Abstract
Bupivacaine, an amide type long-acting local anaesthetic is commonly employed for epidural anesthesia and as well for nerve blockades. However, studies have shown neurotoxicity following local administration of bupivacaine raising concerns over the use of the drug. Compounds that could minimize or inhibit toxic effects of bupivacaine are of high value in operative settings and in pain management. The present study aims to investigate if epigallo catechin gallate (EGCG) could inhibit or prevent bupivacaine toxicity in neuroblastoma cells (N2a and SH-SY5Y). The viability of N2a and SH-SY5Y cells following exposure to EGCG (10-50 µM) were assessed by MTT assay and Annexin V/PI staining. The influence of EGCG on ROS generation was determined. The expression of apoptotic cascade proteins (Caspases-3, -8 and -9, Bcl-xL, Bad, Bax, Bcl-2) and PI3/Akt pathway proteins (Akt, p-Akt, GSK-3β, p-GSK-3β, PTEN) were analyzed by western blotting. EGCG improved the viability of the cells and inhibited apoptosis by potentially decreasing the expression of caspases and pro-apoptotic proteins. Bupivacaine induced ROS generations were reduced on EGCG exposure. EGCG significantly promoted the phosphorylation of Akt and GSK-3β and down-regulated PTEN, thus activating PI3/Akt signalling. EGCG effectively improved the cell viability and inhibited apoptosis of N2a and SH-SY5Y cells via suppression of ROS generation and modulation of PI3K/Akt signalling cascade.
Keywords: Anesthesia, apoptosis, bupivacaine, epigallo catechin gallate, PI3/Akt/PTEN, NF-KB signalling
Introduction
Local anesthetics are a requisite in surgical procedures and also in post-operative pain management. However, accumulating evidences suggests that local anesthetics could possibly cause neural injury and lead to neurological complications [1-4]. Bupivacaine, an amide type local anesthetic is frequently employed in regional anesthesia as spinal or epidural anesthetic for nerve blockade and postoperative pain management in patients. Previous studies have demonstrated that bupivacaine-induced neural injury and neurotoxicity involves necrosis and apoptosis [4-6]. Activation of mitogen activated protein kinases (MAPKs) [7] as p38MAPK [8], disruption of calcium homeostasis [9] and generation of reactive oxygen species (ROS) [10] have been reported to be involved in the pathogenesis of bupivacaine-induced neurotoxicity. Nevertheless, the exact mechanism involved in bupivacaine-induced neuronal injury is yet to be understood completely.
The phosphatidylinositol-3-kinase (PI3K)/threonine serine protein kinase B (Akt) signalling cascade is involved in regulation of cell survival [11,12]. Phosphorylation of Akt and its downstream target glycogen synthase kinase 3β (GSK-3β), a serine/threonine kinase, results in increased cyclin D1 and Myc leading to cell survival [13]. Activation of Akt and GSK-3β has been reported to exhibit neuroprotective effects [14-16]. Akt activates another important signalling factor, nuclear factor-κB (NF-κB). NF-κB is one of the most vital transcription factors that is involved in regulating cell proliferation and cell cycle progression [17,18].
Studies have reported that bupivacaine and lidocaine, two commonly used local anesthetics suppresses the activation of PI3K/Akt signalling in neuroblastoma neuro 2a (N2a) cells [19,20]. Thus compounds that could cause activation of PI3K/Akt signalling cascade could possibly reduce or inhibit anesthetic-induced neuroapoptosis [19,20].
Previous investigations on dietary antioxidants have demonstrated the protective effects against local anesthetic induced neuronal injury [20-22]. Epigallocatechin gallate (EGCG) an ester of epigallocatechin and gallic acid, is a polyphenol present in several plants, mainly in green tea [23]. Previous studies on EGCG have reported that it possesses several beneficial effects under various pathologic conditions including diabetes, stroke and obesity. EGCG is a powerful antioxidant and anti-tumor effects of EGCG have also been well demonstrated [24,25]. Epidemiological and animal studies further indicate that EGCG shows neuroprotective activity in various neurological disorders [26-30]. Considering the health effects of EGCG, in the study we investigated if EGCG could exert neuroprotective effects in bupivacaine-induced neuron injury using mouse neuroblastoma N2a and human neuroblastoma SH-SY5Y cell lines.
Experimental methods
Chemicals and reagents
Bupivacaine and epigallocatechin gallate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for total Akt, phospho-Akt (p-Akt), total GSK-3β and phospho-GSK3β (p-GSK3,β) were obtained from Cell Signalling Technology (Beverly, MA, USA), Antibodies against phosphatase and tensin homolog (PTEN), caspase-3, caspase-8, capse-9, Bad, Bax, Bcl-2 and Bcl-xL were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Annexin V-FITC and propidium iodide (PI) (KeyGEN, China), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-tetrazolium bromide (MTT), 2’,7’-dichlorofluorescein diacetate (DCFH-DA) and Hoechst 33258 (Beyotime, China). Other reagents that were used for the study are of analytical grade and were procured from Sigma-Aldrich (St. Louis, MO, USA) otherwise are specified.
Cell lines
The SH-SY5Y and N2a cell lines was purchased from ATCC and were cultured in DMEM medium that was supplemented with fetal bovine serum (15%), penicillin (100 U/mL) and streptomycin (100 μg/mL) and was incubated in a humidified 5% CO2 incubator at 37°C. The medium was renewed once every 48 h.
MTT assay
SH-SY5Y and N2a cells were placed into 96-well plates having a density of 5×103 cells/well with 100 μL culture medium/well. After reaching a confluence of 70%, the cells were exposed to EGCG at different concentrations (10-50 µM) for 3 h. The cells were then subjected to 1 mM bupivacaine treatment [20] in medium for 24 h. After treatment with bupivacaine, to each well 20 μL MTT was added and incubated further for 4 h at 37°C. The supernatant was discarded after incubation and DMSO (150 μL) was added to each well to liquefy the formazan crystals. The absorbance was read using Synergy HT plate reader at 570 nm (Synergy HT, Bio-Tek, USA).
Assessment of apoptosis by Hoechst nuclear staining
Nuclear staining using Hoechest 33258 was performed to evaluate nuclear morphology. After treatment with EGCG and bupivacaine as in MTT assay, the cells were rinsed thrice using the PBS solution and was stained with Hoechst 33258. The cells were observed under fluorescence microscopy (Nikon ECLIPSE TE2000-u, Japan) at 300-500 nm. Apoptotic cells were counted based on nuclear morphology changes in the form of chromatin condensation and fragmentation.
Annexin V-FITC/PI staining for apoptosis detection
Annexin V-FITC/PI staining is used for detecting externalization of phosphatidylserine, indicator used for early stage apoptosis. Briefly, 1×106 cells treated with EGCG and bupivacaine were subjected to annexin V staining. The cells were washed in PBS (phosphate buffered saline) and resuspended in binding buffer containing (100 μL) annexin V-FITC and propidium iodide incubated for 20 min and analyzed by flow cytometer y (FACS Calibur, BD Biosciences).
Measurement of ROS
The cells following treatment with EGCG were seeded at a density of 5×105 cells/well with 500 μL culture medium into 24-well plates. Following 3 h of bupivacaine exposure, the intracellular reactive oxygen species (ROS) levels were determined using DCFH-DA. Briefly the cells were incubated with 10 μM DCFH-DA at 37°C for 20 min following bupivacaine treatment. The cells were then washed thrice, collected and suspended in PBS. The intensity of fluorescence as a measure of ROS was determined by flow cytometry (BD FACS Calibur, USA) at excitation wavelength of 488 nm and at emission wavelength of 525 nm. The data observed were analyzed with Cell Quest software (Becton Dickinson, USA).
Western blotting
After exposure to EGCG and bupivacaine as described above, the N2a and SH-SY5Y cells were removed and lysed in RIPA lysis buffer containing protease inhibitor cocktail for 30 min on ice. Cellular protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, USA). Extracted proteins were subjected to fractionation by SDS-PAGE and electro transferred to PVDF membrane and blocked with 5% non-fat dry milk in tris-buffered saline. The membranes were then blotted with respective antibodies (cleaved caspase-3, cleaved caspase-8, cleaved caspase-9, Akt, phospho-Akt, GSK-3β, phospho-GSK-3β, Bcl-xL, Bcl-2, Bad, Bax, PTEN and β-actin) and incubated overnight at 4°C. Following incubation, the membranes were rinsed and further incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin at a dilution of 1:1000 for about 1 h. The immunoreactive bands were detected by enhanced chemiluminescence (GE Healthcare). The expression levels of proteins were normalized to that of β-actin.
Statistical analysis
The data are represented as mean ± S.D, taken from three or six individual experiments. The values were subjected to analysis by one-way ANOVA (analysis of variance) followed by Duncan’s multiple range test post-hoc analysis. All statistical analyses were performed using the SPSS software (version 22.0, SPSS, Chicago, IL, USA). The values at P < 0.05 were considered statistically significant.
Results
EGCG improves the viability of N2a and SH-SY5Y cells
We examined the viability of N2a and SH-SY5Y cells by MTT assay. Severe apoptosis of the cells exposed to bupivacaine 24 h was observed (Figure 1). The viability decreased to 24.12% in N2a cells and 20.18% in SH-SY5Y cells as compared to control cells not exposed to bupivacaine. However, pretreatment with EGCG at (10-50 µM) significantly (P < 0.05) improved the viable cell counts in both the cell lines. EGCG at 50 µM brought about significant raise in cell viability percentage as compared to lower doses. The percentage of cell viability increased to 29.82% at 10 µM to 83.6% on exposure to 50 µM EGCG in N2a cells and to 31.88% and 88.66% on exposure to EGCG at 10 and 50 µM respectively in SHSY5Y cells.
Figure 1.
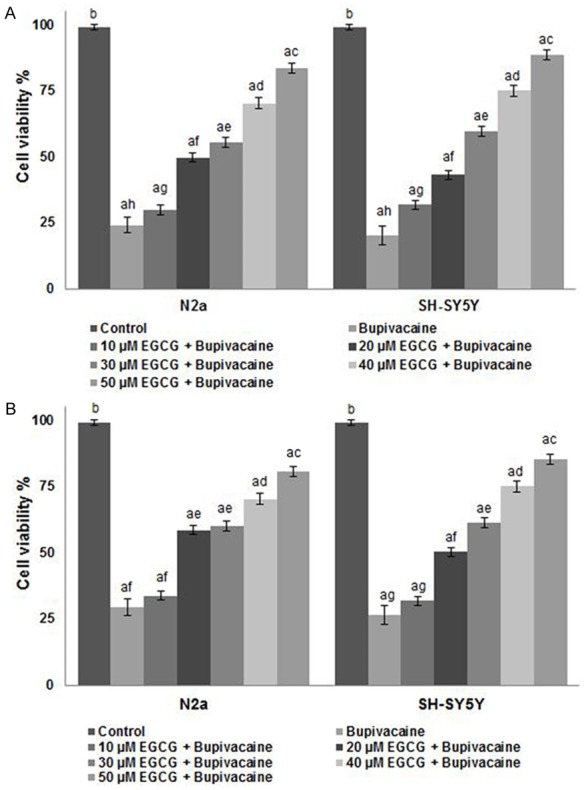
Influence of EGCG on cell viability by MTT assay (A) and annexin V staining (B). EGCG effectively decreased apoptosis and improved the viability of cells. Values are represented as mean ± SD, n = 6. A represents statistical significance at P < 0.05 compared against respective controls and B-H represents significant difference (P < 0.05) between mean values within the groups of same cell line as determined by one-way ANOVA followed by DMRT analysis.
EGCG inhibited bupivacaine-induced neuroapoptosis
Bupivacaine-induced apoptosis was also determined by nuclear condensation and annexin V/PI staining. Incubation of SH-SY5Y and N2a cells with bupivacaine for 24 h resulted in multi-fold increase in nuclear condensation as compared with untreated cells (P < 0.05). Pretreatment with EGCG significantly (P < 0.05) attenuated bupivacaine-induced apoptosis as observed by decrease in nuclear condensation and apoptotic morphology (Figure 1). Also, the nuclear alterations were showed by Hoechst nuclear staining as condensed, and segmented nuclei were more markedly seen in cells exposed to bupivacaine alone (Figure 2). Striking decline in typical signs of apoptosis on EGCG treatment were observed at all the tested concentrations, however decrease in apoptotic cell counts was high at higher concentrations than at 10-30 µM. These observations suggest the efficiency of EGCG in reducing neuroapoptosis induced by bupivacaine.
Figure 2.
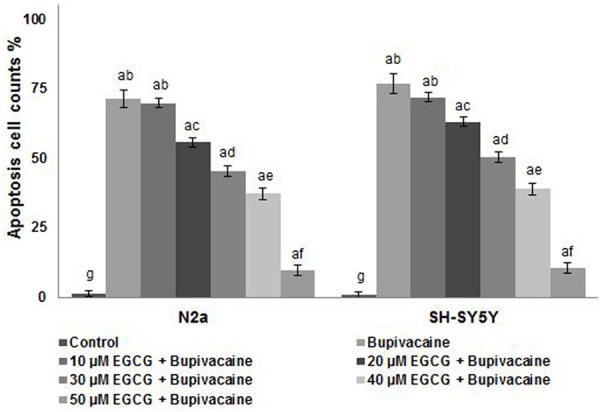
EGCG reduced nuclear condensation and apoptotic morphology. EGCG significantly reduced the nuclear condensation and apoptotic morphology of the cells exposed to bupivacaine. Values are represented as mean ± SD, n = 6. A represents statistical significance at P < 0.05 compared against respective controls and B-H represents significant difference (P < 0.05) between mean values within the groups of same cell line as determined by one-way ANOVA followed by DMRT analysis.
EGCG inhibited ROS generation
Studies have shown the involvement of ROS generation in bupivacaine-induced apoptosis [10]. In line with previous reports, exposure to bupivacaine caused a multi-fold increase in ROS generation in N2a and in SH-SY5Y cells (P < 0.05) as compared to unexposed cells (Figure 3). Rise of ROS levels upto 171.74% in N2a and 186.90% in SH-SY5Y cells was observed. EGCG treatment however caused a marked (P < 0.05) reduction in ROS levels. Further EGCG brought more significant decrease at 50 µM. On treatment with 50 µM, ROS levels decreased to 116.61% and 110.36% in N2a and in SH-SY5Y cells. Antioxidant activities of EGCG have been reported previously [28]. Thus the decrease in ROS levels could be attributed to the antioxidant properties of EGCG.
Figure 3.
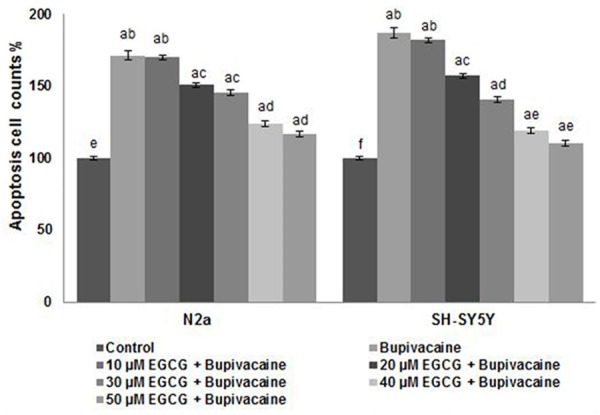
EGCG reduced bupivacaine-induced ROS generation. Values are represented as mean ± SD, n = 6. A represents statistical significance at P < 0.05 compared against respective controls and B-H represents significant difference (P < 0.05) between mean values within the groups of same cell line as determined by one-way ANOVA followed by DMRT analysis.
EGCG modulated expression of apoptotic pathway proteins
Apoptosis plays a crucial role in maintaining tissue homeostasis. Influence of EGCG on the expression of proteins of the apoptotic pathway was analyzed by western blotting. Caspases and Bcl-2 family proteins play important role as regulators of apoptosis [31]. Bupivacaine exposure caused up-regulation of caspases-caspase-3, -8 and -9 (Figure 4A and 4B). EGCG exposure caused a multi-fold decrease in the expression of caspases. Further, EGCG dose dependently up-regulated the expression of anti-apoptotic proteins-Bcl-xL and Bcl-2 with a reduction in the expression levels of Bax and Bad (P < 0.05). Fifty µM EGCG caused nearly two-fold increased expression of Bcl-xL and Bcl-2 in comparison with cells not exposed to EGCG (Figure 4C and 4D).
Figure 4.
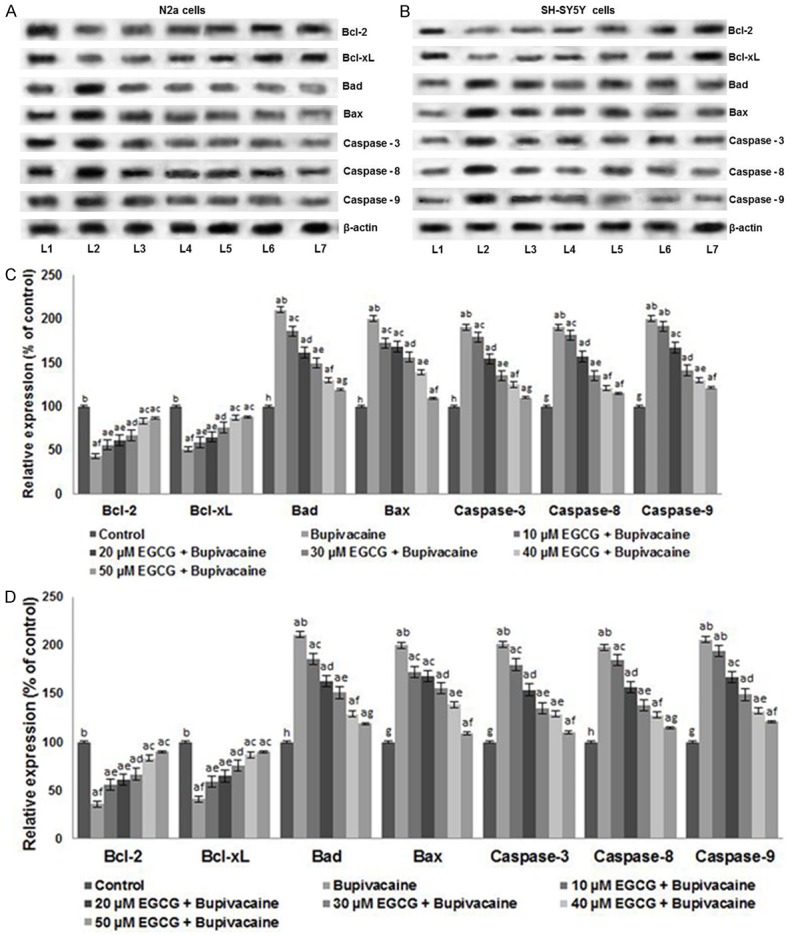
Effect of EGCG on the expressions of apoptotic pathway proteins. EGCG markedly modulated the expression of caspases and apoptotic pathway proteins. EGCG caused significant down-regulation of caspases and pro-apoptotic proteins with enhanced expressions of Bcl-2 and Bcl-xL in N2a cells (A and C), SH-SY5Y cells (B and D). Values are represented as mean ± SD, n = 6. A represents statistical significance at P < 0.05 compared against respective controls and B-H represents significant difference (P < 0.05) between mean values within the groups of same cell line as determined by one-way ANOVA followed by DMRT analysis. (L1-Control; L2-Bupivacaine; L3-10 µM EGCG + Bupivacaine; L4-20 µM EGCG + Bupivacaine; L5-30 µM EGCG + Bupivacaine; L6-40 µM EGCG + Bupivacaine; L7-50 µM EGCG + Bupivacaine).
EGCG potentially regulated the PI3K/Akt signalling
Activation of the PI3K/Akt cascade is vital for cell survival [32,33]. We examined the influence of bupivacaine on PI3K/Akt signalling. Bupivacaine suppressed the expression of Akt and p-Akt, thus inhibiting the activation of Akt signalling. GSK-3β is the down-stream protein that is regulated by Akt. Therefore, the total GSK-3β and p-GSK-3β expression levels were analyzed in N2a and also in SH-SY5Y cells. Significantly (P < 0.05) down-regulated expression of both total and phosphorylated forms of GSK-3β was observed following bupivacaine exposure (Figure 5). However, EGCG pretreatment enhanced the expression of phosphorylated Akt and GSK-3β along with up-regulated Akt and GSK-3β. The elevated expressions of the proteins were observed to be dose-dependent, with the 50 µM concentrations of EGCG exhibiting maximum effects. Further, the expression of PTEN was inhibited significantly (P < 0.05) in both the cells by EGCG. PTEN, a potent negative regulator of Akt is thought to play a decisive role in controlling the activation of PI3K/Akt signalling cascade [34]. Bupivacaine-induced the expression of PTEN which was suppressed by EGCG, thus leading to activation of PI3K/Akt signal.
Figure 5.
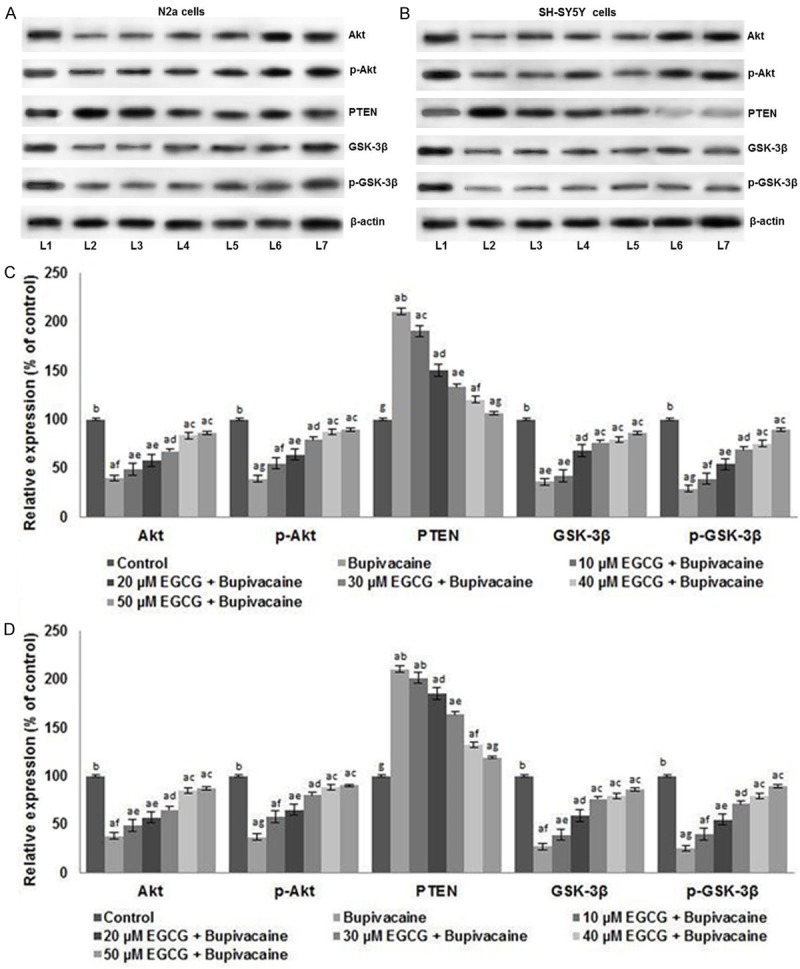
EGCG activated the PI3K/Akt signaling in N2a cells and SH-SY5Y cells. EGCG markedly elevated the expressions of total and phosphorylated Akt and GSK3β, suggesting activation of PI3K/Akt signaling while suppressing PTEN expressions in N2a cells (A and C), SH-SY5Y cells (B and D). Values are represented as mean ± SD, n = 6. A represents statistical significance at P < 0.05 compared against respective controls and B-H represents significant difference (P < 0.05) between mean values within the groups of same cell line as determined by one-way ANOVA followed by DMRT analysis. (L1-Control; L2-Bupivacaine; L3-10 µM EGCG + Bupivacaine; L4-20 µM EGCG + Bupivacaine; L5-30 µM EGCG + Bupivacaine; L6-40 µM EGCG + Bupivacaine; L7-50 µM EGCG + Bupivacaine).
Discussion
Bupivacaine is administrated for regional infiltration, epidural and intrathecal anesthesia [4]. Bupivacaine is a sodium channel blocker and all excitable neurons having action potential may possibly be suppressed by bupivacaine [20]. Numerous studies have reported that bupivacaine-induced neurotoxicity possibly occurs through apoptosis [4-6,10]. The mouse neuroblastoma cell lines (N2a and N1E-115) and human neuroblastoma cell lines (SH-SY5Y and SKN-AS) are employed as in-vitro models for investigation of the local anesthetics - induced neurotoxicity [35,36]. The present study investigated the effect of EGCG on bupivacaine-induced neuronal injury in N2a and SH-SY5Y cells.
Bupivacaine anesthesia caused apoptosis of the neuroblastoma cells. EGCG exposure caused a profound increase in the viability of neuroblastoma cells in a dose-dependent manner. The oxido-redox balance is known to be vital for the cell survival [20]. Studies have demonstrated that bupivacaine-induced neurotoxicity is associated with the generation of ROS [10]. Increased ROS levels on exposure to bupivacaine in Schwann cells [10] and SH-SY5Y cells [37] have been reported. In line with the previous reports, enhanced ROS levels were detected in our study following bupivacaine exposure in N2a and SH-SY5Y cells. Similar raised ROS generation upon exposure to local anesthetics were reported by Grishko et al. [38]. However, the striking reduction observed in ROS levels on pretreatment with EGCG, suggests the capacity of EGCG in curbing bupivacaine-induced neurotoxicity. Also, the antioxidant capacity of EGCG could have possibly contributed in neutralising ROS.
Apoptosis or the programmed cell death can be induced via the intrinsic and extrinsic pathways. Caspases are chief enzymes that are involved in the highly specific proteolytic cleavage of cellular proteins leading to cell death [39]. In our study, local anesthetic, bupivacine-induced caspase expression levels suggests the induction of apoptosis via caspase activation. Perez-Castro et al. [4], demonstrated activation of caspase-3/-7 in human neuroblastoma cells following incubation with local anesthetics-ropivacaine, bupivacaine and lidocaine. Activation of caspase-9 and -8 suggests the involvement of both intrinsic and extrinsic pathways of apoptosis that sequentially lead to the activation of caspase-3 [40]. Nevertheless, EGCG markedly suppressed the expression of caspase-3, 8 and 9.
The Bcl-2 family is composed of pro-apoptotic and anti-apoptotic proteins and the balance between these proteins are largely involved in regulating the apoptotic pathway and the mitochondrial membrane potential [32,41-44]. The pro-apoptotic Bcl-2-family proteins as Bax and Bak are involved in formation of pores in the outer mitochondrial membrane and induce apoptosis, while the anti-apoptotic proteins as Bcl-2 and Bcl-xL inhibit pore formation [45]. The down-regulation of Bcl-xL and Bcl-2 accompanied with enhanced expression of the pro-apoptotic proteins (Bax and Bad) was observed following bupivacaine exposure in N2a and in SH-SY5Y cells. The Bcl-2 and Bax protein levels are directly related to apoptosis regulation. The elevated Bax level promotes apoptosis, while enhanced Bcl-2 expressions inhibited cell apoptosis [46,47]. EGCG treatment almost completely inhibited the expressions of pro-apoptotic proteins and elevated the expressions of Bcl-2 and Bcl-xL suggesting that EGCG prevented bupivacaine-induced apoptosis by modulating caspases and apoptotic protein expression.
PI3K/Akt signalling pathway has been widely found in regulating cell survival. Akt is an essential kinase down stream of PI3K [33,48]. Phosphorylation of Akt is necessary for its activation which subsequently regulates many cellular responses [49]. p-Akt phosphorylates and deactivates downstream effector, GSK-3β [13]. Inactivation of Akt has been shown to be involved in apoptosis induction [14]. In brain ischemia/reperfusion model, calcineurin induced neuron apoptosis via Akt dephosphorylation is reported [50]. Akt also plays a crucial role in suppressing cellular apoptosis. Akt inhibits proapoptotic Bad and Bax levels and elevates Bcl-2 and, Bcl-xL expression [51]. The PI3K/Akt signalling enhances cellular replication and survival and inhibits apoptosis.
Suppression of PI3K/Akt signalling has been reported in myocytes, renal cells and in N2a cells exposed to bupivacaine or lidocaine [19,20,52,53]. Similar to the previous studies, bupivacaine exposure caused down-regulation of the PI3K/Akt signalling. Bupivacaine caused a decline in the expression of Akt, p-Akt, and GSK-3β and enhanced PTEN expressions. PTEN acts as a main negative regulator of the PI3K/Akt pathway [54]. However, significantly up-regulated levels of phosphorylated Akt and GSK-3β with suppression of PTEN were observed on treatment with EGCG, thus activating the PI3K/Akt signalling cascade. The activation of PI3K/Akt signalling plays important roles in the neuroprotection against variant stressors [20].
Collectively, the observations demonstrated neuroprotective effects of EGCG. EGCG at 10-50 µM concentrations potentially inhibited bupivacaine-induced apoptosis of N2a and SH-SY5Y cells by up-regulating the anti-apoptotic proteins and also modulating the expression of caspases and the proteins of PI3K/Akt signalling cascade. Thus, EGCG could be suggested as a potent candidate drug for management of bupivacaine-induced neuronal toxicity.
Disclosure of conflict of interest
None.
References
- 1.Hodgson PS, Neal JM, Pollock JE, Liu SS. The neurotoxicity of drugs given intrathecally (spinal) Anesth Analg. 1999;88:797–809. doi: 10.1097/00000539-199904000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier FJ, Bouaziz H, Samii K. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97:1274–1280. doi: 10.1097/00000542-200211000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Kasaba T, Onizuka S, Takasaki M. Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics. Anesth Analg. 2003;97:85–90. doi: 10.1213/01.ane.0000065905.88771.0d. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ, Xu F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997–1007. doi: 10.1213/ane.0b013e31819385e1. [DOI] [PubMed] [Google Scholar]
- 5.Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg. 2002;94:319–324. doi: 10.1097/00000539-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW, Bauer I, Stevens MF. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718. doi: 10.1093/bja/aep236. [DOI] [PubMed] [Google Scholar]
- 7.Lirk P, Haller I, Colvin HP, Lang L, Tomaselli B, Klimaschewski L, Gerner P. In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaineinduced neurotoxicity. Anesth Analg. 2008;106:1456–1464. doi: 10.1213/ane.0b013e318168514b. [DOI] [PubMed] [Google Scholar]
- 8.Tan Z, Dohi S, Chen J, Banno Y, Nozawa Y. Involvement of the mitogena ctivated protein kinase family in tetracaine-induced PC12 cell death. Anesthesiology. 2002;96:1191–1201. doi: 10.1097/00000542-200205000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Johnson ME, Saenz JA, DaSilva AD, Uhl CB, Gores GJ. Effect of local anesthetic on neuronal cytoplasmic calcium and plasma membrane lysis (necrosis) in a cell culture model. Anesthesiology. 2002;97:1466–1476. doi: 10.1097/00000542-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Park CJ, Park SA, Yoon TG, Lee SJ, Yum KW, Kim HJ. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84:852–857. doi: 10.1177/154405910508400914. [DOI] [PubMed] [Google Scholar]
- 11.Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Sem Immunol. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- 12.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Q, Yang J, Han S, Liu J, Holzbeierlein J, Thrasher JB, Li B. Suppression of glycogen synthase kinase 3 activity reduces tumor growth of prostate cancer in vivo. Prostate. 2011;71:835–845. doi: 10.1002/pros.21300. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Kim YS, Park CH, Chung IY, Yoo JM, Kim JG, Lee BJ, Kang SS, Cho GJ, Choi WS. Protein kinase C-delta mediates neuronal apoptosis in the retinas of diabetic rats via the Akt signalling pathway. Diabetes. 2008;57:2181–2190. doi: 10.2337/db07-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem. 2008;283:15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. J Neurosurg. 2008;109:691–698. doi: 10.3171/JNS/2008/109/10/0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ougolkov AV, Bone ND, Fernandez-Zapico ME, Kay NE, Billadeau DD. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110:735–742. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. NF-kappaB the first quarter-century: remarkable progress and outstanding questions. Genes Develop. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G, Liu L, Ding Z. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience. 2010;167:329–342. doi: 10.1016/j.neuroscience.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhang X, Cheng Y, Li C, Zhang W, Liu L, Ding Z. α-Lipoic acid prevents bupivacaine-induced neuron injury in vitro through a PI3K/Akt-dependent mechanism. Neurotoxicology. 2010;31:101–112. doi: 10.1016/j.neuro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Bai T, Dong DS, Pei L. Resveratrol mitigates isoflurane-induced neuroapoptosis by inhibiting the activation of the Akt-regulated mitochondrial apoptotic signaling pathway. Int J Mol Med. 2013;32:819–826. doi: 10.3892/ijmm.2013.1464. [DOI] [PubMed] [Google Scholar]
- 22.Lei X, Zhang W, Liu T, Xiao H, Liang W, Xia W, Zhang J. Perinatal supplementation with omega-3 polyunsaturated fatty acids improves sevoflurane induced neurodegeneration and memory impairment in neonatal rats. PLoS One. 2013;8:e70645. doi: 10.1371/journal.pone.0070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton EE, Wang Y, Thiessen PA, Bryant SH. PubChem: integrated platform of small molecules and biological activities. In: Ralph AW, David CS, editors. Annual Reports in Computational Chemistry. Vol. 4. Amsterdam, Netherlands: Elsevier; 2008. pp. 217–241. [Google Scholar]
- 24.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan T, Jankovic J, Le W. Potential therapeutic properties of green tea polyphenols in Parkinson’s disease. Drugs Aging. 2003;20:711–721. doi: 10.2165/00002512-200320100-00001. [DOI] [PubMed] [Google Scholar]
- 27.Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MBH. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG) J Alzheimer’s Dis. 2008;15:211–222. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- 28.Menard C, Bastianetto S, Quirion R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front Cell Neurosci. 2013;26:281. doi: 10.3389/fncel.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Wang M, Jing X, Shi H, Ren M, Lou H. (-)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem Res. 2014;39:1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 30.Yao C, Zhang J, Liu G, Chen F, Lin Y. Neuroprotection by (-)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stress. Mol Med Rep. 2014;9:69–76. doi: 10.3892/mmr.2013.1778. [DOI] [PubMed] [Google Scholar]
- 31.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You W, Min X, Zhang X, Qian B, Pang S, Ding Z, Li C, Gao X, Di R, Cheng Y, Liu L. Cardiacspecific expression of heat shock protein 27 attenuated endotoxin-induced cardiac dysfunction and mortality in mice through a PI3K/Akt-dependent mechanism. Shock. 2009;32:108–117. doi: 10.1097/SHK.0b013e318199165d. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi A, Wada Y, Kitagishi Y, Matsuda S. Link between PI3K/AKT/PTEN pathway and NOX proteinin diseases. Aging Dis. 2014;5:203–211. doi: 10.14336/AD.2014.0500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friederich P, Schmitz TP. Lidocaine-induced cell death in a human model of neuronal apoptosis. Eur J Anaesthesiol. 2002;19:564–570. doi: 10.1017/s0265021502000911. [DOI] [PubMed] [Google Scholar]
- 36.Werdehausen R, Braun S, Essmann F, Schulze-Osthoff K, Walczak H, Lipfert P, Stevens MF. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107:136–143. doi: 10.1097/01.anes.0000268389.39436.66. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Xu SY, Zhang QG, Xu R, Lei HY. Bupivacaine induces apoptosis via mitochondria and p38 MAPK dependent pathways. Eur J Pharmacol. 2011;657:51–58. doi: 10.1016/j.ejphar.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 38.Grishko V, Xu M, Wilson G, Pearsall AW. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609–618. doi: 10.2106/JBJS.H.01847. [DOI] [PubMed] [Google Scholar]
- 39.Rao A, Johnston TR, Harris AH, Smith RL, Costouros JG. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anesthetics chondrocyte apoptosis after anesthetics. Am J Sports Med. 2014;42:50–58. doi: 10.1177/0363546513507426. [DOI] [PubMed] [Google Scholar]
- 40.Hartojo W, Silvers AL, Thomas DG, Seder CW, Lin L, Rao H, Wang Z, Greenson JK, Giordano TJ, Orringer MB, Rehemtulla A, Bhojani MS, Beer DG, Chang AC. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl Oncol. 2010;3:99. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following. Experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 42.Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27:6419–6433. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- 43.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 44.Hüttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion. 2011;11:369–381. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Develop. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 46.Xie Z, Koyama T, Suzuki J, Fujii Y, Togashi H, Sawa H, Nagashima K. Coronary reperfusion following ischemia: different expression of Bcl-2 and bax proteins, and cardiomyocyte apoptosis. Japanese Heart J. 2001;42:759–770. doi: 10.1536/jhj.42.759. [DOI] [PubMed] [Google Scholar]
- 47.McClintock DS, Santore MT, Lee VY, Brunelle J, Budinger GR, Zong WX, Thompson CB, Hay N, Chandel NS. Bc1-2 family members and functional electron transport chain regulate oxygen deprivation-induced cell death. Mol Cell Biol. 2002;22:94–104. doi: 10.1128/MCB.22.1.94-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of prosurvival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–1367. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park CH, Kim YS, Kim YH, Choi MY, Yoo JM, Kang SS, Choi WS, Cho GJ. Calcineurin mediates AKT dephosphorylation in the ischemic rat retina. Brain Res. 2008;1234:148–157. doi: 10.1016/j.brainres.2008.07.082. [DOI] [PubMed] [Google Scholar]
- 51.Atif F, Yousuf S, Stein DG. Anti-tumor effects of progesterone in human glioblastomamultiforme: role of PI3K/Akt/mTOR signaling. J Steroid Biochem Mol Biol. 2015;146:62–73. doi: 10.1016/j.jsbmb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Lee HT, Xu H, Siegel CD, Krichevsky IE. Local anesthetics induce human renal cell apoptosis. Am J Nephrol. 2003;23:129–139. doi: 10.1159/000069304. [DOI] [PubMed] [Google Scholar]
- 53.Maurice JM, Gan Y, Ma FX, Chang YC, Hibner M, Huang Y. Bupivacaine causes cytotoxicity in mouse C2C12 myoblast cells: involvement of ERK and Akt signaling pathways. Acta Pharmacol Sin. 2010;31:493–500. doi: 10.1038/aps.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen-Preiss SM, Silva SR, Gillette JM. Role of PTEN and Akt in the regulation of growth and apoptosis in human osteoblastic cells. J Cell Biochem. 2003;90:964–975. doi: 10.1002/jcb.10709. [DOI] [PubMed] [Google Scholar]


