Abstract
Objective: To explore the effect of siRNA-mediated inhibition of lymphocyte-specific protein tyrosine kinase (Lck) on pulmonary inflammation in a mouse model of asthma. Methods: A total of 32 female BABL/c mice were used in the study. The mouse asthma model was established with ovabumin (OVA), and Lck specific siRNA or nonspecific siRNA was transfected through the tail vein before the first OVA challenge. Two days after the last challenge, mice were sacrificed and bronchoalveolar lavage fluid (BALF), plasma and lung tissue were collected. Levels of Lck mRNA and protein in lung were detected by quantitative real-time PCR and western blot. The levels of IL-4 and IgE in BALF and plasma were detected with ELISA. Results: Lck specific siRNA significantly inhibited expression of Lck mRNA and protein in T cells. In vivo transfection of Lck siRNA down regulated the expression of Lck mRNA and protein in lung parenchymal homogenates. Sensitized mice treated with Lck siRNA prior to OVA challenge had fewer eosinophils in BALF and in lung sections and lower levels of IL-4 and IgE in BALF and plasma compared to those treated with nonspecific siRNA. Conclusions: Pretreatment of OVA sensitized mice with Lck siRNA results in attenuation of pulmonary inflammation following OVA challenge. Inhibition of Lck gene expression should be investigated further as a potential therapy for asthma.
Keywords: T lymphocyte, in vivo transfection, IL-4, IgE
Introduction
Asthma is a prevalent chronic disease characterized by airway hyper responsiveness, completely reversible airflow obstruction and bronchial inflammation [1]. Many inflammatory cells, including T cells, B cells, eosinophils and mast cells, participate in the pathogenesis of asthma [2]. Among these cells, activated T cells play a major role, particularly by secreting cytokines such as IL-4, IL-5, IL-13 and IL-17, which mediate inflammatory reactions [3]. Thus, the activation of T cells is a critical step in the development of asthma.
T cell activation is a complex process involving many signaling molecules, including T cell receptor (TCR) and lymphocyte specific tyrosine kinase (Lck). Antigen activation of TCR results in the activation of Lck and further downstream signaling, ultimately leading to T cell differentiation and cytokine secretion. Kemp et al [4] reported that Lck mediates Th2 differentiation. It is clear that asthma was associated with activation of a Th2 type of T cell in the airway. Expression of Th2 cytokines could be related to activity of disease, symptom scores, airway eosinophilia and bronchial hyperresponsiveness [5].
Previous studies have suggested that inhibition Lck might block allergen-induced inflammatory responses and asthma. McRae et al [6] showed that A-420983, aLck specific inhibitor, could block antigen-induced T cell proliferation and the secretion of IFN-γ and IL-4 by inhibiting the TCR signal transduction pathway.
Specific inhibition of gene expression can be achieved using siRNA. Recent developments in siRNA delivery have made it possible to use this technique in vivo. In this study we use in vivo siRNA inhibition to investigate the role of Lck in the development of lung inflammation in a mouse model of asthma.
Methods
Animals
A total of 32 female BABL/c mice, 6-8 weeks old and weighing 18-22 g were used for this study. All mice were housed in a local facility for laboratory animal care and fed ad libitum on stock diet in the animal research center of Fudan University, according to local ethical guidelines. This study was designed according to generally accepted international standards and was approved by the Ethic Committee for Animal Care and Use, Fudan University (Shanghai, China). All procedures involving mice were performed according to the NIH Guiding Principles in the Care and Use of Animals.
Preparation of Lck specific siRNA
Murine Lck specific siRNA fragments were chemically synthesized with help from Shanghai Integrated Biotech Solutions Company (Shanghai, China). Four Lck specific siRNA fragments were synthesized and tested in preliminary experiments, and the sequence of the most effective one was as follow: sense: 5’-GGC UGU GUC UGC AGC UCA AAC-3’; anti-sense: 5’-UUG AGC UGC AGA CAC AGC CCA-3’.
Isolation of T cells
Two mice were sacrificed using sodium pentobarbital, and the spleen was removed, ground and filtered through 100 oculus steel mesh. Supernatant was collected and centrifuged at 1500 rpm for 5 min, the red cells were discarded with the erythrocyte lysate (Beyontime, Nanjing, China), and then the cells were centrifuged again at 1500 rpm for 5 min, supernatant was discarded, and collected cells were re-suspended with RPRI 1640 media. The total T cells were isolated using a nylon T cell separation column (147-06721 Nylon Fiber Column T, Wako, Japan) according to the manufacturer’s protocol. T cells were re-suspended in RPMI medium 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and were cultured in a humidified incubator at 37°C, 5% CO2. Anti mouse CD3e-PE (eBiosecience, 12-0031, Santiago, USA) was used to detect the phenotype of T cells and CD3 positive ones were analyzed by using an Epics XL flow cytometer (Beckman-Coulter, Brea, CA, U.S.A.).
Cell culture and in vitro transfection of siRNA
T cells were plated in 24-well plates (Corning) at a density of 2×105 cells/well in 0.5 ml of RPMI medium 1640. Transfection of Lck specific siRNA was performed using siRNA Transfection Reagent (PolyplusINTERFERinTM) according to the manufacturer’s instructions. Briefly, 2 μl of siRNA solution and 4 μl of the transfection reagent were incubated in 100 μl of serum-free RPMI medium 1640 for 15 minutes. The mixture was added to the T cells in each well and incubated for 48 hours. Nonspecific siRNA transfected T cells and normal untransfected T cells were used as negative control and blank control, respectively. In order to evaluate the transfection rate, the same dose of FAM-siRNA was mixed with transfection reagent and transfected into T cells in one well for 48 hours, and cells were examined and photographed by fluorescence microscopy (Olympus 1×51).
Real-time PCR analysis in T cell
T cells were collected at 48 h after transfection, and total RNA was isolated using TRNzol Reagent (TianGen, DP405, Beijin, China). 1 μg of total RNA from each sample was subjected to first-strand cDNA synthesis using PrimeScriptTM 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). Reverse transcription reactions were done at 42°C for 1 h, followed by 72°C for 5 min. Quantitative real-time PCR was conducted using Eppendorf Realplex4 (Hamburg Germany) according to the following thermal cycle protocol: 94°C for 10 min, followed by 40 cycles of 94°C for 15 s and 60°C for 1 min. Sangon Biotech (Shanghai, China)provided the primers used for the amplification of murine Lck. The primer sequences used were as follows: sense: 5-TTA CCT ACC CGC GCT CCT GTG TCC C-3; anti-sense: 5-CTG GGA AGT CAG TGT CAA ACC A-3; and β-actinwas used as positive control, and its sequence was as follow: sense: 5-CGT TGA CAT CCG TAA AGA CC-3; anti-sense: 5-AAC AGT CCG CCT AGA AGC AC-3.
Western blot analysis in T cell
T cells were collected 48 h after transfection and the nuclear protein was collected by using extraction kit (P0013B, Beyontime, Nanjing, China). Protein concentrations were determined with BCA Protein Assay reagent (Beyontime, Nanjing, China). Equal amounts of protein (40 mg per lane) were loaded. Proteins were separated in 10% SDS-polyacrylamide gels and transferred electrophoretically onto a nitrocellulose membrane. Membranes were blocked overnight at 4°C with blocking buffer, and then incubated with rabbit anti-mouse Lck antibody (Ab18896, AbcamInc, USA) or β-actin antibody (Ab3280, AbcamInc, USA) for 1 h at room temperature, with a dilution of 1:500. Then the membrane was soaked in Ni-enhanced 3,30-diaminobenzidine (DAB) solution until the protein bands could be visualized. Second antibody is goat anti-mouse (A0286, Beyontime, Nanjing, China). The relative intensity of bands was analyzed using Quantity One software and Lck levels were normalized to β-actin levels.
Establishment of mouse asthma model
The mouse model of asthma was established as previously reported [10]. Briefly, micewere randomly divided into 3 groups (ten mice for each group): control, nonspecific siRNA, Lck siRNA. For control group, the mice were intraperitoneally sensitized with 0.2 ml of phosphate-buffered saline (PBS) at day 0 and 14, and then were intranasally challenged with 20 μl of PBS under anesthesia on days 28, 29, 30. For nonspecific siRNA group and Lck siRNA group, mice were intraperitoneally sensitized with 0.2 ml of phosphate-buffered saline (PBS) including 10 mg of ovalbumin (OVA, A5503, Sigma, MO, USA) at day 0 and 14, then were intranasally challenged with 20 μl of PBS containing 0.2 mg of OVA under anesthesia on days 28, 29, 30.
In vivo transfection of siRNA
40 μg siRNA was diluted with 50 μl 10% glucose solution, sterile water was added to 100 μl and mixed gently. 6.4 μl in vivo jetPEI transfection reagents (Polyplus-transfection, ILLKIRCH cedex, France) was diluted with 50 μl 10% glucose solution, sterile water was added to 100 μl and mixed gently. The in vivo jetPEI dilution and the siRNA dilution were mixed quickly and gently, incubated at room temperature for 15 min, then injected into mice through the tail vein one day before the first challenge. 2 days after the last challenge, the mice were sacrificed using sodium pentobarbital and samples were collected.
Collection of samples (plasma, BALF, lung homogenates)
Plasma
Blood was collected by cardiac puncture using a sterile syringe and transferred to heparin-gel vacuum tubes. The blood samples were then centrifuged and plasma was collected and stored at -80°C for further cytokine analysis.
BALF
The left principal bronchus was ligated and the right lung was lavaged three times with 0.5 ml of PBS. Total cell counts were determined using a haemocytometer. Differential cell counts were determined on BALF smear slides that were stained with Giemsa (Sigma). The number of eosinophils was calculated as the percentage of eosinophils multiplied by the total number of cells in the BALF. Collected BALF was then centrifuged at 2500 rpm for 10 min, and the supernatant was collected and stored at -80°C for further use of cytokines analysis.
Lung homogenates
The left lower lobe of the lung was collected for pathology, and the rest of the left lung was collected for lung homogenates. Briefly, 100 μg of lung tissue was transferred to a 1.8 ml micro centrifuge tube containing 1 ml cold PBS with 10 μl protease inhibitors (Sigma Cat. No. A1276). The tissues were homogenized for 30 s in a vortex mixer and kept on ice. Homogenates were centrifuged with 12,000 rpm for 20 min at 4°C. Supernatants were aliquotted into tubes and stored at -80°C for further use of cytokines analysis.
Detection of cytokine levels in plasma, BALF, lung tissues and homogenates
Levels of IL-4, and IgE in plasma, BALF, lung homogenates were detected using corresponding ELISA kits (R&D, USA) according to the manufacturer’s recommendations.
Western blot analysis in tissues
The abdominal aorta and vena cava of mice were severed at the diaphragm, and the pulmonary circulation was perfused through right ventricle of the heart with 10 ml of heparinized saline. The whole right lung was washed with cold PBS and lysed with RIPA buffer (Beyontime, P0013B, China) on ice for 30 min, centrifuged with 1, 2000 rpm for 15 minat 4°C, and then the supernatant was discarded. The total protein was collected and Lck expression was detected by Western blot analysis, as described above.
Real-time PCR analysis in lung tissue
The abdominal aorta and vena cava of mice were severed at the diaphragm, and the pulmonary circulation was perfused through right ventricle of the heart with 10 ml of heparinized saline. The whole right lung was washed with cold PBS and total RNA was isolated using TRNzol Reagent (TianGen, DP405, Beijin, China), 1 ml for 100 mg tissue, repeated pipetting, added 0.2 ml chloroform/ml Trizol, oscillated for 3 min, centrifuged with 15000 rpm for 5 minat 4°C, the supernatant was collected and 0.5 ml isopropanol/ml Trizol was added, standed for 15 minutes at room temperature, centrifuged again with 15000 rpm for 5 min at 4°C, the deposition was washed with 1 ml 75% cold alcohol, centrifuged again with 15000 rpm for 5 min at 4°C, the deposition was collected as the total RNA. Lck gene expression was detected by Real-time PCR analysis, as described above.
Histopathology
Left lower lobes of lung were fixed in 4% formaldehyde, embedded in paraffin, and cut into 4 μm thick sections. Sections were stained with hematoxylin and eosin (HE), and images were taken under an Olympus light microscope (BX51) equipped with a digital camera (Olympus dp71; Olympus, Tokyo, Japan). The slides were analyzed by a histologist without knowledge of the identity of the samples.
Statistical analysis
The SPSS 17.0 software package was used to process the data. To calculate a P value for comparisons between two samples, statistical analyses were performed using Student’s t-test. For multiple comparisons, data were analyzed using one-way analysis of variance (ANOVA). The threshold of significance was set at P < 0.05.
Results
In vitro transfection of Lck siRNA inhibits Lck expression in mouse T cells
Before investigating the effect of Lck siRNA transfection in vivo, we verified that our siRNA inhibited Lck expression in T cells. Primary T cells were isolated, cultured and transfected with Lck siRNA and non-specific siRNA. Control transfection with a fluorescent siRNA showed that our transfection efficiency was about 70% (Figure 1). 48 hours after transfection RNA and protein were collected and tested for Lck expression by quantitative real time PCR and immunoblotting, respectively. Expression of both Lck protein (Figure 2A, 2B) Lck mRNA (Figure 2C) were significantly decreased after Lck siRNA transfection, but were not affected by transfection with nonspecific siRNA.
Figure 1.
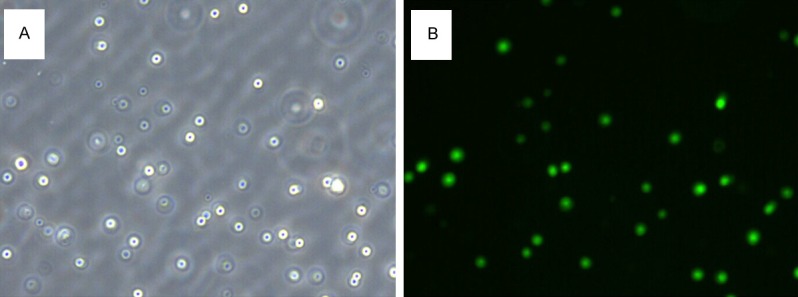
Primary T cells were transfected with Lck FAM-siRNA and observed by common light microscopy (A) and fluorescent microscopy (B). The magnification is 200×.
Figure 2.
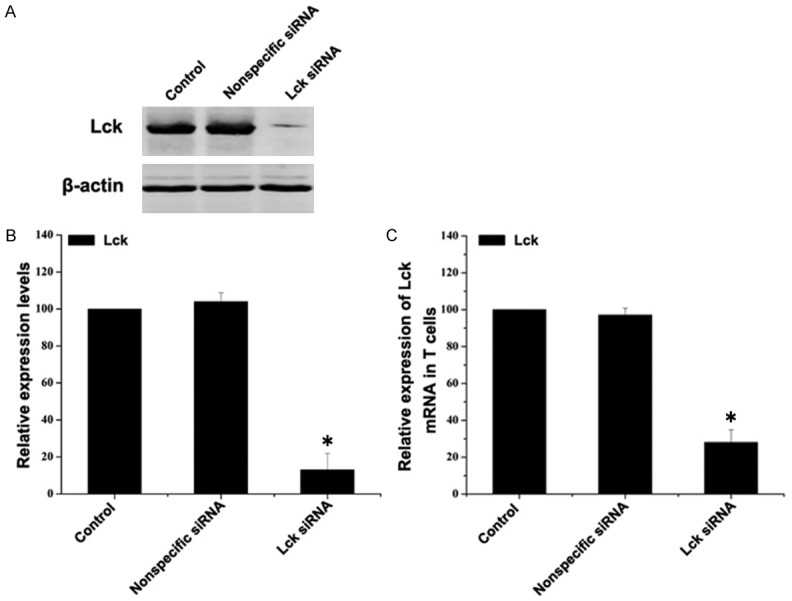
In vitro transfection of Lck siRNA decreases Lck expression in cultured primary T cells. Primary T cells were transfected with FAM-siRNA, nonspecific siRNA or Lck siRNA. A. Expression levels of Lck in primary T cell were detected by western blot. β-actin served as the loading control. B. Quantitative analysis of the changes of Lck. Data represent mean ± SEM (n=3, *P < 0.05 vs control group). C. Total RNA was extracted 48 hours after transfection and quantitative real time PCR was performed using Lck primers and β-actin primers. The amount of Lck mRNA relative to β-actin is shown. Data represents mean ± SEM (n=3 *P < 0.05 vs control group).
In vivo transfection of Lck siRNA inhibits Lck expression in mouse lung tissue
The mouse model of asthma was established by sensitization and challenge with OVA, as described in the Methods section. One day before the OVA challenges were initiated, mice were transfected with Lck siRNA or nonspecific siRNA via tail vein injection. When the OVA challenges were completed, mice were sacrificed, lungs were harvested, mRNA and protein extracts were prepared and Lck levels determined. Both Lck protein expression (Figure 3A, 3B) and Lck mRNA (Figure 3C) levels were significantly inhibited in mice treated with Lck siRNA compared to mice treated with nonspecific siRNA.
Figure 3.
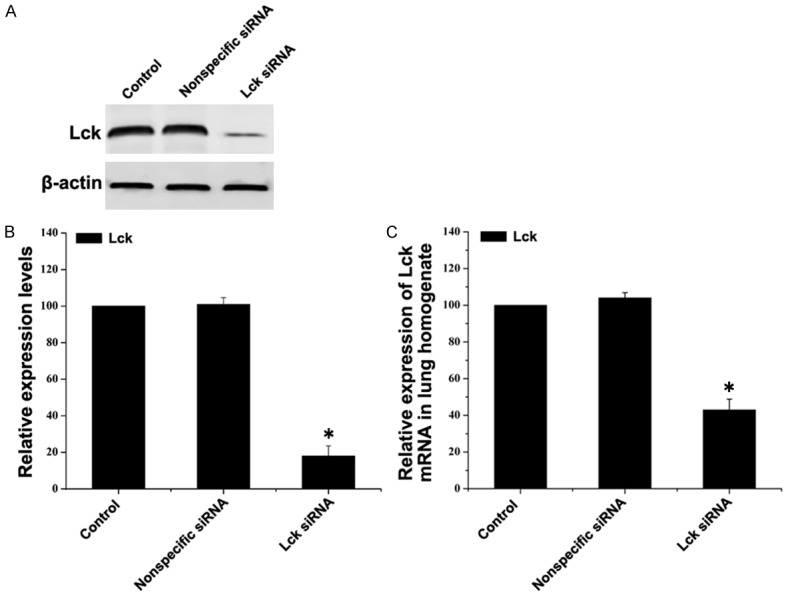
In vivo transfection of Lck siRNA reduces Lck expression in mouse lung tissue. Mice were sensitized with OVA and transfected via tail vein injection with Lck siRNA or nonspecific siRNA and then challenged with OVA. Control mice were sensitized and challenged with PBS and not transfected. Mice were sacrificed and lung tissue was removed following the final OVA challenge. A. Expression levels of Lck in lung tissue were detected by western blot. β-actin served as the loading control. B. Quantitative analysis of the changes of Lck. Data represent mean ± SEM (n=3, *P < 0.05 vs control group). C. Total RNA was extracted from lung and expressions of Lck mRNA levels were detected by quantitative real-time PCR. The amount of Lck mRNA relative to β-actin is shown. Data represent mean ± SEM (n=3, *P < 0.05 vs control group).
Inflammation phenotypes are decreased following inhibition of Lck expression in asthmatic mice
To investigate the effect of Lck inhibition on inflammation in asthmatic mice, we prepared BALF from mice after in vivo siRNA transfection and OVA challenge and determined the number of inflammatory cells and the levels of proinflammatory cytokines IL-4 and IgE. BALF from mice sensitized and challenged with OVA and transfected with nonspecific siRNA contained increased numbers of inflammatory cells (Figure 4A, 4B) and increased levels of IL-4 and IgE (Figure 4C, 4D) compared to BALF from control untransfected mice sensitized and challenged with PBS. BALF from mice sensitized and challenged with OVA and transfected with Lck siRNA contained significantly lower numbers of inflammatory cells and lower levels of IL-4 and IgE compared to BALF from OVA sensitived mice transfected with nonspecific siRNA (Figure 4A-D).
Figure 4.
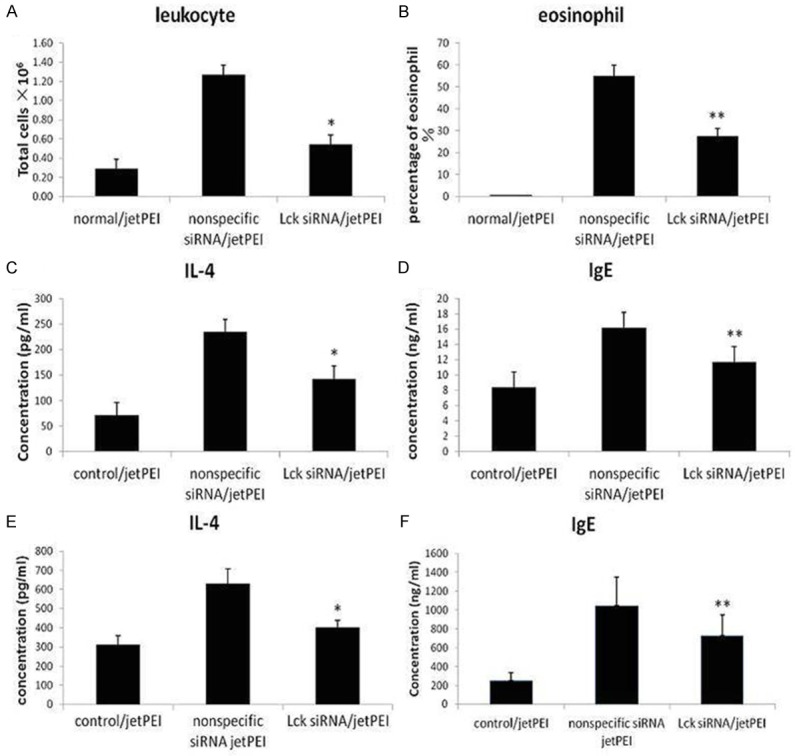
Effect of Lck siRNA on markers of inflammation. Mice were sensitized with OVA and transfected via tail vein injection with nonspecific siRNA or Lck siRNA, and then challenged with OVA. Control mice were sensitized and challenged with PBS and untransfected. Following OVA challenge, mice were sacrificed and BALF and plasma were collected. The total number of leukocytes (A) and the percentage of eosinophils (B) in BALF were determined. BALF was analyzed by ELISA to determine the concentration of IL-4 (C) and IgE (D). Plasma was analyzed by ELISA to determine the concentration of IL-4 (E) and IgE (F). *P < 0.05, **P < 0.01, compared to nonspecific siRNA (n=9 per group).
We also measured levels of IL-4 and IgE in plasma isolated from transfected and sensitized mice. As seen in the BALF samples, levels of IL-4 and IgE in plasma were significantly decreased in mice transfected with Lck siRNA and sensitized with OVA compared to mice transfected with non-specific siRNA and sensitized with OVA (Figure 4E, 4F).
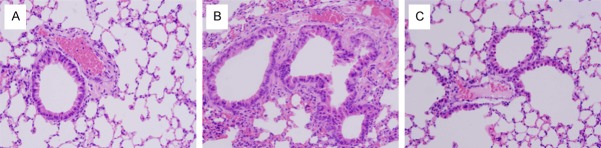
Finally, we determined the number of inflammatory cells in lung sections from control, nonspecific siRNA/OVA sensitized and Lck siRNA/OVA sensitized mice. As shown in Figure 5, the number of eosinophils and goblet cells is increased in nonspecific siRNA/OVA sensitized lung tissue compared to control, but not in Lck siRNA/OVA sensitized lung tissue compared to control.
Figure 5.
Effect of Lck siRNA on lung histology. Lung tissue was taken from control mice (A) sensitized and challenged with PBS and untransfected, or from mice sensitized with OVA and transfected via tail vein injection with nonspecific siRNA (B) or Lck siRNA (C), then challenged with OVA. Tissues were stained with hematoxylin and eosin (H&E). Being compared with control mice (A), the number of the inflammatory cells such as eosinophils in the lung tissues increased in nonspecific siRNA mice (B), while they decreased in Lck siRNA mice (C). Magnification is 200×.
Taken together, these results suggest that inhibition of Lck expression blocks the inflammatory response caused by allergen challenge in the mouse model of asthma.
Discussion
T cells and their subsets, as well as inflammatory cytokines they secrete, are the root of the inflammatory reaction and series of clinical symptoms in asthma [7]. Activation of T cells is a critical step to the initiation and maintenance of airway inflammation [8], while the activation of T cell receptor (TCR) is the first step.
Briefly, when TCR is engaged by the specific antigen presented by major histocompatibility complex (MHC), Lck phosphorylates the intracellular chains of the CD3 and ζ-chains of the TCR-MHC- peptide complex [9-11], allowing another cytoplasmictyrosinekinase called ZAP-70 to bind to them, and then the TCR signaling pathway is activated [12]. Furthermore, Lck phosphorylates and activates ZAP-70, which in turn phosphorylates LAT, and precipitates signalosome and possibly synapse formation, followed by T cell activation and a series of intracellular signal transduction [13,14]. Once T cells were activated, they differentiated into Th2, Th9, Th17, Th22 cells et al, which produced IL-4, IL-5, IL-7, IL-9, IL-10, IL-13, IL-17 and other cytokines [3]. Other immune cells were also activated and released inflammatory mediators. For example, B cells secreted IgE and mast cells secreted histamine, and all these lead to asthma [15].
RNA interference technology has been highlighted as a powerful research method in the study of gene function, anti-viral, anti-tumor gene target treatment [16]. However, the delivery of siRNA into T cell lines is exceedingly challenging, because T cells are resistant to transfection by conventional reagents. For this reason, the initial study on T cells was limited at in vitro level. Recently, concomitant with the development of biotechnology, the efficient and low toxicity transfection reagent appeared, such as in vivo jetPEI transfection reagent, and it becomes possible that siRNA could be used in in vivo studies.
McRae et al [6] has been reported that A-420983, Lck specific inhibitor, could block the antigen-induced T cell proliferation and the secretion of IFN-γ and IL-4 through inhibiting TCR associated signal transduction pathway. SKemp KL et al [4] has shown that Lck mediated Th2 differentiation. According to their studies, we designed and synthesized Lck specific siRNA and transfected them into mice with asthma through tail vein, and our results showed that pretreatment of Lck specific siRNA down-regulated the expression levels of Lck in lung parenchymal homogenates, and the levels of IL-4 and IgE were decreased in both BALF and plasma. Histological assessment of mice lung tissues revealed that Lck specific siRNA attenuated the pulmonary inflammation in asthma mice.
In summary, we investigated the effect of Lck siRNA on pulmonary inflammation in a mouse model of asthma. We found that sensitized mice treated with Lck siRNA prior to allergen challenge had fewer eosinophils in BALF and lung tissues, and lower levels of IL-4 and IgE in BALF and plasma compared to sensitized mice treated with nonspecific siRNA prior to allergen challenge. These finding suggested that Lck play a key role in the T cell activation pathway and that Lck might be a potential therapeutic target for asthma.
Disclosure of conflict of interest
None.
References
- 1.Fitzgerald JM, Bateman E, Hurd S, Boulet LP, Haahtela T, Cruz AA, Levy ML. The GINA Asthma Challenge: reducing asthma hospitalisations. Eur Respir J. 2011;38:997–8. doi: 10.1183/09031936.00114511. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–19. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS. The role of the T cell in asthma. J Allergy ClinImmunol. 2010;126:1081–91. doi: 10.1016/j.jaci.2010.06.025. quiz 1092-3. [DOI] [PubMed] [Google Scholar]
- 4.Kemp KL, Levin SD, Bryce PJ, Stein PL. Lck mediates Th2 differentiation through effects on T-bet and GATA-3. J Immunol. 2010;184:4178–84. doi: 10.4049/jimmunol.0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson DS. The role of the T cell in asthma. J Allergy ClinImmunol. 2010;126:1081–91. doi: 10.1016/j.jaci.2010.06.025. quiz 1092-3. [DOI] [PubMed] [Google Scholar]
- 6.McRae BL, Wallace C, Dixon KF, Roux A, Mohan S, Jia Y, Presky DH, Tracey DE, Hirst GC. Suppression of CD4+ T cell activation by a novel inhibitor of Src family kinases. IntImmunopharmacol. 2005;5:667–77. doi: 10.1016/j.intimp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Shin IS, Shin NR, Jeon CM, Kwon OK, Sohn KY, Lee TS, Kim JW, Ahn KS, Oh SR. EC-18, a synthetic monoacetyldiglyceride (1-palmitoyl-2-linoleoyl-3-acetylglycerol), attenuates the asthmatic response in an aluminum hydroxide/ovalbumin-induced model of asthma. IntImmunopharmacol. 2014;18:116–23. doi: 10.1016/j.intimp.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Finiasz M, Otero C, Bezrodnik L, Fink S. The role of cytokines in atopic asthma. Curr Med Chem. 2011;18:1476–87. doi: 10.2174/092986711795328346. [DOI] [PubMed] [Google Scholar]
- 9.Davis SJ, van der Merwe PA. Lck and the nature of the T cell receptor trigger. Trends Immunol. 2011;32:1–5. doi: 10.1016/j.it.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Shalaby KH, Martin JG. Overview of asthma; the place of the T cell. Curr Opin Pharmacol. 2010;10:218–25. doi: 10.1016/j.coph.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Höfer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–77. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephen TL, Wilson BS, Laufer TM. Subcellular distribution of Lck during CD4 T-cell maturation in the thymic medulla regulates the T-cell activation threshold. Proc Natl Acad Sci U S A. 2012;109:7415–20. doi: 10.1073/pnas.1119272109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kittipatarin C, Tschammer N, Khaled AR. The interaction of LCK and the CD4 co-receptor alters the dose response of T-cells to interleukin-7. Immunol Lett. 2010;131:170–81. doi: 10.1016/j.imlet.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahon RN, Sande OJ, Rojas RE, Levine AD, Harding CV, Boom WH. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol. 2012;275:98–105. doi: 10.1016/j.cellimm.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldizsar F, Szabo M, Kvell K, Czompoly T, Talaber G, Bjorkan J, Bartis D, Nemeth P, Berki T. ZAP-70 tyrosines 315 and 492 transmit nongenomic glucocorticoid (GC) effects in T cells. MolImmunol. 2013;53:111–7. doi: 10.1016/j.molimm.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Ramakrishna L, de Vries VC, deLafaille MAC. Cross-roads in the lung: immune cells and tissue interactions as determinants of allergic asthma. Immunol Res. 2012;53:213–28. doi: 10.1007/s12026-012-8296-4. [DOI] [PubMed] [Google Scholar]