Abstract
Posterior capsular opacification (PCO), the main complication of cataract surgery, is mainly caused by the proliferation, migration, and epithelial-mesenchymal transition (EMT) of the residual lens epithelial cells (LECs).Vitamin C was reported to reduce the risk of forming a cataract. However, there has been no study showing the association between vitamin C and PCO. In this study, we found that vitamin C could inhibit the migration and proliferation of human lens epithelial cells. We also found that vitamin C could increase the proline hydroxylation of HIF-1α and reduce the activity of HIF-1α. Moreover, vitamin C could not inhibit the activity of proline-mutant HIF-1α (402/564). Overexpression of wild-type HIF-1α or proline-mutant HIF-1α was found to increase the proliferation and migration of human lens epithelial cells. Differently, vitamin C could inhibit the proliferation and migration in wild-type HIF-1α-overexpressing lens epithelial cells but not the proline-mutant HIF-1α-overexpressing cells. Additionally, vitamin C was also found to inhibit the expression of EMT transcription factors TWIST. We then found that vitamin C could repress the EMT phenotypes induced by the overexpression of wild-type HIF-1α but not the proline-mutant HIF-1α. These results provide evidence that vitamin C plays a role in the repression of proliferation, migration, and EMT of human lens epithelial cells by destabilizing HIF-1α.
Keywords: Vitamin C, posterior capsular opacification, proliferation, migration, epithelial-mesenchymal-transition, Lens epithelial cells, HIF-1α
Introduction
Posterior capsular opacification (PCO), associated with fibrosis, opacification and contraction of posterior lens capsule, is the main complication of cataract surgery [1-3]. Thus, it’s important to investigate new drugs or materials to reduce the incidence of PCO. PCO is mainly caused by the proliferation, migration, and epithelial-mesenchymal transition (EMT) of the residual lens epithelial cells (LECs) after cataract surgery [4,5]. Epithelial wound healing process and fibrogenic reactions were initiated after cataract surgery [6,7]. The opacification of the posterior capsule appears to form Soemmerring’s rings or Elschnig’s pearls that were initiated from the proliferation, migration, and epithelial-mesenchymal transition (EMT) of LECs [8].
EMT is a process a biologic process by which epithelial cells lose their cell polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells [9,10]. EMT has also been shown to occur in wound healing and fibrosis [11]. E-cadherin is a central component of the cell-cell adhesion junctions that play a principal role in maintaining epithelial cell morphology [12,13]. The loss of E-cadherin expression is a hallmark of EMT [14,15]. Several transcription factors are the key inducers of EMT that repress E-cadherin expression. TWIST (a basic helix-loop-helix transcription factor) down-regulates the expression of E-cadherin [16,17]. Moreover, HIF-1α (Hypoxia-inducible factor-1α) was reported to up-regulate the expression of TWIST by binding directly to the hypoxia-response element (HRE). Meanwhile, overexpression of HIF-1α was also found to promote the process of EMT [18-20].
HIF-1α is targeted for ubiquitin-dependent degradation by an E3 ubiquitin ligase VHL (Von Hippel-Lindau tumor suppressor protein) [21]. Proline hydroxylation of HIF-1α is required for the interaction of HIF-1α with VHL. Prolyl-hydroxylase (PHD1/2/3) modifies HIF-1α, allowing it to interact with the VHL [22-24]. Vitamin C (ascorbate) is one of the cofactor of the prolyl-hydroxylases. Moreover, vitamin C acts as an electron-donor keeping Fe iron in the ferrous (Fe2+) state which is necessary to achieve full activity of prolyl-hydroxylase [25,26]. Vitamin C was reported to reduce the risk of forming a cataract [3,27-29]. In the present study, we investigated whether vitamin C could affect the stability of HIF-1α, proliferation, migration and EMT process of lens epithelial cells.
Material and methods
Cell culture
The human HLE-B3 lens epithelial cells lines were obtained from American Type Culture Collection (Manassas, VA, USA). HEK293T cells were obtained from the Institute of Cell and Biochemistry Research of Chinese Academy of Science (Shanghai, China). These cell lines were cultured with DMEM medium (Invitrogen, Carlsbad, CA) and supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Cells were cultured at 37°C in an incubator containing 5% CO2.
Reagents
Antibody against HIF-1α, TWIST and HIF-1α (hydroxy P402/P564) were purchased from Abcam (Cambridge, Mass, USA). The β-Actin antibody was from Cell Signaling Technology (Beverly, MA, USA). Anti-E-cadherin and anti-N-cadherin antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Vitamin C was from Sigma-Aldrich (Sigma-Aldrich, St Louis, MO, USA). HRE-driven luciferase lentiviral expression vector was from Genomeditech (Shanghai, China).
Lentivirus transduction
HIF-1α and proline-mutant HIF-1α (402/564) in pCDHlentiviral expression vectors were from Genesent (Shanghai, China). HEK293T cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Lentiviral vectors are typically produced in HEK 293T cells with packaging plasmids. The recombinant viral supernatants were harvested from HEK 293T cells and then used to infect target cells in the presence of 8 μg/ml polybrene. After selected with puromycin-containing media for 48 hours, the expression of HIF-1α was analyzed by Western Blot on the third day after infection.
RNA preparation and quantitative real time PCR analysis
After the treatments, HLE-B3 cells were harvested and total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized with PrimeScript RT reagent kit (Takara, Dalian, China). Quantitative real time PCR analysis was performed by using SYBR green I dye (Takara, Dalian, China). The β-actin was used as an endogenous control to standardize the amount of the sample mRNA. Primers for HIF-1α were as follows: 5’-TGCTTGCCAAAAGAGGTGGA-3’ (sense); 5’-GGGGCCAGCAAAGTTAAAGC-3’ (antisense), primers for β-Actin were as follows: 5’-GAGCACAGAGCCTCGCCTTT-3’ (sense); 5’-AGAGGCGTACAGGGATAGCA-3’ (antisense). The relative mRNA expression was calculated by the ΔΔCt method.
Western blot analysis
Total protein was obtained cells were suspended with RIP lysis buffer containing protease/phosphatase inhibitors mixture (Genesent, Shanghai, China). The protein concentration was measured with Bradford method. The proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then blotted onto aPVDF membrane (Merck Millipore Bioscience, Germany). The membranes were blocked with 5% BSA for 1 hour at room and then the primary antibodies were added for incubation overnight at 4°C. After washing thrice with TBS-Tween, peroxidase conjugated secondary antibodies were incubated with the membrane. The blots were incubated with were detected by Chemiluminescent HRP Substrate (Cwbiotech, Beijing, China) and photographed by Bio-Rad’. sChemiDoc XRS system (Hercules, CA, USA).
Colony formation assays
Cells were seeded at a density of 250 cells per well in 6-cm well plates. 24 hours later, cells were incubated with vehicle control or 100 μM vitamin C. Ten days later, the cells were then stained with crystal violet, photographed and counted.
In vitro migration assay
Equal numbers of cells (2 × 105) in DMEM complemented with 2% FBS were added to the upper compartment of a Transwell chamber (8 μm, Costar, Cambridge, MA, USA) and cultured at 37°C for 48 h. The cells from the upper surface of the filter were wiped off by a cotton swab. Cells on the lower surface of the membrane were fixed with 2% formaldehyde and then stained with hematoxylin-eosin (H&E), counted.
Luciferase activity assay
To directly evaluate the activity of HIF-1α, we used the luciferase assay. HLE-B3 cells expressing an HRE-driven luciferase expression vector or co-expressing wild-type HIF-1α or proline-mutant HIF-1α were treated with or without vitamin C for 12 h. Total cell lysates were harvested by passive lysis buffer containing luciferin (Promega, Madison, WI, USA). Luciferase activity was measured using a luminometer (Perkin-Elmer, Waltham, MA, USA).
Statistical analysis
Statistical analyses were presented as mean ± SD and analyzed using GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA). P values less than 0.05 were considered statistically significant. The results were reproduced in three repeated experiments.
Results
Vitamin C inhibits the proliferation and migration of lens epithelial cells
Previous studies have demonstrated that vitamin C inhibited the proliferation and migration [30,31]. In the present study, we determine whether vitamin C could affect the proliferation and migration of lens epithelial cells. To determine whether vitamin C affect the migration of lens epithelial cells, we did transwell migration assay. As shown in Figure 1A, vitamin C inhibited the migration of HLE-B3lens epithelial cells. Moreover, we examined the effects of vitamin C on the proliferation of HLE-B3 cells by the colony formation assay. Treatment of HLE B-3 cells with vitamin C significantly inhibited the proliferation (Figure 1B).
Figure 1.
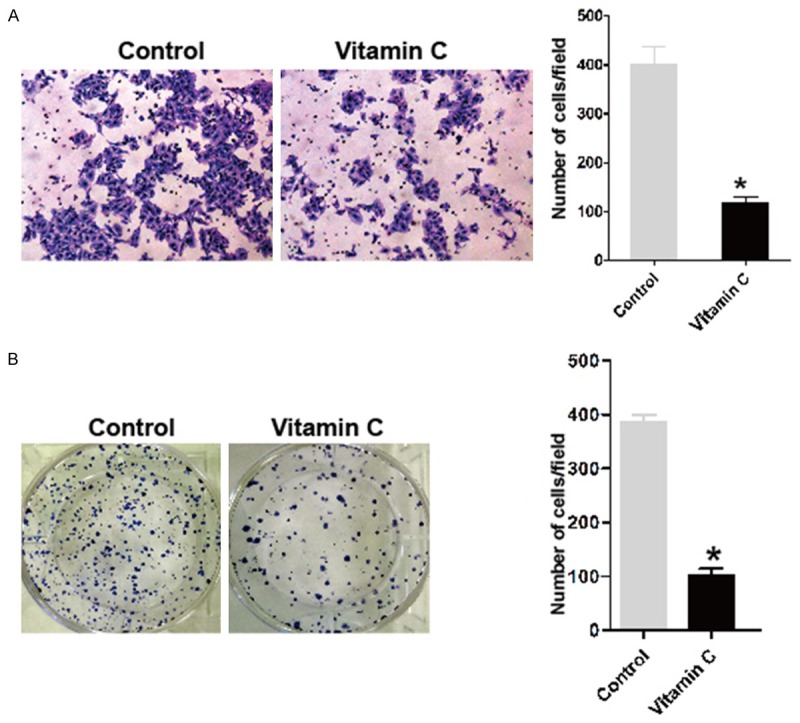
Vitamin C inhibits the proliferation and migration of lens epithelial cells. A. The migration potential of HLE-B3 cells was determined by transwell assay with PBS vehicle control or vitamin C (100 μM) during the experiments. The representative images are shown on the left, and the quantification of selected fields is shown on the right; *P<0.001 compared with the control group. B. HLE-B3 cells were treated with PBS vehicle control or vitamin C (100 μM) during the experiments. Representative photographs of colony formation in HLE-B3 cells are shown on the left. Quantification of the colony formation rate is shown on the right; *P<0.001 compared with the control group.
Vitamin C inhibits the activity of HIF-1α in lens epithelial cells
HIF-1α was reported to up-regulate the expression of TWIST by binding directly to the hypoxia-response element (HRE). Meanwhile, overexpression of HIF-1α was also found to promote the process of EMT [18]. Vitamin C was found to inhibit the proliferation and migration of lens epithelial cells. Moreover, proline hydroxylation of HIF-1α byprolyl-hydroxylases is required for the interaction of HIF-1α with VHL for ubiquitin-dependent degradation. Vitamin C is one of the cofactor of the prolyl-hydroxylases. Therefore, we suppose that vitamin C might stimulate the full activity of prolyl-hydroxylase and promote the degradation of HIF-1α. HIF-1α protein is remarkably unstable and undetectable under normoxia [32,33]. Thus, we first performed quantitative RT-PCR to examine the mRNA levels of HIF-1α under the treatment of vitamin C. As shown in Figure 2A, vitamin C did not affect the mRNA expression of HIF-1α. Then we detected the levels of HIF-1α target protein under the treatment of vitamin C. GLUT1 (Glucose transporter 1) and VEGF (human vascular endothelial growth factor), possessing HRE elements, are the well-known targets of HIF-1α [34]. The data showed that vitamin C could inhibit the expression of GLUT1 and VEGF (Figure 2B). In addition, we measured the transcriptional activity of HIF-1α using luciferase reporter assays and found a significant decrease in HIF-1α activity in HLE-B3 cells when treated with vitamin C (Figure 2C).
Figure 2.
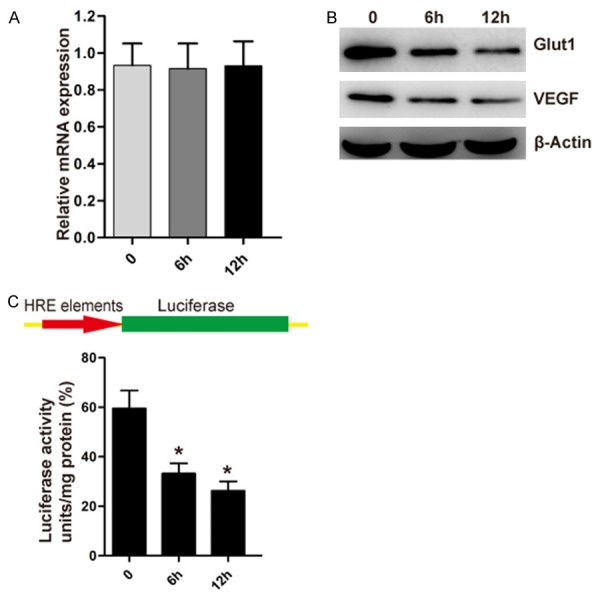
Vitamin C inhibits the activity of HIF-1α in lens epithelial cells. A. With the treatment of vitamin C for 6 or 12 h, the levels of HIF-1α mRNA were examined by real-time PCR from HLE-B3 cells. B. With the treatment of vitamin C for 6 or 12 h, the levels of GLUT1, VEGF and β-actin protein were examined by Western Blot from HLE-B3 cells. C. HLE-B3 cells stably expressing HRE-driven luciferase expression vector were treated with vitamin C for 6 or 12 h. Relative luciferase units were subsequently measured using a luminometer; *P<0.02 compared with the control untreated group.
Vitamin C increases the proline hydroxylation of HIF-1α
To further investigate the affection of vitamin C to HIF-1α, we detected the levels of proline hydroxylation of HIF-1α in the present of proteasomal inhibitor MG-132 and vitamin C with hydroxylation-specific antibodies against the two proline-hydroxylation site sat both VHL binding sites (Pro402 and Pro564). We found that vitamin C significantly increased the levels of proline hydroxylation of HIF-1α compared to the control untreated HLE-B3 cells (Figure 3A). We then introduced point mutations of prolyl residues 402 and 564 (Pro402 and Pro564) of HIF-1α (Figure 3B). Furthermore, luciferase reporter assays showed that vitamin C did not significantly decrease the activity of mutant HIF-1α (Figure 3C). These findings imply that vitamin C inhibits the activity and proline hydroxylation of the wild-type HIF-1. The up-regulated levels of proline-hydroxylated forms of HIF-1α determine the unstable forms of HIF-1α [35]. Thus, vitamin C could destabilize HIF-1α protein of lens epithelial cells.
Figure 3.
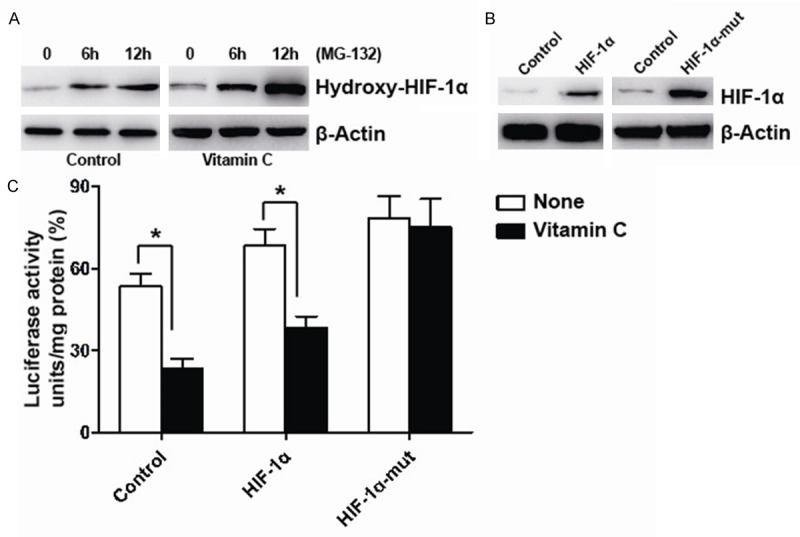
Vitamin C increases the proline hydroxylation of HIF-1α. A. HLE-B3 cells were pretreated with MG-132 and then treated without or with vitamin C plus MG-132 for 6 or 12 h, the levels of proline hydroxylation of HIF-1α (hydroxy P402/564) and β-actin were examined by Western Blot from HLE-B3 cells. B. HLE-B3 cells were stably transfected with wild-type HIF-1α/proline-mutant HIF-1α or negative control. Expression of HIF-1α and β-actin were examined by Western Blot. C. HLE-B3 cells stably co-expressing an HRE-driven luciferase expression vector and wild-type HIF-1α or proline-mutant HIF-1α or negative control were treated without or with vitamin C for 12 h. Relative luciferase units were subsequently measured using a luminometer; *P<0.008 compared with each of the control untreated group.
Vitamin C inhibits HIF-1α-induced proliferation and migration of lens epithelial cells
HIF-1α was reported to stimulate the proliferation and migration [36]. Here, we found that overexpression of wild-type HIF-1α or proline-mutant HIF-1α promoted the proliferation and migration of HLE-B3 cells (Figure 4A and 4B). Next, we found that vitamin C significantly inhibited wild-type HIF-1α-induced proliferation and migration of lens epithelial cells (Figure 4A and 4B). Differently, vitamin C could not affect the proliferation and migration of lens epithelial cells induced by proline-mutant HIF-1α. These results further support that vitamin C inhibits HIF-1α-induced proliferation and migrationofHLE-B3 cells by enhancing the proline hydroxylation and then destabilizing HIF-1α protein.
Figure 4.
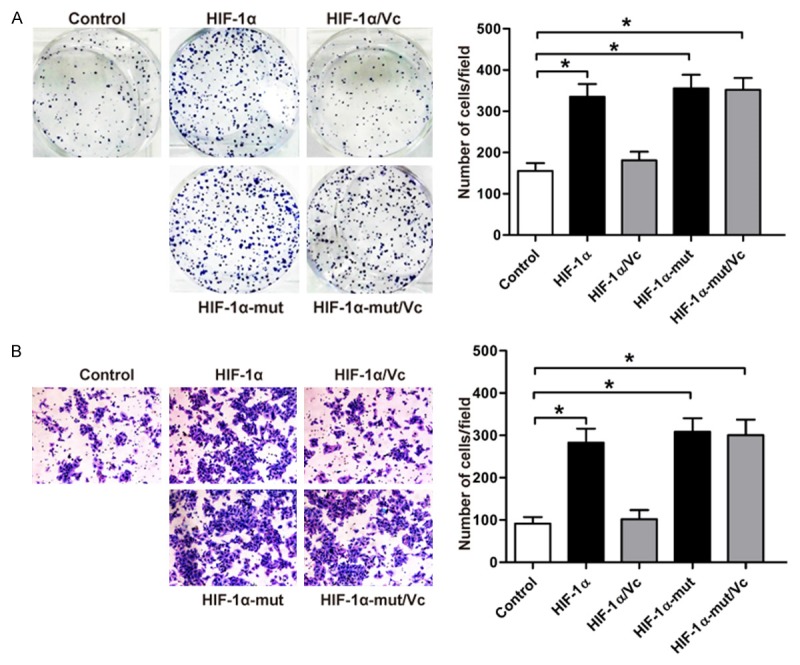
Vitamin C inhibits HIF-1α-induced proliferation and migration of lens epithelial cells. HLE-B3 cells were stably transfected with wild-type HIF-1α or proline-mutant HIF-1α or negative control. Cells expressing wild-type HIF-1α or proline-mutant HIF-1α were treated with PBS vehicle control or vitamin C (100 μM) during the experiments. A. Representative photographs of colony formation in HLE-B3 cells are shown on the left. Quantification of the colony formation rate is shown on the right; *P<0.003 compared with the control group. B. The migration potentials of HLE-B3 cells with different treatments were determined by transwell assay. The representative images are shown on the left, and the quantification of selected fields is shown on the right; *P=0.002 compared with the control group.
Vitamin C inhibits HIF-1α-induced epithelial-mesenchymal transition
HIF-1α could promote the EMT process by up-regulating the expression of TWIST [18]. We then stably transfected HLE-B3 cells with wild-type HIF-1α or proline-mutant HIF-1α. We found that overexpression of wild-type HIF-1α or proline-mutant HIF-1α could promote EMT-associated morphology change in HLE-B3 cells (Figure 5A). Furthermore, vitamin C could inhibit epithelial-mesenchymal transformation induced by wild-type HIF-1α but not the proline-mutant HIF-1α (Figure 5A). To confirm the effect of vitamin C on epithelial-mesenchymal transformation, we examined the expression of TWIST on the treatment of vitamin C in HLE-B3 cells. The results showed that vitamin C decreased the expression of TWIST (Figure 5B). Twist is a transcriptional repressor of E-cadherin gene expression. We also found the expression of E-cadherin was up-regulated by the stimulation of vitamin C (Figure 5B). Meanwhile, the expression of mesenchymal markers N-cadherin was also found decreased (Figure 5B). Furthermore, we found that overexpression of wild-type HIF-1α or proline-mutant HIF-1α could up-regulate the expression of TWIST (Figure 5C). We also found that vitamin C inhibited the expression of TWIST induced by the overexpression of wild-type HIF-1α but not the proline-mutant HIF-1α. The expression of E-cadherin was also found to be conversely correlated with the expression of TWIST (Figure 5C).
Figure 5.
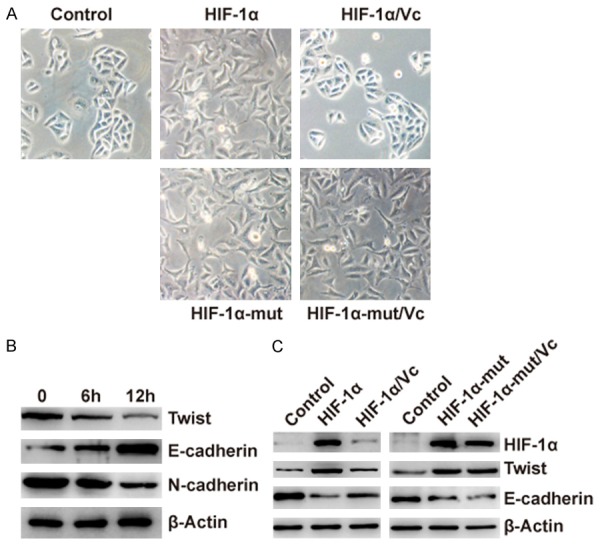
Vitamin C inhibits HIF-1α-induced epithelial-mesenchymal transition. A. HLE-B3 cells were stably transfected with wild-type HIF-1α or proline-mutant HIF-1α or negative control. HLE-B3 cells stably expressing wild-type HIF-1α or proline-mutant HIF-1α were treated with PBS vehicle control or vitamin C (100 μM) for 24 h. Morphological changes supporting EMT were observed in HLE-B3 cells expressing wild-type HIF-1α or proline-mutant HIF-1α. No morphological changes supporting EMT were observed in HLE-B3 cells expressing wild-type HIF-1α treated with vitamin C. B. HLE-B3 cells were treated without or with vitamin C for 6 or 12 h. Expression of E-cadherin, N-cadherin and β-actin were examined by Western Blot. C. HLE-B3 cells were stably transfected with negative control or wild-type HIF-1α/proline-mutant HIF-1α or wild-type HIF-1α/proline-mutant HIF-1α plus vitamin C (100 μM) for 12 h. Expression of HIF-1α, TWIST, E-cadherin and β-actin were examined by Western Blot.
Discussion
Our data show that vitamin C increases the levels of proline-hydroxylated HIF-1α, which is the unstable forms of HIF-1α, in lens epithelial cells. Meanwhile, the expression levels of HIF-1α target protein such as GLUT1 and VEGF were also found to be inhibited by vitamin C. TWIST (a basic helix-loop-helix transcription factor), the key inducers of EMT that repress the epithelial marker E-cadherin expression, was reported to be up-regulated by HIF-1α [18]. Our results indicate that the expression of TWIST can be also inhibited by vitamin C in HLE-B3 cells. Moreover, the expression of E-cadherin can be up-regulated by vitamin C. This is conversely correlated with the expression of TWIST. Accordingly, EMT-associated morphology change was also found by the stimulation by vitamin C in HLE-B3 cells. HIF-1α was reported to increase the proliferation and migration of different types of cells [36]. Here, we also found vitamin C inhibit the proliferation and migration of HLE-B3 cells.
HIF-1α is targeted for ubiquitin-dependent degradation by an E3 ubiquitin ligase VHL. Proline hydroxylation of HIF-1α by prolyl-hydroxylase (PHD1/2/3) is required for the interaction of HIF-1α with VHL [37]. Vitamin C acts as an electron-donor keeping Fe iron in the ferrous (Fe2+) state which is necessary to achieve full activity of prolyl-hydroxylase [25]. Point mutations of prolyl residues 402 and 564 (Pro402 and Pro564) of HIF-1α destroy the proline hydroxylation and stabilize the HIF-1α protein [38]. HIF-1α protein is unstable and undetectable under normoxia [32,33]. Instead, we detected the levels of HIF-1α target protein under the treatment of vitamin C. We found that the expression levels of HIF-1α target protein such as GLUT1 and VEGF were inhibited by vitamin C. Similarly, the HIF-1α activity in HLE-B3 cells was also shown to be inhibited with the treated of vitamin C using hypoxia response element (HRE) luciferase reporter assays. In the present study, we also found that the regulation of vitamin C to the activity of HIF-1α was lost in proline-mutant HIF-1α (402/564). Additionally, the regulation of vitamin C to the proliferation, migration, and epithelial-mesenchymal transition (EMT) of the residual lens epithelial cells also depends on the proline hydroxylation of HIF-1α. These findings show that vitamin C inhibit the proliferation, migration and Epithelial-Mesenchymal-Transition of lens epithelial cells by destabilizing HIF-1α.
PCO is mainly caused by the proliferation, migration, and epithelial-mesenchymal transition of the residual lens epithelial cells after cataract surgery [1,39]. A great many of genes are involved in the epithelial-mesenchymal transition of lens epithelial cells [6,40-42]. Moreover, some inhibitors targeting to these genes were found to inhibit the proliferation, migration, and epithelial-mesenchymal transition of the lens epithelial cells [5,43,44]. Recent studies have suggested the critical role of HIF-1α to the epithelial-mesenchymal transition [18-20]. Vitamin C was reported to reduce the risk of forming a cataract [3,27-29]. Here, vitamin C was proved to destabilize HIF-1α and inhibit the proliferation, migration of lens epithelial cells. Hence, vitamin C represents its possibility for therapeutic intervention and the prevention of PCO after cataract surgery. While further studies will be required to evaluate the effect of vitamin C in animal model of PCO.
Acknowledgements
This work was supported by the grants Shanxi Province Science and Technology Development Research Project (2012K19-05-05).
Disclosure of conflict of interest
None.
References
- 1.Raj SM, Vasavada AR, Johar SR, Vasavada VA, Vasavada VA. Post-operative capsular opacification: a review. Int J Biomed Sci. 2007;3:237–250. [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Montanes J, Alvarez A, Maldonado MJ. Objective quantification of posterior capsule opacification after cataract surgery, with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:3999–4006. doi: 10.1167/iovs.04-1531. [DOI] [PubMed] [Google Scholar]
- 3.Dherani M, Murthy GVS, Gupta SK, Young IS, Maraini G, Camparini M, Price GM, John N, Chakravarthy U, Fletcher AE. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a north Indian population. Invest Ophthalmol Vis Sci. 2008;49:3328–3335. doi: 10.1167/iovs.07-1202. [DOI] [PubMed] [Google Scholar]
- 4.Su Y, Wang F, Yan Q, Teng Y, Cui H. Inhibition of proliferation of rabbit lens epithelial cells by S-phase kinase-interacting protein 2 targeting small interfering RNA. Mol Vis. 2010;16:907–915. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Xiao W, Chen W, Luo L, Ye S, Liu Y. The epigenetic modifier trichostatin A, a histone deacetylase inhibitor, suppresses proliferation and epithelial-mesenchymal transition of lens epithelial cells. Cell Death Dis. 2013;4:e884. doi: 10.1038/cddis.2013.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao XL, Song H, Chen Z, Tang X. Wnt3a promotes epithelial-mesenchymal transition, migration, and proliferation of lens epithelial cells. Mol Vis. 2012;18:1983–1990. [PMC free article] [PubMed] [Google Scholar]
- 7.Saika S. TGFbeta pathobiology in the eye. Lab Invest. 2006;86:106–115. doi: 10.1038/labinvest.3700375. [DOI] [PubMed] [Google Scholar]
- 8.Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK. The roles of alphaV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med. 2014;18:656–670. doi: 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gervasi M, Bianchi-Smiraglia A, Cummings M, Zheng Q, Wang D, Liu S, Bakin AV. JunB contributes to Id2 repression and the epithelial-mesenchymal transition in response to transforming growth factor-beta. J Cell Biol. 2012;196:589–603. doi: 10.1083/jcb.201109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 13.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R, Yang Q, Lee JD. BMK1 kinase suppresses epithelial-mesenchymal transition through the Akt/GSK3beta signaling pathway. Cancer Res. 2012;72:1579–1587. doi: 10.1158/0008-5472.CAN-11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim J, Cha HJ. Loss of E-cadherin activates EGFRMEK/ERK signaling, which promotes invasion via the ZEB1/MMP2 axis in non-small cell lung cancer. Oncotarget. 2013;4:2512–2522. doi: 10.18632/oncotarget.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YN, Liu Y, Lee HJ, Hsu YH, Chen JH. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol. 2008;28:7096–7108. doi: 10.1128/MCB.00449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 20.Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 21.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 23.Park MH, Choi KY, Jung Y, Min DS. Phospholipase D1 protein coordinates dynamic assembly of HIF-1alpha-PHD-VHL to regulate HIF-1alpha stability. Oncotarget. 2014;5:11857–72. doi: 10.18632/oncotarget.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Q, Liu M, Han WQ, Li PL, Wang Z, Li N. Overexpression of HIF prolyl-hydoxylase-2 transgene in the renal medulla induced a salt sensitive hypertension. J Cell Mol Med. 2012;16:2701–2707. doi: 10.1111/j.1582-4934.2012.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010;70:5749–5758. doi: 10.1158/0008-5472.CAN-10-0263. [DOI] [PubMed] [Google Scholar]
- 26.Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1 alpha differentially in cancer and ischemia. Mol Cell Biol. 2008;28:5106–5119. doi: 10.1128/MCB.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida M, Takashima Y, Inoue M, Iwasaki M, Otani T, Sasaki S, Tsugane S, Group JS. Prospective study showing that dietary vitamin C reduced the risk of age-related cataracts in a middle-aged Japanese population. Eur J Nutr. 2007;46:118–124. doi: 10.1007/s00394-006-0641-8. [DOI] [PubMed] [Google Scholar]
- 28.Pastor-Valero M. Fruit and vegetable intake and vitamins C and E are associated with a reduced prevalence of cataract in a Spanish Mediterranean population. BMC Ophthalmol. 2013;13:52. doi: 10.1186/1471-2415-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA. Development of cataract and associated risk factors: the Visual Impairment Project. Arch Ophthalmol. 2006;124:79–85. doi: 10.1001/archopht.124.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Venturelli S, Sinnberg TW, Berger A, Noor S, Levesque MP, Bocker A, Niessner H, Lauer UM, Bitzer M, Garbe C, Busch C. Epigenetic impacts of ascorbate on human metastatic melanoma cells. Front Oncol. 2014;4:227. doi: 10.3389/fonc.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikirova NA, Casciari JJ, Riordan NH. Ascorbate inhibition of angiogenesis in aortic rings ex vivo and subcutaneous Matrigel plugs in vivo. J Angiogenes Res. 2010;2:2. doi: 10.1186/2040-2384-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toh YM, Li TK. Mitoxantrone inhibits HIF-1alpha expression in a topoisomerase IIindependent pathway. Clin Cancer Res. 2011;17:5026–5037. doi: 10.1158/1078-0432.CCR-11-0235. [DOI] [PubMed] [Google Scholar]
- 34.Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, Simon MC. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–282. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Bienes-Martinez R, Ordonez A, Feijoo-Cuaresma M, Corral-Escariz M, Mateo G, Stenina O, Jimenez B, Calzada MJ. Autocrine stimulation of clear-cell renal carcinoma cell migration in hypoxia via HIFindependent suppression of thrombospondin-1. Sci Rep. 2012;2:788. doi: 10.1038/srep00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 38.Andre H, Pereira TS. Identification of an Alternative Mechanism of Degradation of the Hypoxia-inducible Factor-1 alpha. J Biol Chem. 2008;283:29375–29384. doi: 10.1074/jbc.M805919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian F, Dong L, Zhou Y, Shao Y, Li W, Zhang H, Wang F. Rapamycin-Induced apoptosis in HGF-stimulated lens epithelial cells by AKT/mTOR, ERK and JAK2/STAT3 pathways. Int J Mol Sci. 2014;15:13833–13848. doi: 10.3390/ijms150813833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamiya S, Liu LH, Kaplan HJ. Epithelial-Mesenchymal Transition and Proliferation of Retinal Pigment Epithelial Cells Initiated upon Loss of Cell-Cell Contact. Invest Ophthalmol Vis Sci. 2010;51:2755–2763. doi: 10.1167/iovs.09-4725. [DOI] [PubMed] [Google Scholar]
- 41.Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WWY, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho HJ, Yoo JY. Rho activation is required for transforming growth factor-beta-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol Int. 2007;31:1225–1230. doi: 10.1016/j.cellbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Dong N, Xu B, Benya SR, Tang X. MiRNA-26b inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Mol Cell Biochem. 2014;396:229–238. doi: 10.1007/s11010-014-2158-4. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa M, Hishikawa K, Marumo T, Fujita T. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta 1 in human renal epithelial cells. J Am Soc Nephrol. 2007;18:58–65. doi: 10.1681/ASN.2005111187. [DOI] [PubMed] [Google Scholar]


