Sham; *P < 0.05, LIPC versus IR.
Chemiluminescent results
As we can see from Figure 5, the IR and the LIPC group both showed an increased level of the free oxygen species in comparison to the sham operated animals, but this increment was significantly milder in the LIPC group.
Figure 5.
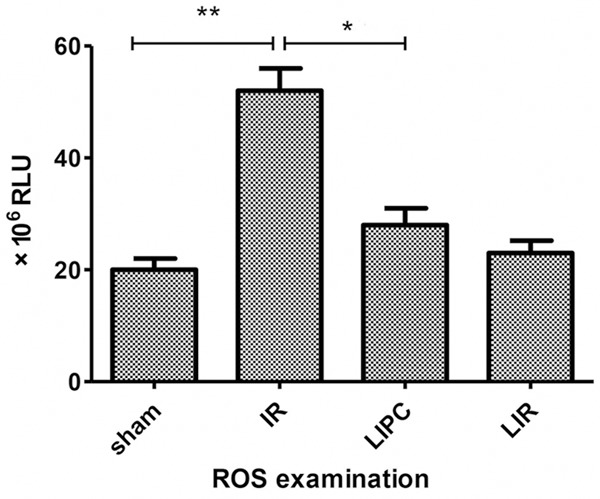
Antioxidant state was measured by Heide-Boegl luminometry. Chemiluminescent intensity (expressed in RLU, relative light units) is in direct proportion to the concentration of the reactive oxygen-derivatives found in the sample. A significant reduction in the plasma reactive oxygen radicals’ level can be seen in the PostC group referring to a less impaired redox homeostasis. Data are mean ± S.D., n = 8 per group. **P < 0.05 or P < 0.01, IR versus Sham; *P < 0.05, LIPC versus IR.
Expression of MCP-1, p38, JNK, p-JNK, NF-κB and p-NF-κB
The expressions of MCP-1, p38, JNK, p-JNK, NF-κB and p-NF-κB in the IR group were significantly increased when compared with the sham group (P < 0.05 or P < 0.01; Figure 6). The increase of these protein expressions were prevented by post conditioned IR intervention.
Figure 6.
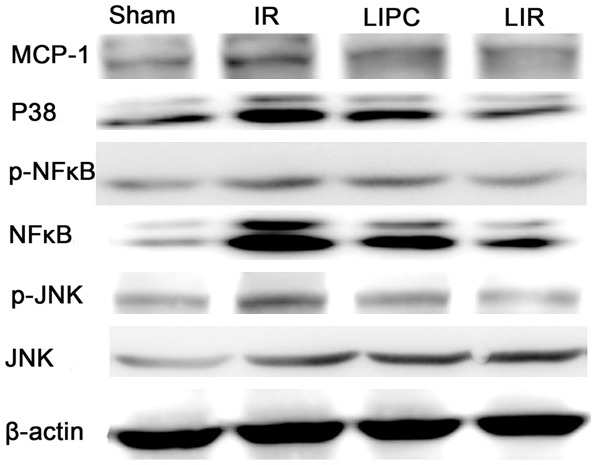
Effects of limb IR with post-conditioning on lung tissue monocyte chemoattractant protein-1 (MCP-1), p38, Jun N-terminal kinase (JNK), phosphorylated JNK (p-JNK), nuclear factor kappa-B (NF-κB) and phosphor-NF-κB (p-NF-κB) after 105 min of reperfusion. Sham, IR, LIPC, and LIR indicate sham control, ischemic post conditioning, limb IR with post conditioning, and limb ischemic-reperfusion, respectively.
Post conditioning significantly reduced the p-JNK and p-NF-κB, but not JNK and NF-κB, which demonstrated that post-conditioning more prefer to inhibit the kinase activity but not influencing the normal expression of above kinase.
Discussions
Our study confirm that LIPC could improve pulmonary oxygenation, reduce lung wet-to-dry weight ratio and reduce systemic inflammatory response, which is manifested as significantly reduced plasma levels of pro inflammatory cytokines TNF-α, IL-6 and reactive oxygen radicals’ level, in addition, our study indicated that LIPC could ameliorate histologic damage and inhibit the kinase activity.
Reperfusion has the potential to introduce additional injury such as reperfusion injury, this injury is characterized as endothelial and microvascular dysfunction, impaired blood flow, metabolic dysfunction, cellular necrosis, and apoptosis. Research indicated post conditioning could mechanically alter the hydrodynamics of early reperfusion [19]. In our study, we found a net amelioration of arterial PaO2 and PaCO2 levels, lung tissue W/D, and lung injury score in LIPC group compared with IR group, these results indicate that lower limb post conditioning has a certain protective effect against reperfusion injury in lung tissue. It is believed that lung IR is an injury factor and a pathophysiological process, lung IR is dynamically developed by the protective mechanism of the organism.
Zhao et al. [10] and Halkos et al. [20] indicated that post conditioning reduced reperfusion injury could through inhibiting the inflammatory response to reperfusion. A most recent study shows that post conditioning caused a significant reduction in systemic inflammatory response [18]. In this study, we found limb ischemic post-conditioning markedly reduced the level of TNF-α and IL-6, which may indicate that remote limb post conditioning attenuated lung-IR injury. Our results are consistent with the previous studies which suggested that the pro-inflammatory cytokines tissue necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) can be reduced by post conditioning [21]. However, the functional role for cytokines and inflammatory factors has not yet to be demonstrated. In addition, it is unknown whether there is a cause and effect link between reduced cytokines or inflammatory factors, and the protection of the lung IR injury exerted by post conditioning.
Reperfusion generates an overproduction of reactive oxygen species (ROS) or free radicals, leading to reperfusion injury [22]. In this study, we found the lung-IR and the LIPC group showed an increased level of the reactive oxygen compared with the sham group animals, but this increment was significantly milder in the LIPC group, which may indicate that lung-IR lead to the increment of ROS or free radicals but is compromised by limb ischemic post conditioning. Our results are compatible with the previous report, which showed that post conditioning inhibited oxidant generation and oxidant-mediated injury in myocardial IR injury [9].
Previous studies have shown that generation of abundant reactive oxygen species during early reperfusion contributes to tissue injury; these are secondary to ischemia and reperfusion. Oxygen radicals trigger the release of pro-inflammatory mediators and transcription factors such as NF-κB, and stimulate the surface expression of adhesion molecules on coronary vascular endothelium [23]. Post conditioning stimulates endogenous mechanisms that attenuate the multiple manifestations of reperfusion injury. These mechanisms include ligands that act as proximal triggers to stimulate molecular pathways involving mediators such as protein kinase C, mitochondrial ATP-sensitive potassium channels, and survival kinases. Post conditioning may also inhibit deleterious pathways involving p38 and JNK mitogen-activated protein (MAP) kinases, and Post conditioning may attenuate the damage to endothelial cells and cardio myocytes from oxidants, cytokines, proteases, and inflammatory cells [19]. We found the expressions of MCP-1, p38 in the IR group was significantly increased compared with those in the sham group and the increase of all protein expressions was prevented by limb ischemic post conditioning intervention. In addition, we found post conditioning significantly reduced the p-JNK and p-NF-κB, but not JNK and NF-κB, which showed that post-conditioning may inhibit the kinase activity but not influence the normal expression of above kinase.
There are several limitations in present study. Firstly, we did not determine whether the radicals involved in limb post conditioning were liberated from the ischemic hind limb or other mechanisms, e.g. the activation of opioid and/or other g-protein-coupled receptors by remote post conditioning generated free radicals in the heart resulting in cardio protection. Secondly, we did not determine whether limb post conditioning reduces free radicals by regulating the release of triggers such as endogenous adenosine. Finally, the protective effect was demonstrated in a rat model of ischemia. Whether this can be transferred to other animal models or even humans needs further study. Although these limitations, limb ischemic post conditioning appears to be a simple and promising strategy to reduce or prevent lung-IR injury, and has potential for future clinical application, because it induces endogenous protective mechanisms that may be components of a general protective strategy.
Conclusions
Present study demonstrates that limb ischemic post conditioning reduces IR injury of the lung in a simulated limb ischemia-reperfusion injury rat model; this process involves the release of systemic pro inflammatory cytokine and inhibition of the kinase activity but not influencing the normal expression of above kinase. The phenomenon of limb post conditioning implies that ischemic post conditioning has systemic effects in protecting distant other organ undergoing ischemia-reperfusion injury. Further studies are necessary to investigate and determine whether other mechanism involved in the reduction of long-term damages of the lung after lower limb ischemic-reperfusion injury.
Acknowledgements
This project was supported by the grant from China Nature Science Foundation Committee (#81070041), the grant from Beijing health system special foundation for building high-level health personnel (#2013-2-002), and the grant from the Beijing Science and Technology Project (#Z121107001012067).
Disclosure of conflict of interest
None.
References
- 1.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 2.Xia ZY, Gao J, Ancharaz AK, Liu KX, Xia Z, Luo T. Ischaemic post-conditioning protects lung from ischaemia-reperfusion injury by upregulation of haeme oxygenase-1. Injury. 2010;41:510–516. doi: 10.1016/j.injury.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol. 2008;294:L632–L641. doi: 10.1152/ajplung.00262.2007. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Ozasa H, Kojima N, Miura M, Iwa T, Senoo H, Horikawa S. Pharmacological preconditioning protects lung injury induced by intestinal ischemia/reperfusion in rat. Shock. 2003;19:462–468. doi: 10.1097/01.shk.0000055240.25446.16. [DOI] [PubMed] [Google Scholar]
- 5.Turan NN, Demiryurek AT. Preconditioning effects of peroxynitrite in the rat lung. Pharmacol Res. 2006;54:380–388. doi: 10.1016/j.phrs.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Turan NN, Yildiz G, Gumusel B, Demiryurek AT. Ischemic and peroxynitrite preconditioning effects in chronic hypoxic rat lung. Exp Lung Res. 2008;34:325–341. doi: 10.1080/01902140802093212. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz G, Demiryurek AT, Gumusel B, Lippton H. Ischemic preconditioning modulates ischemia-reperfusion injury in the rat lung: role of adenosine receptors. Eur J Pharmacol. 2007;556:144–150. doi: 10.1016/j.ejphar.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, Jugdutt CE. Agingrelated early changes in markers of ventricular and matrix remodeling after reperfused STsegment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010;122:341–351. doi: 10.1161/CIRCULATIONAHA.110.948190. [DOI] [PubMed] [Google Scholar]
- 9.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Shi E, Nakajima Y, Sato S. Postconditioning, a series of brief interruptions of early reperfusion, prevents neurologic injury after spinal cord ischemia. Ann Surg. 2006;244:148–153. doi: 10.1097/01.sla.0000217608.08582.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister SE, Ashrafpour H, Cahoon N, Huang N, Moses MA, Neligan PC, Forrest CR, Lipa JE, Pang CY. Postconditioning for salvage of ischemic skeletal muscle from reperfusion injury: efficacy and mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295:R681–689. doi: 10.1152/ajpregu.90303.2008. [DOI] [PubMed] [Google Scholar]
- 13.Szwarc I, Soullier S, Gayrard N, Mejean C, Mourad G, Argiles A. Ischemic postconditioning prevents ischemic acute renal failure. Transplant Proc. 2007;39:2554–2556. doi: 10.1016/j.transproceed.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Lu JG, He XL, Li N, Qiao Q, Yin JK, Ma QJ. Effects of ischemic postconditioning on reperfusion injury in rat liver grafts after orthotopic liver transplantation. Hepatol Res. 2009;39:382–390. doi: 10.1111/j.1872-034X.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 15.Szijártó A, Hahn O, Lotz G, Schaff Z, Madarász E, Kupcsulik PK. Effect of ischemic preconditioning on rat liver microcirculation monitored with laser Doppler flowmetry. J Surg Res. 2006;131:150–157. doi: 10.1016/j.jss.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Xia ZY, Wang XY, Chen X, Xia Z. Effect of NO donor sodium nitroprusside on lipopolysaccharide induced acute lung injury in rats. Injury. 2007;38:53–59. doi: 10.1016/j.injury.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 17.King J, Deboisblanc BP, Mason CM, Onofrio JM, Lipscomb G, Mercante DE, Summer WR, Nelson S. Effect of granulocyte colonystimulating factor on acute lung injury in the rat. Am J Respir Crit Care Med. 1995;151:302–309. doi: 10.1164/ajrccm.151.2.7531097. [DOI] [PubMed] [Google Scholar]
- 18.Gyurkovics E, Aranyi P, Stangl R, Onody P, Ferreira G, Lotz G, Kupcsulik P, Szijarto A. Postconditioning of the lower limb-protection against the reperfusion syndrome. J Surg Res. 2011;169:139–147. doi: 10.1016/j.jss.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Sun HY, Wang NP, Halkos M, Kerendi F, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11:1583–1593. doi: 10.1007/s10495-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 22.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 23.Miki T, Cohen M, Downey J. Failure of N-2-mercaptopropionyl glycine to reduce myocardial infarction after 3 days of reperfusion in rabbits. Basic Res Cardiol. 1999;94:180–187. doi: 10.1007/s003950050141. [DOI] [PubMed] [Google Scholar]


