Abstract
Luteolin (LUT), a flavone, which is universally present as constituent of medicinal plants as well as some vegetables and spices, has been demonstrated display specific anti-carcinogenic effects. However, the mechanisms by which LUT inhibits human osteosarcoma growth remain unknown. The effects of LUT on cell growth in human osteosarcoma U2OS cells were measured by MTT assay and flowcytometry. The effects of LUT on morphological markers of autophagy in U2OS were analyzed by fluorescence microscopy and electron microscopy. Autophagic markers, beclin1 and LC3 were detected by western blotting. Here, we found that LUT induced autophagy in U2OS and acted as an enhancer to sensitize doxorubicin (DOX)-mediated autophagy signaling. The combined treatment of LUT and DOX greatly decreases the growth of U2OS, showing synergistic cytotoxicity. Our results indicate that LUT in combination with DOX maybe a novel strategy for the treatment of human osteosarcoma.
Keywords: Luteolin, doxorubicin, autophagy, human osteosarcoma
Introduction
The flavonoids which are polyphenolic compounds, have been widely investigated for their anticancer effects [1]. Among flavonoids, luteolin is most often found in plant materials. Recent research suggests that luteolin has cancer preventive and therapeutic potential [2]. Remarkerblely, the activities of flavonoids appear to be very cell type dependent [3]. So, we try to do some research about the effect of luteolin on human osteosarcoma.
Sarcomas are a broad group of mesenchymal tumors that are notoriously chemoresistant. More than 50 histological types of sarcoma are described [4] with an overall 5-year survival for all stages of 50% to 60% [5-7]. Only 20% of sarcomas respond to doxorubicin, which is the current standard of systemic therapy care for these tumors [8]. Unfortunately, the clinical utility of doxorubicin, which is also used to treat a wide range of solid and nonsolid tumors, is limited by significant side effects, particularly irreversible cardiac toxicity [9]. For these reasons, efforts to improve the efficacy of doxorubicin are essential and could benefit many patients suffering from a variety of cancers.
It has been demonstrated synergistic cytotoxicity by doxorubicin and roscovitine is associated with autophagy in U2OS-LC3-GFP cells [10]. And accumulating evidence also shows that chemotherapeutic agents elicit other forms of non-apoptotic cell death including necrosis, mitotic catastrophe, autophagy and senescence. Among them, autophagy is a well-conserved mechanism by which some parts of the cytoplasm and organelles are sequestered by a double membrane-bound vacuole, known as an autophagosome, and delivered to the lysosome for degradation. Autophagy is a physiological process for degrading long-lived organelles in eukaryotic cells, and also serves as a survival mechanism by removing toxic substances and damaged proteins from cells which are under certain conditions of environmental stress [11,12]. Meanwhile, it is also known to function as a death mechanism by an excessive activation of the self-degrading system [13,14]. Consistently, autophagy is classified as type II programmed cell death (PCD), while apoptosis, characterized by DNA fragmentation, chromatin condensation, cell shrinkage, membrane blebbing and apoptotic body formation, is referred to as type I PCD. Therefore, understanding the complexity of the relationship between apoptosis and autophagy in human osteosarcoma U2OS cells by the treatment of LUT and DOX is required for better management and to tip the balance from cell survival to death.
Materials and methods
Cell culture
Human osteosarcoma cell lines U2OS was purchased from ATCC. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% Newborn Calf Serum (GIBCO), 4 mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin.
Reagents and antibodies
Luteolin, Doxorubicin, rhodamine 123 (Rh123), dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased Promega (Madison, Wisconsin, USA). Luteolin and doxorubicin was dissolved in DMSO and kept protected from light. The concentrations LUT used in the experiments varied between 10-200 µM and the concentrations DOX used in the experiments varied between 10-100 µM. In case not specified in the figure legend, values refer to concentration in µM. We purchased the beclin1 and LC3 antibodies from CST (Cell Signal Technology) companies. Antibodies against GAPDH, β-actin and the secondary HRP-labeled goat-anti-mouse and goat-anti-rabbit antibodies were obtained from Byotime Biotechnology.
Cell viability assay (MTT assay)
Cell viability was measured by the (MTT) method [15]. In brief, cells were collected and seeded in 96-well plates at a density of 5×105 cells/cm2. Different seeding densities were optimized at the beginning of the experiments. 20 µL of MTT tetrazolium salt (Sigma) dissolved in Hanks’ balanced solution at a concentration of 5 mg/mL was added to each well with the indicated treatment and incubated in CO2 incubator for 4 h. Finally, the medium was aspirated from each well, and 150 µL of DMSO (Sigma) was added to dissolve formazan crystals, and the absorbance of each well was obtained using a Dynatech MR5000 plate reader at a test wave-length of 490 nm with a reference wavelength of 630 nm. The following formula was used to calculate cell viability: percentage cell viability = (absorbance of the experiment samples/absorbance of the control)×100%.
Measurement of mitochondrial membrane potential (MMP)
MMP was measured by flow cytometer using the cationic lipophilic green fluorochrome Rh123 [16]. Cells were harvested, washed twice with PBS, incubated with 1 µM Rh123 at 37°C for 30 min, and washed twice with PBS. Fluorescence was determined by flow cytometer with an excitation wavelength of 480 nm at FL-1 filter.
Determination of cellular reactive oxygen species (ROS)
Intracellular ROS was determined by flow cytometer and staining with DCFH-DA [17]. DCFH-DA is deacetylated by intracellular esterase and converted to nonfluorescent dichlorodihydrofluorescein, which is oxidized rapidly to the highly fluorescent compound dichlorofluorescein (DCF) in the presence of ROS. Cellular ROS content was measured by incubating the cells with 10 µM DCFH-DA at 37°C for 30 min. After incubation with the fluorochrome, cells were washed with PBS and analyzed immediately by flow cytometer through FL-1 filter with an excitation wavelength of 480 nm.
Annexin V-FITC/PI staining
Apoptosis was determined by translocation of phosphatidylserine to the cell surface using an Annexin V-FITC apoptosis detection kit (Nanjing KeyGen Biotech. Co. Ltd., China) according to the manufacturer’s protocol. Briefly, after treatment for 24 h, cells were harvested and washed twice with ice-cold PBS, then evaluated for apoptosis using a FACSCalibur flow cytometer (BD Biosciences) with Annexin V-FITC and PI double staining. Fluorescence was measured with an excitation wavelength of 480 nm through FL-1 filter (530 nm) and FL-2 filter (585 nm).
Cell morphology assessment
For microscope observation, cells were cultured overnight in 24-well dishes with glass slides inside and then incubated with LUT, DOX and the combination for 24 h. After treatment, the cellular morphology was observed directly by light microscope. To observe the autophagic vascular organelle (AVO), cells were stained with serum-free medium containing 10 µg/mL of a acridine orange (AO) (sigma) [10]. After 30 min of incubation, cells were washed with PBS and fixed with 4% formaldehyde for 30 min. After fixation, cells were washed with PBS and observed by fluorescence microscope. For nuclear morphology observation, cells were fixed with 4% formaldehyde for 30 min, washed with PBS, then incubated with DAPI for 10 min, washed with PBS three times, and observed by fluorescence microscope.
And for Electron microscopy, cells were fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), followed by 1% OsO4. After dehydration, thin sections were stained with uranyl acetate and lead citrate for observation under a JEM 100 CX electron microscope (JEOL, Peabody, MA) [18].
Western blot analysis
Cells at 1×107 cells/ml were treated with DMSO, LUT alone, DOX alone and the combination of LUT and DOX and harvested after the indicated times. After the lysis procedure, the lysates were centrifuged at 12000 g for 10 min at 4°C. The determination of the protein concentration of supernatants using the BCA Protein Assay Reagent (Pierce Chemical Company, Rockford, IL, USA), equal amounts of protein (50 mg) from each sample were separated by electrophoresis through SDS-PAGE gels (4-12% Tris-HCl, Nu, Invitrogen, Merelbeke, Belgium) and transferred to Hybond-C Super membrane (Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical analysis
The data were expressed as means ± S.D. Statistical analysis was performed by using Student’s t-test (two-tailed). The criterion for statistical significance was taken as P < 0.05.
Results
Combination treatment of LUT and DOX exhibits additive effect in U2OS
Recent studies indicate that LUT has potential anticancer effects in different tumor cell types, however these effects have not been investigated in human osteosarcoma U2OS cells. To address the therapeutic effects of LUT in U2OS, we first investigated the cell death inducing effects of LUT with different concentration (10-200 µM).
MTT analysis showed the cell toxicity of LUT alone, DOX alone and the combination of LUT and DOX. As shown in Figure 1A, LUT nearly did not induce U2OS cells death below 100 µM. These observations indicate that nearly no apoptotic effect was initiated in U2OS cells in response to the LUT treatment below 100 µM. And according to Figure 1A, we infer that in the concentration range above 200 µM LUT was clearly cytotoxic in U2OS cells.
Figure 1.
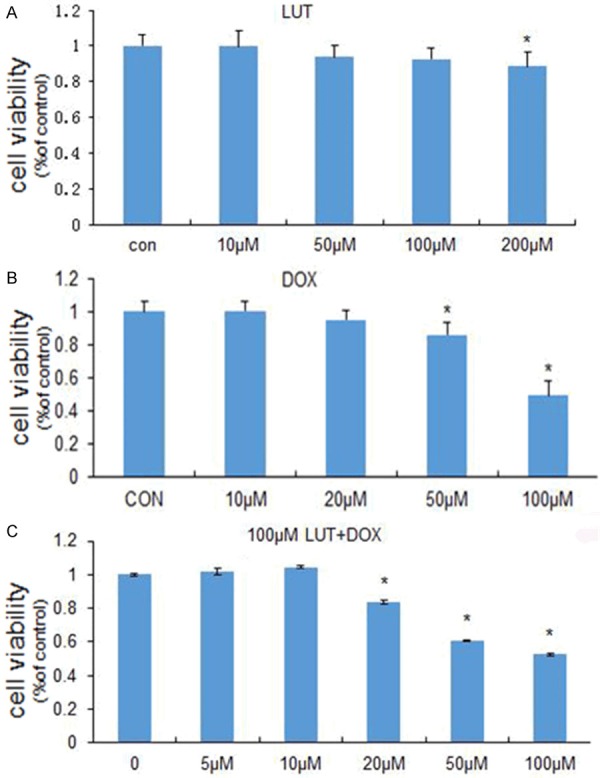
Combined treatment with LUT and DOX greatly enhances human osteosarcoma U2OS cell death. A. U2OS cells were grown in 96-well plates until 50% confluent, and then treated with 1 µL DMSO (control), 10 µM, 50 µM, 100 µM, 200 µM LUT for 24 h, respectively. Cells were incubated with MTT, and then the absorbance was detected at 490 nm. B. U2OS cells were grown in 96-well plates until 50% confluent, and then treated with 10 µM, 20 µM, 50 µM, 100 µM DOX for 24 h, respectively. Cells were incubated with MTT, and then the absorbance was detected at 490 nm. C. U2OS cells were grown in 96-well plates until 50% confluent, and then treated with 10 µM, 20 µM, 50 µM, 100 µM DOX for 24 h, plus 100 µM LUT respectively. Cells were incubated with MTT, and then the absorbance was detected at 490 nm. Each bar represents the mean ± SD from three experiments (*P < 0.05 versus DMSO control).
Figure 1B showed that DOX did not induce greatly U2OS cells growth below 50 µM, and the IC50 of DOX was about 100 µM. As shown in Figure 1A and 1B, LUT or DOX single treatment for 24 h inhibited the growth of U2OS cells in a concentration-dependent manner, and the combination treatment produced additive effect on cell growth (Figure 1C).
LUT in combination with DOX induces MMP disruption in U2OS cells
To evaluate the effect of LUT alone, DOX alone and the combination of LUT and DOX on mitochondrial membrane potential (MMP), we measured MMP by flow cytometer using cationic lipophilic green fluorochrome Rh123. After the treatment with 100 µM LUT, mitochondrial activity was decreased 39% compared with the control. Mitochondrial activity dropped significantly to 43.2% after 100 µM DOX treatment. The impact of the combination of 100 µM LUT and 100 µM DOX on mitochondria (as shown in Figure 2) suggested that LUT can significantly enhance the mitochondrial activity decline induced by DOX. Meanwhile, significant depolarization of MMP occurred after 200 µM LUT treatment for 24 h (data not show), followed by an increased depolarization peak corresponding to a much lower fluorescence intensity after plus 50 µM DOX treatment (data not show), suggesting the collapse of the inner mitochondrial membrane and mitochondrial dysfunction.
Figure 2.
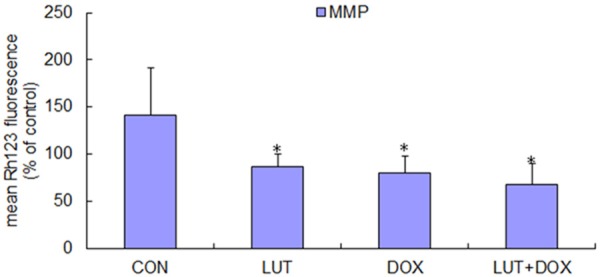
Effects of combined treatment with LUT and DOX on MMP levels. U2OS cells were grown at a concentration of 1×106 cells/ml, and then treated with 100 µM LUT, 100 µM DOX, 100 µM DOX plus 100 µM LUT for 24 h (PBS as a bank control). After three washes, cells were incubated for 37°C for 30 min with 10 µM Rh123 in PBS. The fluorescencewasmeasured using flowcytometry (excitation wavelengths were both 488 nm, and emission wavelengths were both 525 nm). Each bar represents the mean ± SD from three experiments (*P < 0.05 versus PBS control).
LUT in combination with DOX induces ROS burst in U2OS cells
ROS are indices of cell redox status. The generation of intracellular ROS and depletion of GSH are always associated with MMP disruption and cell apoptosis [19,20]. Therefore, we examined the levels of ROS in U2OS cells treated with DMSO (as control), LUT alone, DOX alone and the combination of LUT and DOX. ROS was monitored by the oxidation sensitive fluorescent dye DCFH-DA. Rapid generation of ROS, up to 1.30 to 1.89 fold faster than the control, was detected after drug treatment, as shown in Figure 3. And LUT alone or DOX alone induced less ROS change on U2OS cells after 24 h treatment compared to the combination of both. These results indicate that LUT help to promote the ROS level induced by DOX in U2OS cells.
Figure 3.
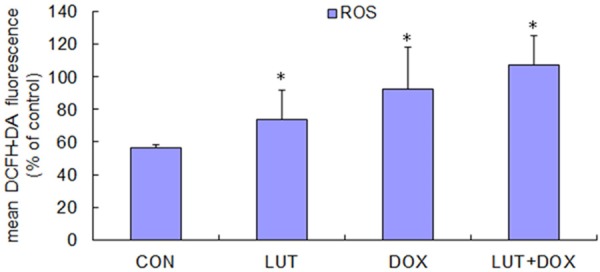
The treatment of DOX plus LUT increases ROS. U2OS cells were grown at a concentration of 1×106 cells/ml, and then treated with 100 µM LUT, 100 µM DOX, 100 µM DOX plus 100 µM LUT for 24 h (or PBS as a bank control, 10 µM H2O2 for 2 h as a positive control). After three washes, cells were incubated for 37°C or 30 min with 10 µM DCFH-DA in PBS. The fluorescencewasmeasured using flowcytometry (excitation wavelengths were both 488 nm, and emission wavelengths were both 525 nm). Each bar represents the mean ± SD from three experiments (*P < 0.05 versus PBS control).
LUT in combination with DOX induces PCD including autophagy and apoptosis in U2OS cells
Non-apoptotic programmed cell death is principally attributed to autophagy (type II programmed cell death). Autophagy is a series of biochemical steps through which eukaryotic cells commit suicide by degrading their own cytoplasm and organelles through a process in which these components are engulfed and then digested in double membrane bound vacuoles called autophagosomes [20]. To determine whether the LUT treatment will induce U2OS autophagy, cells were stained with AO (acridine orange) to detect AVO formation. Figure 4A shows the punctuated AO positive cells at 100 µM LUT treatment as compared to control. Figure 4B shows that after treatment with 100 µM DOX, AVO formation increased. The AVO increased sharply after 24 h of treatment with the combination of LUT and DOX. And to further demonstrate the induced autophagy, we did electron microscopy experiment, which is the Gold Standard of assurance of autophagy. We found that in all four treatments, LUT in combination with DOX treatment exhibited more autophagic vacuoles compared with control, LUT alone and DOX alone treatment (Figure 4B).
Figure 4.
The combined administration of LUT and DOX enhances autophagy and apoptosis in human osteosarcoma U2OS cells. A. U2OS cells were grown on glass slides in 24-well plates at 50% confluent, and then treated with 100 µM LUT, 100 µM DOX, 100 µM DOX plus 100 µM LUT for 24 h (PBS as a bank control). After treatment, cells were stained with AO and observed with fluorescence microscopy to detect the presence of AO puncta. B. Electron micrographs of U2OS cells after treatment for 24 h with 100 µM LUT, 100 µM DOX, 100 µM DOX plus 100 µM LUT for 24 h (PBS as a bank control). C. Annexin V-FITC and PI staining for apoptosis. X-axis, Annexin V-FITC; Y-axis, DNA content by propidium iodide. The test was repeated three times and the image presented was typical of these three independent tests.
In order to identify whether the combination of LUT and DOX-induced cell death in apoptotic mode, we observed the combination-treated U2OS cells by staining with Annexin V-FITC/PI (Figure 4C). When cells were stained with both Annexin V-FITC and PI, the cell membrane showed PS externalization. Compared to the control, cells treated with the combination of LUT and DOX for 24 h had an increase in the percentage of apoptotic cells from 0.06% to 31.72%.
LUT enhances DOX induced-autophagy in U2OS cells through upregulating beclin1
Beclin1 induces autophagy and inhibits tumorigenesis [21]. To examine whether beclin1 was upregulated in LUT combined with DOX-induced U2OS cell autophagy, Western blot analyses were made of the cell lysate. As expected, an increased amount of beclin1 was detected after 24 h treatment with the combination of LUT and DOX (Figure 5A) compared with the control and LUT or DOX alone. Besides, we analyzed LC3, an autophagic marker, which plays an essential role in the expansion of autophagosome [22,23]. Figure 5B shows the combination treatment of LUT and DOX converts LC3 from its soluble, cytoplasmic form (LC3-I) to the membrane-bound, autophagosome-associated form (LC3-II). These results provide further evidence for the enhancement of induction of autophagy by DOX treatment plus LUT in human osteosarcoma U2OS cells.
Figure 5.
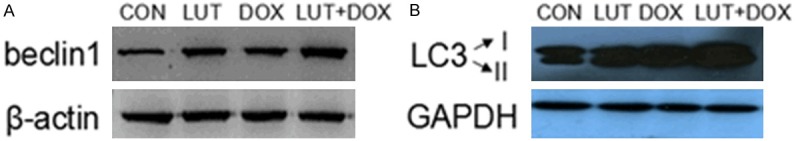
Effects of the combination of LUT and DOX on the beclin1, LC3 in U2OS cells. U2OS cells were treated with100 µM LUT, 100 µM DOX, 100 µM DOX plus 100 µM LUT for 24 h (PBS as a bank control). The lysates were measured by Western blotting for Beclin1 (A) and LC3 (B). The test was repeated three times and the image presented was typical of these three independent tests.
Discussion
LUT has been reported to have many anticancer effects including preventing carcinogen metabolic activation, inhibiting cancer cell proliferation, and eliminating transformed cells by induction of apoptosis, anti-angiogenesis, anti-metastasis [24]. In our study, we first combined LUT and DOX as a treatment and the results shown LUT enhanced DOX induced autophagic cell death. According to Figure 1, LUT indeed enhance the toxicity of DOX, while LUT combined with DOX is not just an additive effect. Cell death has various forms and characters. Mitochondrial play a major role in apoptosis triggered by many stimuli. During the process of apoptosis, mitochondrial membrane potential (MMP) reduces. The combination treatment of LUT and DOX to U2OS cells decreased the MMP. This pattern has been associated with mitochondrial uncoupling and increased mitochondrial production of ROS [25]. We subsequently tested whether ROS production increased in this model. We tested the production of ROS, compared with each group, found that LUT help to increase the DOX induced-ROS. But the MMP decrease and ROS increase is not strong compared with the treatment of LUT alone or DOX alone (Figure 2). Besides, the LUT plus DOX had minor effect of the increase of apoptosis, only 8.22% (from 23.5% to 31.72%), indicating another cell death mechanism exists.
Since apoptosis is referred to as type I programmed cell death, then type II programmed cell death, which is autophagy, probably lead to U2OS cell death after treatment of LUT and DOX. Fluorescence microscope and electron microscope showed autophagic morphology in our study (Figure 4A and 4B), and the combination treatment displayed obvious and strong autophagic phenomenon compared with the treatment of DOX alone. Autophagy is a complex physiological process, including many pathways. Here, we found LUT combined with DOX induced autophagy through increasing beclin1 (Figure 5A), which has structural similarity to the yeast autophagy gene, apg6/vps30 [26,27]. Although the function of autophagy has been debated, our results showed that LUT lead to the death mechanism. And the treatment of DOX plus LUT to U2OS cells leading to death is mainly through autophagic cell death. So we can conclude LUT may synergies with DOX in therapy of human osteosarcoma.
Disclosure of conflict of interest
None.
References
- 1.Kanadaswami C, Lee LT, Lee PPH, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 2.López-Lázaro M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev Med Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 3.Verschooten L, Barrette K, Van Kelst S, Romero NR, Proby C, De Vos R, Agostinis P, Garmyn M. Autophagy Inhibitor Chloroquine Enhanced the Cell Death Inducing Effect of the Flavonoid Luteolin in Metastatic Squamous Cell Carcinoma Cells. PLoS One. 2012;7:e48264. doi: 10.1371/journal.pone.0048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A High-grade Sarcoma Arising in a Patient With Recurrent Benign Giant Cell Tumor of the Proximal Tibia While Receiving Treatment With Denosumab. Clin Orthop Relat Res. 2015;473:3050–5. doi: 10.1007/s11999-015-4249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asada H, Tomiyasu H, Goto-Koshino Y, Fujino Y, Ohno K, Tsujimoto H. Evaluation of the drug sensitivity and expression of 16 drug resistance- related genes in canine histiocytic sarcoma cell lines. J Vet Med Sci. 2015;77:677–684. doi: 10.1292/jvms.14-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacalbasa N, Balescu I. Atypical Paracaval Recurrence of Uterine Endometrial Stromal Sarcoma: A Case Report (vol 35, pg 3405, 2015) Anticancer Res. 2015;35:4371–4371. [PubMed] [Google Scholar]
- 7.Denniston K, Lin C, Brown CK, Zhen W, Enke C. Factors Influencing 5-year Overall Survival in Patients With the Ewing Sarcoma Family of Tumors. Int J Radiat Oncol Biol Phys. 2012;84:S637–S638. [Google Scholar]
- 8.Angelini A, Conti P, Ciofani G, Cuccurullo F, Di Ilio C. Modulation of Multidrug Resistance P-Glycoprotein Activity by Antiemetic Compounds in Human Doxorubicin-Resistant Sarcoma Cells (Mes-Sa/Dx-5): Implications on Cancer Therapy. J Biol Regul Homeost Agents. 2013;27:1029–1037. [PubMed] [Google Scholar]
- 9.Chawla SP, Blay JY, Italiano A, Gutierrez M, Le Cesne A, Gomez-Roca CA, Gouw LG, von Mehren M, Wagner A, Maki RG, Higgins B, Middleton S, Nichols GL, Geho D, Blotner S, Zhi JG, Chen LC. Phase Ib study of RG7112 with doxorubicin (D) in advanced soft tissue sarcoma (ASTS) J. Clin. Oncol. 2013:31. [Google Scholar]
- 10.Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, Keyomarsi K. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res. 2008;68:7966–7974. doi: 10.1158/0008-5472.CAN-08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453–458. doi: 10.1016/j.bbrc.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 14.Pyo JO, Nah J, Jung YK. Molecules and their functions in autophagy. Exp Mol Med. 2012;44:73–80. doi: 10.3858/emm.2012.44.2.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Wang CH, Gong XG. Apoptosis-inducing effects of two anthraquinones from Hedyotis diffusa WILLD. Biol Pharm Bull. 2008;31:1075–1078. doi: 10.1248/bpb.31.1075. [DOI] [PubMed] [Google Scholar]
- 16.Lu M, Gong X, Lu Y, Guo J, Wang C, Pan Y. Molecular cloning and functional characterization of a cell-permeable superoxide dismutase targeted to lung adenocarcinoma cells. Inhibition cell proliferation through the Akt/p27kip1 pathway. J Biol Chem. 2006;281:13620–13627. doi: 10.1074/jbc.M600523200. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Xu Z, Tan M, Su W, Gong XG. 3-(4-(Benzo[d] thiazol-2-yl)-1-phenyl-1H-pyrazol-3-yl) phenyl acetate induced Hep G2 cell apoptosis through a ROS-mediated pathway. Chem Biol Interact. 2010;183:341–348. doi: 10.1016/j.cbi.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Gong K, Chen C, Zhan Y, Chen Y, Huang Z, Li W. Autophagy-related gene 7 (ATG7) and reactive oxygen species/extracellular signal-regulated kinase regulate tetrandrine-induced autophagy in human hepatocellular carcinoma. J Biol Chem. 2012;287:35576–88. doi: 10.1074/jbc.M112.370585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y, Liu X, Lee CP, Chua BH, Ho YS. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic Biol Med. 2006;41:46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Lopez E, Arce C, Oset-Gasque MJ, Canadas S, Gonzalez MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 22.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–10. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pieme CA, Kumar SG, Dongmo MS, Moukette BM, Boyoum FF, Ngogang JY, Saxena AK. Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement Altern Med. 2014;14:516. doi: 10.1186/1472-6882-14-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C-elegans. Science. 2003;301:1387–91. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 27.Koukourakis MI, Giatromanolaki A, Sivridis E, Pitiakoudis M, Gatter KC, Harris AL. Beclin1 over- and underexpression in colorectal cancer: distinct patterns relate to prognosis and tumour hypoxia. Br J Cancer. 2010;103:1209–1214. doi: 10.1038/sj.bjc.6605904. [DOI] [PMC free article] [PubMed] [Google Scholar]



