Abstract
Objective: The revised International Prognostic Index (R-IPI) aids in predicting the prognosis of patients with diffuse large B cell lymphoma (DLBCL), but R-IPI yields no significant differences in assessing different subtypes of DLBCL. It is necessary to identify patients with a high-risk of DLBCL and alternative therapy should be delivered as early as possible. Methods: In total, 144 patients newly diagnosed with DLBCL including 63 GCB-DLBCL and 81 non-GCB-DLBCL and 30 healthy controls were enrolled. Peripheral monocytic myeloid-derived suppressor cells (M-MDSC) (CD14+HLA-DRlow/-) were detected by flow cytometry and the percentage of monocytes (MΦ) was evaluated by completed blood count (CBC). The correlation between M-MDSC% and MΦ% was statistically analyzed. Results: Compared with healthy controls, significant increase was observed in M-MDSC% and MΦ% in DLBCL patients (both P<0.001). Significant difference of M-MDSC% was found between GCB-DLBCL and non-GCB-DLBCL patients in both poor (P<0.001) and very good groups (P=0.03), whereas no statistical significance in the good group (P>0.05). The MΦ% in non-GCB-DLBCL patients was significantly higher than that in GCB-DLBCL counterparts merely in the poor group (P<0.001). Positive correlation was noted between MΦ% and M-MDSC in all DLBCL patients rather than in healthy controls (r=0.227 P=0.229). Conclusion: The percentage of peripheral MΦ was positively correlated with M-MDSC% in patients with different subtypes and risks of DLBCL. Peripheral MΦ% and M-MDSC% combined with R-IPI score may be useful for predicting the prognosis of patients newly-diagnosed with DLBCL.
Keywords: Monocytic myeloid-derived suppressor cell, monocyte, diffuse large B-cell lymphoma, revised international prognostic index
Introduction
Diffuse large B cell lymphoma (DLBCL) [1] is the most common and aggressive type of non-Hodgkin lymphoma with complex histological, immunophenotypic and cytogenetic features. Thus, it is extremely challenging to yield accurate clinical prognosis. The causes of DLBCL are not well understood. DLBCL constantly arises from normal B cells, but it can also represent a malignant transformation of other types of lymphoma or leukemia. An underlying immunodeficiency has been proven to be a significant risk factor [2]. Infection with Epstein-Barr virus has been found to promote the development of certain subtypes of DLBCL [3]. DLBCL is diagnosed by tissue biopsy of the tumor through a biopsy, and then examining this tissue under a microscope [4]. Several molecular subtypes of DLBCL have been identified including germinal B-cell-like DLBCL, activated B-cell-like DLBCL and primary mediastinal B-cell lymphoma, each having a different clinical presentation and prognosis. However, conventional treatment is chemotherapy, often in combination with an antibody targeted at the cancer cells. Through these treatments, more than half of patients with DLBCL can be cured, and overall 5-year survival for elderly adults is approximately 58% [5].
The International Prognostic Index (IPI) is designed to predict the prognostic outcomes of DLBCL patients based on clinical parameters or tumor characteristics of the patients. In 2007 [6], these factors were redistributed into the revised IPI (R-IPI) for the DLBCL patients who were treated with immuno-chemotherapy (R-CHOP). Recent studies [7,8] have demonstrated that the ratio of lymphocyte to monocyte (LMR) calculated from complete blood count (CBC) at the time DLBCL was diagnosed could predict the clinical outcomes of DLBCL patients undergoing R-CHOP, especially in the population with a high risk of DLBCL. LMR is utilized as a simple biomarker combining with an estimate of host immune homeostasis and tumor microenvironment. Luis et al. concluded that an ALC/AMC-DX ratio of ≥1.1 was associated with overall survival, lymphoma-specific survival, progression-free survival, and time to progression [9].
In recent years, as a subpopulation of immunosuppressive cells [10-13], the myeloid-derived suppressor cells (MDSCs) have been described in a variety of cancers. Human MDSCs are a heterogeneous population composing of cells at several differentiation stages of the myeloid lineage (CD33+CD11b+Lin-HLA-DR-/low). Different types of tumors harbor distinct subsets of MDSCs, which can be further divided into CD15+ granulocytic (CD15+HLA-DR-/low G-MDSC) and CD14+ monocytic (CD14+HLA-DR-/low M-MDSC) subsets. Recent study [14] has identified the existence of a monocytic subset of MDSCs with the phenotype CD14+HLA-DR-/low in patients with non-Hodgkin’s lymphoma (NHL), which function to suppress the proliferation of normal T cells.
The purpose of this study was to investigate the proportion of peripheral M-MDSC and MΦ% in patients with different subtypes and risks of DLBCL, aiming to analyze the correlation between M-MDSC and MΦ% and provide prognostic significance at diagnosis by combining with R-IPI score.
Materials and methods
Patients
In this study, 144 DLBCL patients were enrolled, 75 male and 69 female, aged 20-80 years (median age: 61 years) upon diagnosis, and divided into germinal center B-cell-like DLBCL (GCB-DLBCL, n=63) and non-germinal center B-cell-like DLBCL (non-GCB-DLBCL, n=81) groups assessed by immunohistochemistry (IHC) according to the Choi algorithm. Clinical and laboratory data were obtained from consecutive medical records of patients with newly diagnosed DLBCL at our institution during June 2011 and October 2014. Heparinized venous blood samples were collected from patients newly diagnosed with DLBCL (n=144) and healthy controls (n=30). Patients with positive HIV, transformed lymphoma or any associated immunodeficiency disease were excluded. According to the R-IPI, all patients were classified into three groups: very good (R-IPI=0: 9 GCB-DLBCL 12 non-GCB-DLBCL), good (R-IPI=1 or 2: 27 GCB-DLBCL, 30 non-GCB-DLBCL) and poor prognosis groups (R-IPI>2: 27 GCB-DLBCL, 39 non-GCB-DLBCL). Clinical characteristics of the patients at time of diagnosis are listed in Table 1. Written informed consent was obtained from all participants. This study adhered to the Declaration of Helsinki and was approved by the Clinic Institutional Review Board of the Second Hospital of Lanzhou University.
Table 1.
General data of DLBCL patients and healthy controls
| Healthy individuals | DLBCL patients | |
|---|---|---|
| Age (year) | 61 (20-80) | 60 (21-80) |
| Gender | 15 (50.0%) for male | 75 (52.1%) for male |
| Histological subtype | ||
| R-IPI (GCB-DLBCL) | ||
| Very good prognosis (0) | 9 (6.3%) | |
| Good prognosis (1, 2) | 27 (18.8%) | |
| Poor prognosis (3, 4, 5) | 27 (18.8%) | |
| R-IPI (non-GCB-DLBCL) | ||
| Very Good prognosis (0) | 12 (8.3%) | |
| Good prognosis (1, 2) | 30 (20.8%) | |
| Poor prognosis (3, 4, 5) | 39 (27.1%) | |
| MΦ (%) (GCB-DLBCL) | ||
| <0.08 | 0.06±0.011 | 24 (16.7%) |
| ≥0.08 | 0.09±0.010 | 39 (27.1%) |
| MΦ (%) (non-GCB-DLBCL) | ||
| <0.08 | 0.07±0.007 | 27 (18.8%) |
| ≥0.08 | 0.12±0.042 | 54 (37.5%) |
| MΦ (%) | ||
| <0.08 | 0.05±0.016 (27 (90.0%)) | |
| ≥0.08 | 0.08±0.00 (3 (10.0%)) |
Peripheral MΦ% and immunophenotype of M-MDSC
The proportion of peripheral MΦ was calculated from the routine automated CBC at the time of diagnosis in 144 DLBCL patients and 30 healthy counterparts. Monocytosis was defined as the MΦ% was ≥8% higher, which was the upper limit of monocytes in routine blood test (3%-8%). Peripheral blood sampling was conducted for subsequent surface staining and flow cytometry. M-MDSC was identified as CD14+HLA-DR-/low phenotype by flow cytometry in agreement with the findings obtained by Lin et al. [13]. M-MDSC% and MΦ% in the peripheral blood from patients with different subtypes and risks of DLBCL and age-matched healthy counterparts were calculated.
Statistical analysis
All data analyses were performed using SPSS 20.0 statistical software (SPSS, Chicago, IL, USA). M-MDSC% and MΦ% were expressed as x̅ ± standard deviation (S.D.). Comparison of the mean values among different groups if the data were normally distributed was assessed by one-way ANOVA. Pair-wise comparison of the difference between two groups was evaluated by LSD-t test. Pearson correlation coefficient was used to describe the correlation among different quantitative variables. A P value <0.05 was considered to be statistically significant.
Results
Patients’ characteristics
All patients were divided into the GCB (63/43.8%) and non-GCB subgroups (81/56.2%). According to the R-IPI, 21 (14.6%) patients were assigned in the very good group, 57 (39.6%) in the good and 66 (45.8%) in poor prognosis groups. Ninety three (64.6%) patients (GCB-DLBCL 27.1% vs non-GCB-DLBCL 37.5%) and 3 controls (10%) had peripheral MΦ%≥0.08, and 51 (35.4%) patients and 27 (90%) controls presented with MΦ%<0.08. When newly diagnosed, the median MΦ% was 0.078 (range: 0.04-0.12) in GCB-DLBCL patients and 0.104 (range: 0.05-0.19) in non-GCB-DLBCL counterparts. Patients’ basic characteristics are shown in Table 1.
Comparison of peripheral M-MDSC% and MΦ% between DLBCL patients and healthy controls
M-MDSC% significantly differed between DLBCL patients and normal counterparts (P<0.001), whereas no significant difference was noted between GCB- and non-GCB-DLBCL patients (P=0.076) (Figure 1). Stratification analysis showed that M-MDSC% significantly differed between non-GCB- and GCB-DLBCL patients in the poor (P<0.001) and very good groups (P=0.03) rather than in the good group (P>0.05). Compared with healthy controls, the M-MDSC% of both GCB- and non-GCB-DLBCL patients was significantly elevated in three risk groups (all P<0.001). Similar to non-GCB-DLBCL counterparts, the M-MDSC% in the GCB-DLBCL patients significantly differed among each risk group (P<0.001) (Table 2).
Figure 1.
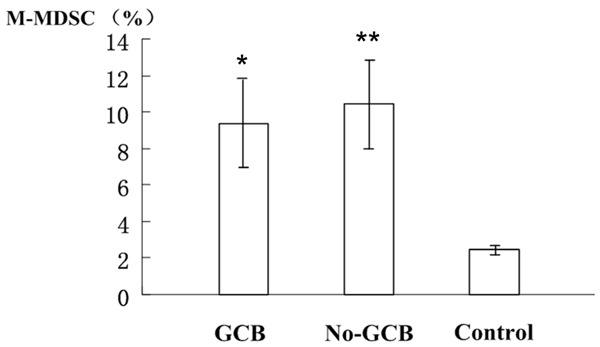
M-MDSC% in subtype DLBCL patients and healthy controls. *Denotes statistical significance between GCB-DLBCL patients and healthy controls (P<0.001); **represents statistical significance between non-GCB-DLBCL patients and healthy controls (P<0.001).
Table 2.
Stratification analysis of M-MDSC and MΦ in subtype DLBCL patients and healthy controls
| GCB-DLBCL (n=63) | Non-GCB-DLBCL (n=81) | Control (n=30) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Very good prognosis (9) | Good prognosis (27) | Poor prognosis (27) | Very good prognosis (12) | Good prognosis (30) | Poor prognosis (39) | ||
| M-MDSC (%) | 5.53±1.00 | 8.25±0.71 | 11.08±1.49 | 6.98±0.86 | 8.84±0.50 | 12.68±0.96 | 2.46±0.26 |
| MΦ (%) | 0.050±0.010 | 0.073±0.015 | 0.089±0.013 | 0.063±0.010 | 0.077±0.011* | 0.139±0.042* | 0.053±0.018 |
denotes statistical significance compared with GCB-DLBCL counterparts.
Compared with healthy controls, peripheral MΦ% was significantly increased in both GCB- (P=0.013) and non-GCB-DLBCL patients (P<0.001), and MΦ% in non-GCB-DLBCL patients was significantly higher than that in the GCB-DLBCL counterparts (P=0.006) (Figure 2). Stratification analysis revealed significant difference between non-GCB and GCB patients in the poor group (P<0.001) rather than in the good and very good groups (both P>0.05). In the GCB-DLBCL patients, the MΦ% in the poor group was significantly elevated compared with that in the very good group (P=0.010), whereas no significant differences were noted between the poor and good prognosis groups and between the good and very good groups (both P>0.05). In the non-GCB-DLBCL patients, the MΦ% in the poor group was dramatically increased than that in the good and very good groups (both P<0.001), whereas no significant difference was noted between the good and very good groups (P>0.05). Compared with healthy controls, the MΦ% was significantly higher in the poor and good groups (both P<0.001), whereas no significant difference was observed in the very good group in the non-GCB-DLBCL counterparts (P>0.05), as illustrated in Table 2. The MΦ% of GCB group in poor group were higher than healthy controls though there were no difference between good group, very good group and controls.
Figure 2.

MΦ% in subtype DLBCL patients and healthy controls. *DENOTES statistical significance between GCB-DLBCL patients and healthy controls (P=0.013); △represents statistical significance between non-GCB-DLBCL patients and healthy controls (P<0.001).
Correlation between peripheral M-MDSC and MΦ% in DLBCL patients and healthy controls
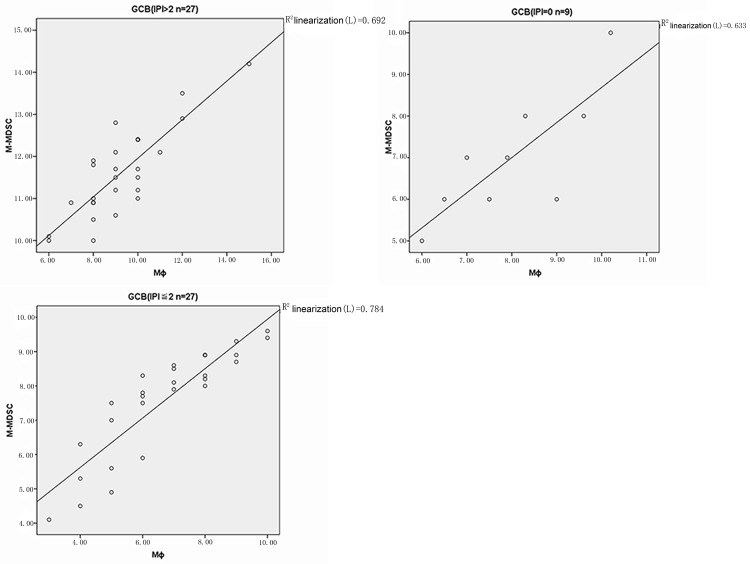
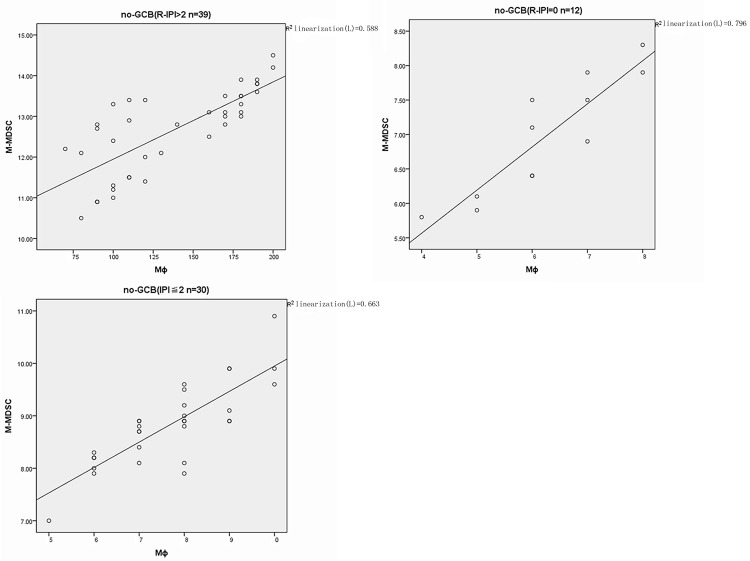
In GCB-DLBCL patients, the proportion of MΦ was significantly correlated with the increased percentage of M-MDSC in the poor, good and very good prognosis groups (r=0.832 P=0.000; r=0.886 P=0.000; r=0.795 P=0.010) (Figure 3). In the non-GCB controls, similar findings were obtained in the poor, good and very good prognosis groups (r=0.767 P=0.000; r=0.814 P=0.000; r=0.892 P=0.000) (Figure 4). No significant correlation was observed between the MΦ% and M-MDSC in the healthy controls (r=0.215 P=0.255) (Figure 5).
Figure 3.
Correlation analysis between M-MDSC% and MΦ% in different risks of GCB-DLBCL patients.
Figure 4.
Correlation analysis between M-MDSC% and MΦ% in different risk of non-GCB-DLBCL patients.
Figure 5.
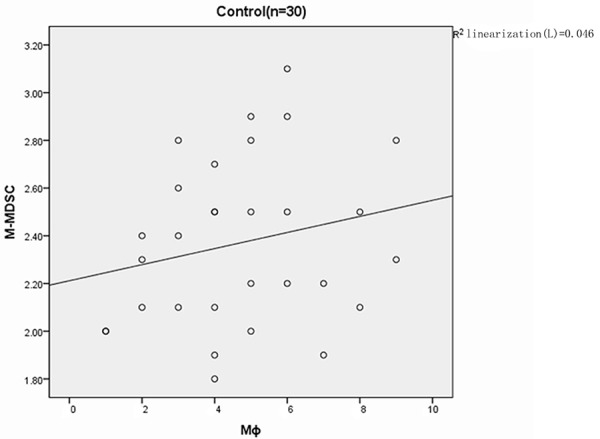
Correlation analysis between M-MDSC% and MΦ% in healthy controls.
Discussion
DLBCL is characterized with diverse clinical presentations and pathological features. During the widespread application of rituximab therapy, R-CHOP has been considered as the gold standard immunochemotherapy against DLBCL. R-IPI scoring system [6] has been widely employed as a prognostic index in DLBCL patients of chemoimmunotherapy since the year of 2007. However, the role of host immunity has not been taken into consideration when using R-IPI and no statistical significance has been noted in the score of R-IPI among different subtypes of DLBCL. Recent studies [7,8] have demonstrated that tumor microenvironment and host immunity play a pivotal role in the therapeutic outcomes of DLBCL. Hence, it is of high necessity to identify the patients with a high-risk of DLBCL (at least two factors of poor prognosis) during the course of treatment and alternative therapy should be administered accordingly.
Gene-expression profiling [15] showed that tumor-infiltrating lymphocytes and myeloid-derived cells collectively play an important role in the pathogenesis of lymphoma and exert an impact upon the prognosis of lymphoma due to their functions in the innate and adaptive immune responses. In 2011, Wilcox et al. [8] reported that the combination of the absolute lymphocyte count and the absolute monocyte count at diagnosis exerted a significant effect upon the prognosis of DLBCL patients independently of the IPI. More evidence has demonstrated a correlation between LMR and the prognostic outcomes of DLBCL patients [16,17]. In this study, the MΦ% was analyzed based upon the parameter of complete blood count and demonstrated that the proportion of MΦ in non-GCB and GCB patients with monocytosis was apparently higher compared with that in healthy donors. The mean value of MΦ% in the non-GCB patients was significantly increased than that in GCB-DLBCL and healthy counterparts. Stratification analysis demonstrated that statistical significance existed between non-GCB- and GCB-DLBCL patients in the poor group (P=0.001) rather than in the good and very good groups. In the GCB-DLBCL patients, the MΦ% in the poor group was significantly enhanced than that in the very good group, no significant differences were found between the poor and good groups, and between the good and very good groups. In non-GCB-DLBCL patients, the ratio of MΦ% in the poor group was significantly increased compared with those in the good and very good groups, whereas no statistical significance was found between the poor and very good groups. Taken together, peripheral MΦ% of CBC probably had disadvantages in terms of evaluating the prognostic profile in conjunction with the R-IPI for patients with non-GCB-DLBCL or GCB-DLBCL patients in the poor group. Whether peripheral MΦ% can stratify the subtypes of GCB-DLBCL in the good group remains to be elucidated.
The high incidence of lymphoma reported in the immunocompromised patients indicates the importance of host immune system in the occurrence of lymphoma. Human MDSCs are a population of immune-suppressive cells (CD33+CD11b+HLA-DRlo/-) originating from circulating myeloid progenitor and immature myeloid cells, as precursors of DCs, macrophage and/or granulocytes. The activated MDSCs could suppress the anti-cancer immunity of the host via multiple underlying mechanisms and promote the growth of tumors. It is also associated with the size and grading of the malignancy [18-21].
In this study, the proportion of peripheral M-MDSC in the DLBCL patients was significantly elevated compared with that in the healthy controls, while no statistical significance was noted in terms of M-MDSC between the GCB- and non-GCB-DLBCL patients. Stratification analysis revealed that the M-MDSC significantly differed between the GCB- and non-GCB-DLBCL patients from the poor and very good prognosis groups, whereas no statistical significance was noted in the good prognosis group. M-MDSC in the GCB-DLBCL patients significantly differed among the poor, good and very good prognosis subgroups, and similar findings were noted in the non-GCB-DLBCL counterparts. M-MDSC may possess similar prognostic value as R-IPI in DLBCL patients at diagnosis and is likely to be a stratified factor combined with R-IPI for patients newly-diagnosed with DLBCL in the poor and very good subgroups. The optimal cut-off values for M-MDSC% and MΦ% deserve further studies with a larger sample size.
Peripheral MΦ% of CBC was positively correlated with M-MDSC in both GCB- and non-GCB-DLBCL patients with different risk of DCBCL at diagnosis, whereas no significant correlation was noted in the healthy controls. Considering objective, reproducible, low cost and accessible advantages of the test, detection of peripheral MΦ% will probably be one of the immune biomarkers reflecting the immune status of the host, applicable to conventional examination. Flow cytometry has a higher specificity in detecting M-MDSC%, whereas it is more expressive. Hence, the combination of these two methods may yield more convincing and meaningful outcomes. The mutual interaction between peripheral MΦ and M-MDSC in the DLBCL patient needs to be further evaluated in a larger population.
To sum up, peripheral MΦ% and M-MDSC% significantly differed in different risk and subtypes of DLBCL patients at diagnosis, especially in the non-GCB-DLBCL patients from the poor group. Peripheral MΦ% was positively correlated with M-MDSC in patients newly-diagnosed with DLBCL. Combined with R-IPI score at the onset of DLBCL, MΦ% and M-MDSC%, especially MΦ% may be utilized as an indicator of poor prognosis of DLBCL patients in routine clinical practice to identify the high-risk population. Moreover, M-MDSC probably possessed more significance in evaluating the prognosis between GCB- and non-GCB-DLBCL patients in the very good prognosis group.
Acknowledgements
This project was supported by grants from the Fundamental Research Funds for the Central Universities of Lanzhou University (Lzujbky-2012-198).
Disclosure of conflict of interest
None.
References
- 1.Habermann T. New developments in the management of diffuse large B-cell lymphoma. Hematology. 2012;17(Suppl 1):S93–7. doi: 10.1179/102453312X13336169156014. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Jaffe ES. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2010. p. 233. [Google Scholar]
- 3.Goldman LS, Andrew I. Goldman’s Cecil Medicine. 24th edition. 2012. p. 1222. [Google Scholar]
- 4.Akyurek N, Uner A, Benekli M, Barista I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2011;118:4173–4183. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 5.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E, Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht C, Reyes F, Coiffier B. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients with Diffuse Large B-Cell Lymphoma: A Study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 6.Sehn L, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 7.Porrata L, Ristow K, Habermann T, Witzig TE, Colgan JP, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski G, Thompson CA, Markovic SN. Peripheral blood absolute lymphocyte/monocyte ratio during rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone treatment cycles predicts clinical outcomes in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;19:19. doi: 10.3109/10428194.2014.893313. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox R, Ristow K, Habermann T, Inwards DJ, Micallef IN, Johnston PB, Colgan JP, Nowakowski GS, Ansell SM, Witzig TE, Markovic SN, Porrata L. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-Bcell lymphoma. Leukemia. 2011;25:1502–1509. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 9.Luis FP, Kay R, Joseph PC, Habermann TM, Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski GS, Thompson C, Markovic SN. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica. 2012;97:262–269. doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favaloro J, Liyadipitiya T, Brown R, Yang S, Suen H, Woodland N, Nassif N, Hart D, Fromm P, Weatherburn C, Gibson J, Ho PJ, Joshua D. Myeloid derived suppressor cells are numerically, functionally and phenotypically different in patients with multiple myeloma. Leuk Lymphoma. 2014;12:1–8. doi: 10.3109/10428194.2014.904511. [DOI] [PubMed] [Google Scholar]
- 11.Nagaraj S, Gabrilovich D. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich D, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b (+)/Gr-1(+)/CD31 (+) myeloid progenitor capable of activating or suppressing CD8 (+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Gustafson M, Bulur P, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, Vose J, Bast M, Fu K, Weisenburger DD, Greiner TC, Armitage JO, Kyle A, May L, Gascoyne RD, Connors JM, Troen G, Holte H, Kvaloy S, Dierickx D, Verhoef G, Delabie J, Smeland EB, Jares P, Martinez A, Lopez-Guillermo A, Montserrat E, Campo E, Braziel RM, Miller TP, Rimsza LM, Cook JR, Pohlman B, Sweetenham J, Tubbs RR, Fisher RI, Hartmann E, Rosenwald A, Ott G, Muller-Hermelink HK, Wrench D, Lister TA, Jaffe ES, Wilson WH, Chan WC, Staudt LM Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Huang J, Xia Y, Sun J, Huang Y, Wang Y, Zhu YJ, Li YJ, Zhao W, Wei WX, Lin TY, Huang HQ, Jiang WQ. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One. 2012;7:23. doi: 10.1371/journal.pone.0041658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porrata L, Inwards D, Ansell S, Micallef IN, Johnston PB, Hogan WJ, Markovic SN. Newonset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant. 2010;16:376–383. doi: 10.1016/j.bbmt.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Akyurek N, Uner A, Benekli M, Barista I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2011;118:4173–4183. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 19.Sehn LH. Paramount prognostic factors that guide therapeutic strategies in diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:402–409. doi: 10.1182/asheducation-2012.1.402. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S, Mate JL, Sancho JM, Arenillas L, Serrano S, Escoda L, Martínez S, Valera A, Martínez A, Jares P, Pinyol M, García-Herrera A, Martínez-Trillos A, Giné E, Villamor N, Campo E, Colomo L, López-Guillermo A Grup per l’Estudi dels Limfomes de Catalunya I Balears (GELCAB) Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117:4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 21.De Jong D, Xie W, Rosenwald A, Chhanabhai M, Gaulard P, Klapper W, Lee A, Sander B, Thorns C, Campo E, Molina T, Hagenbeek A, Horning S, Lister A, Raemaekers J, Salles G, Gascoyne RD, Weller E. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: Validation of tissue microarray as a prerequisite for broad clinical applications (a study from the Lunenburg Lymphoma Biomarker Consortium) J Clin Pathol. 2008;62:128–138. doi: 10.1136/jcp.2008.057257. [DOI] [PubMed] [Google Scholar]