Abstract
Mesangial cells (MCs) proliferation and extracellular matrix (ECM) accumulation are early features of diabetic nephropathy. Follistatin-like 3 (FSTL3), a member of follistatin family, has been shown to regulate insulin and glucagon sensitivities in diet-induced obesity and insulin resistance. However, the role of FSTL3 in diabetic nephropathy is still unclear. Therefore, in this study, we investigated the effects of FSTL3 on cell proliferation and ECM accumulation expression in rat MCs cultured under high glucose, and elucidated the underlying mechanism. We found that the expression of FSTL3 was decreased significantly in MCs cultured high glucose condition. Overexpression of FSTL3 inhibited high glucose-induced MC proliferation and blocked the G1/S phase transition under high glucose condition. And, FSTL3 overexpression also reduced the expression of α-smooth muscle actin (α-SMA) and fibronectin (FN) induced by high glucose. Furthermore, overexpression of FSTL3 suppressed high-glucose-induced p38 phosphorylation in MCs. Taken together, our present study demonstrated that FSTL3 suppressed high glucose-induced MC proliferation and ECM accumulation via inhibiting the p38MAPK signaling pathway, and that FSTL3 may be a potential therapeutic target for the treatment of diabetic nephropathy.
Keywords: Follistatin-like 3 (FSTL3), mesangial cells (MCs) proliferation, extracellular matrix (ECM), diabetic nephropathy
Introduction
Diabetic nephropathy, one of the most serious microvascular complications of diabetes mellitus, is the main cause of chronic renal failure [1]. The pathological changes of diabetic nephropathy include kidney hypertrophy, glomerulus and tubular basement membrane thickening, tubular interstitial fibrosis and arteriosclerosis [2]. Growing evidence indicated that mesangial cells (MCs) proliferation contributes to the development and progression of diabetic nephropathy [3-5]. In addition, MCs proliferation was accompanied by the accumulation of extracellular matrix (ECM) [4]. Therefore, inhibiting MCs proliferation and ECM accumulation is a promising approach for treating diabetic nephropathy.
Follistatin-like 3 (FSTL3) is a highly conserved 27-39 kDa monomeric glycoprotein [6]. It is structurally and functionally distinct from the other follistatin (FST) family member, as it contains only two follistatin domains and is present in the nucleus in a glycosylated form. A growing body of evidence has revealed FSTL3 involve in the development of human diseases including preeclampsia [7], obesity [8], musculoskeletal disorder [9] and heart remodeling [10]. Most recently, one study showed that overexpression of FSTL3 regulates insulin and glucagon sensitivities through increased muscular insulin action, as well as increased hepatic glucagon sensitivity and pancreatic glucagon content [11]. However, the role of FSTL3 in diabetic nephropathy is still unclear. Therefore, in this study, we investigated the effects of FSTL3 on cell proliferation and ECM accumulation expression in rat MCs cultured under high glucose, and elucidated the underlying mechanism.
Materials and methods
Cell culture
The rat mesangial cell line (HBZY-1) was from Center of Type Culture Collection, Wu Han University, Wuhan, China. High glucose (25 mM) has been considered as an important stimulus to increase the proliferation of MCs. MCs were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin under 95% air/5% CO2 condition.
Construction of plasmids and transfection
Full-length FSTL3 cDNA was amplified and subcloned into pAdTrack-cytomegalovirus (CMV), whereas green fluorescent protein (GFP) was used as a non-specific control. Then, the recombinant shuttle plasmids pAdTrack-CMV and pAdEasy-1 were then recombined in Escherichia coli strain BJ5183. The obtained recombinant plasmids were transfected into 293 cells to generate recombinant adenovirus.
For in vitro transfection, MCs were seeded in each well of 96-well microplates, grown for 24 h to reach 60% confluence, and transfected with Ad-FSTL3 or Ad-GFP using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
RNA extraction and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA from the MCs was prepared with Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Then, cDNA was synthesized with a two-step reverse transcription reaction kit (Takara, Dalian, China). The levels of gene mRNA transcripts were analyzed by using specific primers and SYBR Green I reagent and the RT-PCR kit, according to the manufacturer’s instructions, using the Bio-Rad iQ5 Quantitative PCR System (Takara, Dalian, China). The specific primers for FSTL3 were sense 5’-AGCCTGGTGCTCCAGACTGATGTCA-3’ and antisense, 5’-TCCACGCCGTCGCACGAATCTTT-3’. The cycling conditions included a holding step at 94°C for 10 min, and 35 cycles of 94°C for 15 s, 59°C for 30 s, and 70°C for 30 s. The average threshold cycle (Ct) of fluorescence units was used to analyze the mRNA level. The relative amount of mRNA was calculated using the comparative Ct (ΔΔCt).
Western blot
MCs cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease and phosphatase inhibitors (5 mM EDTA, 1 mM PMSF, and 1 mM sodium orthovanadate) for 30 min on ice. The protein content was determined by BCA protein assay (Pierce, Rockford, IL). Protein (20 μg) was loaded on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were blocked in 5% skim milk in TBS containing 0.1% Tween-20 (TBST) for 1 h at room temperature and then incubated with different primary antibodies (anti-FSTL3, anti-p21Cip1, anti-p27Kip1, anti-α-smooth muscle actin (α-SMA), anti-fibronectin (FN), anti-p-p38, anti-p38 and anti-GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After washing and incubating with HRP-conjugated secondary antibodies for 1 h at room temperature, the blots were detected with an ECL detection kit according to the manufacturer’s procedure. GAPDH was used as the reference control.
Cell proliferation assay
The MTT assay was used to measure cell proliferation. Briefly, MCs were seeded at 1×104 cells/well in 96-well plates. Then cells were incubated with Ad-FSTL3 or Ad-GFP in the presence or absence of high glucose for 24 h. Then 20 μL of MTT (5 mg/ml) was added to each well and incubation continued at 37°C for additional 4 h. After the medium was discarded, 150 μl DMSO was added to each well, followed by vibration for 15 min to completely dissolve the crystals. The optical density was determined at 570 nm using a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT).
Cell cycle assay
Cell cycle analysis was performed using flow cytometry. After all the cells reached 70%-90% confluence, a serum-free medium was applied for synchronization. After 24 h culturing, cells were harvested by trypsinization, centrifuged, and suspended with 1 mL cold PBS and then fixed in methanol for 30 min on ice. Fixed cells were washed with PBS twice, then incubated in RNase (25 μg/mL) at 37°C for 30 min, followed by DNA staining with propidium iodide (PI, 50 μg/mL) at 4°C for 30 min in the dark. Finally, samples were analyzed using a Coulter Epics XL Flow Cytometer.
Statistical analysis
The results were presented as means ± SD. Statistical analysis was performed by one-way ANOVA. Statistical significance was defined as P<0.05.
Results
The expression of FSTL3 in high-glucose-induced MCs
In order to investigate the role of FSTL3 in diabetic nephropathy, we detected the changes in FSTL3 mRNA and protein by RT-qPCR and Western blot at 0, 6, 12, 24 and 48 h in MCs cultured under hyperglycemic condition. As shown in Figure 1A, FSTL3 mRNA level was decreased significantly in MCs cultured high glucose condition. Consistent with the results of RT-qPCR, Western blot assay showed that FSTL3 protein level was also obviously reduced with prolonged culture time (Figure 1B).
Figure 1.
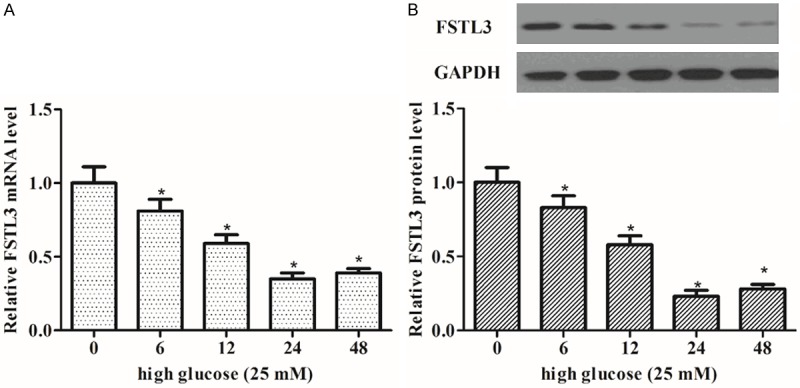
The expression of FSTL3 in high-glucose-induced MCs. MCs were incubated with high glucose (25 mM) at the indicated times (0-48 h). A. The expression of FSTL3 mRNA was detected by RT-qPCR; B. The expression of FSTL3 protein was analyzed by Western blot. The expression of FSTL3 was decreased in high-glucose-induced MCs. Values are expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 vs. control.
Effect of FSTL3 on cell proliferation in high-glucose-induced MCs
To investigate the effect of FSTL3 on the proliferation of MCs cultured under high glucose, we generated FSTL3 overexpressing MCs. As shown in Figure 2A and 2B, overexpression of FSTL3 significantly increased the expression levels of FSTL3. Then, the cell proliferation was detected by MTT assay. The results showed that compared with the high glucose group, 25 mM glucose alone increased MC proliferation. However, overexpression of FSTL3 inhibited high glucose-induced MC proliferation (Figure 2C).
Figure 2.
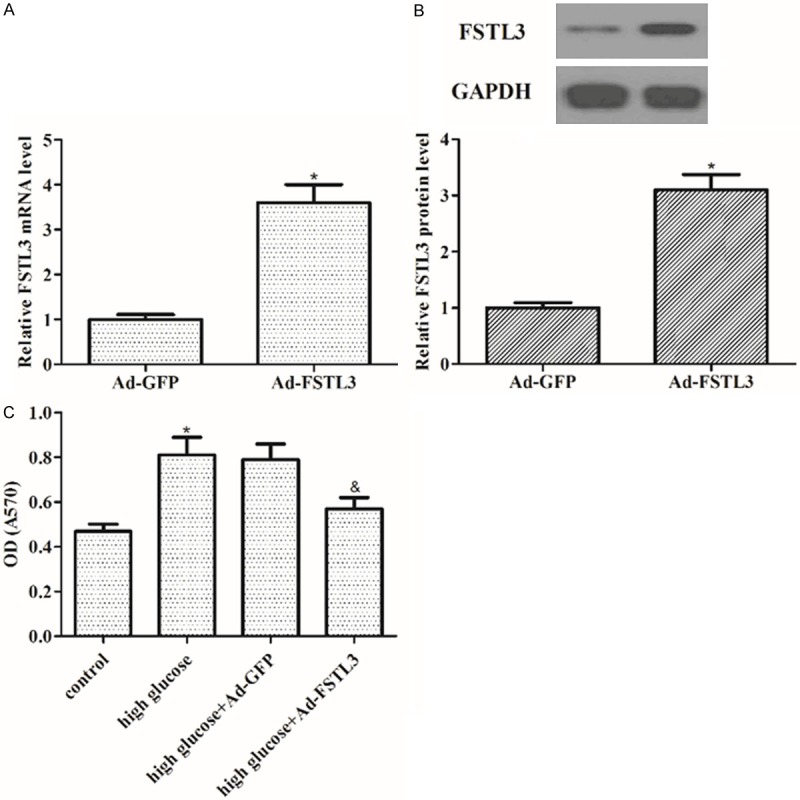
Effect of FSTL3 on cell proliferation in high-glucose-induced MCs. MCs were transfected with Ad-GFP and Ad-FSTL3, respectively. The corresponding transfection efficiency was detected by RT-qPCR and Western blot. A. FSTL3 mRNA expression in MCs; B. FSTL3 protein expression in MCs. Values are expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 vs. control. C. MTT assay showed that overexpression of FSTL3 inhibited high glucose-induced MC proliferation. Values are expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 vs. control group; &P<0.05 vs. high glucose group.
Effect of FSTL3 on cell cycle in high-glucose-induced MCs
Then, we evaluated the effect of FSTL3 on the cell cycle of MCs cultured under high glucose. As indicated in Figure 3A, the proportion of MCs was decreased in G1 phase and increased in S phase by high glucose treatment. However, overexpression of FSTL3 increased the proportion of MCs in G1 phase and decreased the proportion of MCs in S phase. These results demonstrate that FSTL3 blocks high glucose-induced cell cycle progression by inhibiting S phase entry and inducing cell cycle arrest in G1 phase. Moreover, we examined the effects of FSTL3 on high glucose-regulated expression of cell regulatory molecules. Western blot analysis demonstrated high glucose significantly decreased the expression of p21Cip1 and p27Kip1, as compared with control group. However, FSTL3 significantly reversed high glucose-inhibited expression of p21Cip1 and p27Kip1 (Figure 3B).
Figure 3.
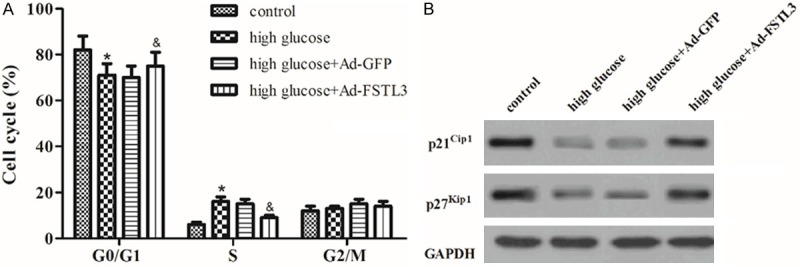
Effect of FSTL3 on cell cycle in high-glucose-induced MCs. A. Cell cycle progression was evaluated by flow cytometry after 24 h of Ad-GFP or Ad-FSTL3 transfection. FSTL3 blocks high glucose-induced cell cycle progression by inhibiting S phase entry and inducing cell cycle arrest in G1 phase. B. Western blot to detect the protein levels of p21Cip1 and p27Kip1 in different treated cell groups with the indicated antibodies. FSTL3 significantly reversed high glucose-inhibited expression of p21Cip1 and p27Kip1. Values are expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 vs. control group; &P<0.05 vs. high glucose group.
Effect of FSTL3 on the expression of α-SMA and FN in high-glucose-induced MCs
It is believed that ECM accumulation play crucial roles in early renal hypertrophy and later glomerular sclerosis in diabetic nephropathy. So, we evaluated the effect of FSTL3 on the expression of α-SMA and FN in high-glucose-induced MCs. As shown in Figure 4, the expression of α-SMA and FN was significantly induced by high glucose in MCs; whereas, overexpression of FSTL3 significantly reduced the expression of α-SMA and FN induced by high glucose.
Figure 4.
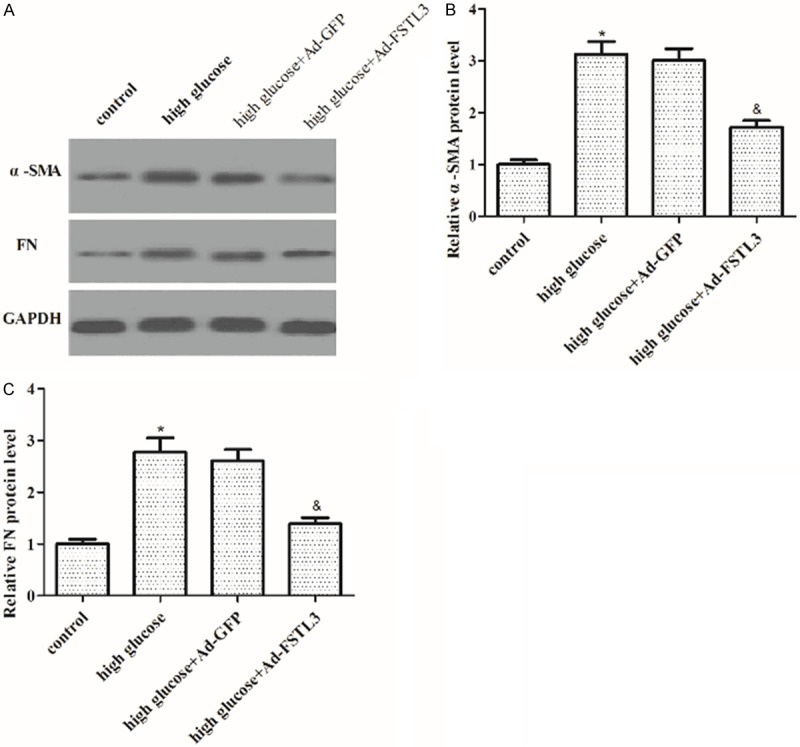
Effect of FSTL3 on the expression of α-SMA and FN in high-glucose-induced MCs. A. Western blot to detect the protein levels of α-SMA and FN in different treated cell groups with the indicated antibodies. B. Protein expression of α-SMA was analyzed using BandScan 5.0 software and normalized to GAPDH. C. Protein expression of FN was analyzed using BandScan 5.0 software and normalized to GAPDH. Values are expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 vs. control group; &P<0.05 vs. high glucose group.
Effect of FSTL3 on the phosphorylation level of p38MAPK induced by high glucose in MCs
Since the activation of p38MAPK signaling is a key step in the proliferation process of MCs, therefore, we investigated the effect of FSTL3 on the phosphorylation level of p38MAPK in high-glucose-induced MCs. As shown in Figure 5, compared with those of the high-glucose groups, the phosphorylation level of p38 significantly increased in high-glucose group; whereas, overexpression of FSTL3 significantly suppressed high-glucose-induced p38 phosphorylation in MCs.
Figure 5.
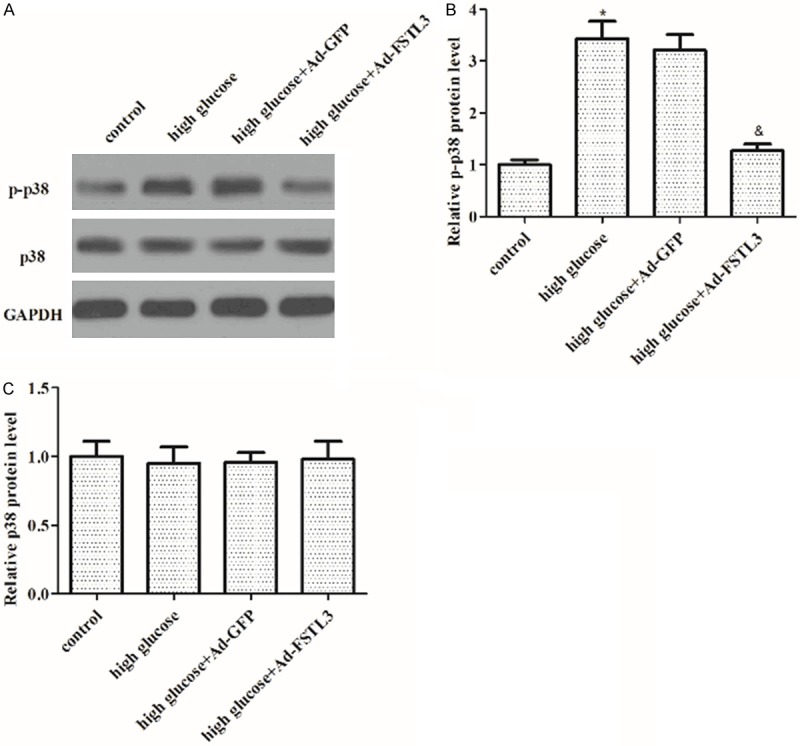
Effect of FSTL3 on the phosphorylation level of p38MAPK induced by high glucose in MCs. A. Western blot to detect the protein levels of p-p38 and p38 in different treated cell groups with the indicated antibodies. B. Protein expression of p-p38 was analyzed using BandScan 5.0 software and normalized to GAPDH. C. Protein expression of p38 was analyzed using BandScan 5.0 software and normalized to GAPDH. Values are expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 vs. control group; &P<0.05 vs. high glucose group.
Discussion
In the present study, we found that the expression of FSTL3 was decreased significantly in MCs cultured high glucose condition. Overexpression of FSTL3 inhibited high glucose-induced MC proliferation and blocked the G1/S phase transition under high glucose condition. And overexpression of FSTL3 also reduced the expression of α-SMA and FN induced by high glucose. Furthermore, FSTL3 suppressed high-glucose-induced p38 phosphorylation in MCs.
It is believed that MC proliferation plays a critical role in early renal hypertrophy and later glomerular sclerosis in diabetic nephropathy. MC proliferative responses to a variety of stimuli are associated with the development of diabetic nephropathy. High glucose is the most potent stimulus for MC proliferation in diabetes [12]. In this study, we employed high glucose to investigate the effects of FSTL3 on MCs in vitro. We found that FSTL3 was decreased significantly in MCs cultured high glucose condition, and overexpression of FSTL3 inhibited high glucose-induced MC proliferation. All these findings indicated that FSTL3 may play an important role in the development of diabetic nephropathy.
It has been reported that the cell cycle is dysregulated in the diabetic state, and G1-phase cell cycle arrest is responsible for the high glucose-induced proliferation [13]. Over-expression of p21 using adenovirus vectors ameliorated serum and PDGF-induced cell proliferation of rat mesangial cells [14,15]. In this study, we found that the proportion of MCs was decreased in G1 phase and increased in S phase by high glucose treatment. However, overexpression of FSTL3 increased the proportion of MCs in G1 phase and decreased the proportion of MCs in S phase. Several studies showed that p21Cip1 and p27Kip1 were involved in the G1-phase cell cycle arrest in MCs while the cells were exposed to high glucose in diabetic mice [16,17]. In this study, we found that overexpression of FSTL3 significantly reversed high glucose-inhibited expression of p21Cip1 and p27Kip1. Taken together, these data indicate that FSTL3 inhibited high glucose-induced MC proliferation by inhibiting cell cycle progression.
FN is a main ingredient of ECM, and its excessive synthesis contributes to glomerular basement membrane thickening as well as ECM deposition in the mesangium [18]. Several studies showed that high glucose stimulated the protein and gene expression of fibronectin and type IV collagen in the expanded glomerular mesangial matrix [19,20]. In agreement with the previous studies, in this study, we found that high glucose increased the expression of α-SMA and FN in MCs. This stimulation was significantly inhibited by FSTL3 overexpression, indicating that inhibitory effects of FSTL3 overexpression on high glucose-stimulated MC proliferation may be mediated, at least in part, by inhibition of the excessive accumulation of ECM.
The mitogen activated protein kinases family including extracellular signal regulated kinase 1/2 (ERK1/2), p38MAPK and c-Jun NH2-terminal kinase (JNK) play important roles in the development of diabetic nephropathy [21,22]. Previous studies reported that glomerular p38MAPK activity was increased early in diabetic rats [23], as well as in various cells cultured under high glucose condition [24,25]. Moreover, activated p38MAPK could increase the protein expression of CTGF, which stimulated the cell proliferation and ECM accumulation [26], and the blockade of the p38MAPK pathway by SB203580 inhibited high glucose-induced fibronectin expression [23]. Consistent with previous reports, in this study, we found that overexpression of FSTL3 significantly suppressed high-glucose-induced p38 phosphorylation in MCs, suggesting that FSTL3 suppressed high glucose-induced MC proliferation and ECM accumulation via inhibiting the activation of p38MAPK.
In summary, our present study demonstrated that FSTL3 suppressed high glucose-induced MC proliferation and ECM accumulation via inhibiting the p38MAPK signaling pathway, and that FSTL3 may be a potential therapeutic target for the treatment of diabetic nephropathy.
Disclosure of conflict of interest
None.
References
- 1.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q. Prevalence of diabetes among men and women in China. New Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 3.Schöcklmann HO, Lang S, Sterzel RB. Regulation of mesangial cell proliferation. Kidney Int. 1999;56:1199–1207. doi: 10.1046/j.1523-1755.1999.00710.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishimura E, Sterzel RB, Budde K, Kashgarian M. Formation of extracellular matrix by cultured rat mesangial cells. Am J Pathol. 1989;134:843–855. [PMC free article] [PubMed] [Google Scholar]
- 5.Maeshima Y, Kashihara N, Yasuda T, Sugiyama H, Sekikawa T, Okamoto K, Kanao K, Watanabe Y, Kanwar YS, Makino H. Inhibition of mesangial cell proliferation by E2F decoy oligodeoxynucleotide in vitro and in vivo. J Clin Invest. 1998;101:2589–2597. doi: 10.1172/JCI429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneyer A, Tortoriello D, Sidis Y, Keutmann H, Matsuzaki T, Holmes W. Follistatin-related protein (FSRP): a new member of the follistatin gene family. Mol Cell Endocrinol. 2001;180:33–38. doi: 10.1016/s0303-7207(01)00501-9. [DOI] [PubMed] [Google Scholar]
- 7.Founds SA, Ren D, Roberts JM, Jeyabalan A, Powers RW. Follistatin-Like 3 Across Gestation in Preeclampsia and Uncomplicated Pregnancies Among Lean and Obese Women. Reprod Sci. 2015;22:402–9. doi: 10.1177/1933719114529372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown ML, Bonomi L, Ungerleider N, Zina J, Kimura F, Mukherjee A, Sidis Y, Schneyer A. Follistatin and Follistatin Like-3 Differentially Regulate Adiposity and Glucose Homeostasis. Obesity. 2011;19:1940–1949. doi: 10.1038/oby.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam J, Perera P, Gordon R, Jeong Y, Blazek A, Kim D, Tee B, Sun Z, Eubank T, Zhao Y. Follistatin-like 3 is a mediator of exercise-driven bone formation and strengthening. Bone. 2015;78:62–70. doi: 10.1016/j.bone.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panse KD, Felkin LE, López-Olañeta MM, Gómez-Salinero J, Villalba M, Muñoz L, Nakamura K, Shimano M, Walsh K, Barton PJ. Follistatin-like 3 mediates paracrine fibroblast activation by cardiomyocytes. J Cardiovasc Transl Res. 2012;5:814–826. doi: 10.1007/s12265-012-9400-9. [DOI] [PubMed] [Google Scholar]
- 11.Brandt C, Hansen RH, Hansen JB, Olsen CH, Galle P, Mandrup-Poulsen T, Gehl J, Pedersen BK, Hojman P. Over-expression of Follistatinlike 3 attenuates fat accumulation and improves insulin sensitivity in mice. Metabolism. 2015;64:283–295. doi: 10.1016/j.metabol.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Sodhi CP, Phadke SA, Batlle D, Sahai A. Hypoxia and high glucose cause exaggerated mesangial cell growth and collagen synthesis: role of osteopontin. Am J Physiol Renal Physiol. 2001;280:F667–F674. doi: 10.1152/ajprenal.2001.280.4.F667. [DOI] [PubMed] [Google Scholar]
- 13.Griffin SV, Pichler R, Wada T, Vaughan M, Durvasula R, Shankland SJ. The role of cell cycle proteins in glomerular disease. Semin Nephrol. 2003;23:569–582. doi: 10.1053/s0270-9295(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 14.Terada Y, Yamada T, Nakashima O, Tamamori M, Ito H, Sasaki S, Marumo F. Overexpression of cell cycle inhibitors (p16INK4 and p21Cip1) and cyclin D1 using adenovirus vectors regulates proliferation of rat mesangial cells. J Am Soc Nephrol. 1997;8:51–60. doi: 10.1681/ASN.V8151. [DOI] [PubMed] [Google Scholar]
- 15.Monkawa T, Pippin J, Yo Y, Kopp JB, Alpers CE, Shankland SJ. The cyclin-dependent kinase inhibitor p21 limits murine mesangial proliferative glomerulonephritis. Nephron Exp Nephrol. 2005;102:e8–18. doi: 10.1159/000088311. [DOI] [PubMed] [Google Scholar]
- 16.Okada T, Wada J, Hida K, Eguchi J, Hashimoto I, Baba M, Yasuhara A, Shikata K, Makino H. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes. 2006;55:1666–1677. doi: 10.2337/db05-1285. [DOI] [PubMed] [Google Scholar]
- 17.Xu ZG, Yoo TH, Ryu DR, Park HC, Ha SK, Han DS, Adler SG, Natarajan R, Kang SW. Angiotensin II receptor blocker inhibits p27Kip1 expression in glucose-stimulated podocytes and in diabetic glomeruli. Kidney Int. 2005;67:944–952. doi: 10.1111/j.1523-1755.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 18.Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995;47:935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]
- 19.Nahman N, Leonhart KL, Cosio FG, Hebert CL. Effects of high glucose on cellular proliferation and fibronectin production by cultured human mesangial cells. Kidney Int. 1992;41:396–402. doi: 10.1038/ki.1992.55. [DOI] [PubMed] [Google Scholar]
- 20.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adhikary L, Chow F, Nikolic-Paterson D, Stambe C, Dowling J, Atkins R, Tesch G. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47:1210–1222. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- 22.Ha H, Kim MS, Park J, Huh JY, Huh KH, Ahn HJ, Kim YS. Mycophenolic acid inhibits mesangial cell activation through p38 MAPK inhibition. Life Sci. 2006;79:1561–1567. doi: 10.1016/j.lfs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Liu W, Wang Q, Liu P, Deng Y, Lan T, Zhang X, Qiu B, Ning H, Huang H. Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol Cell Endocrinol. 2009;307:157–162. doi: 10.1016/j.mce.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Kang MJ, Wu X, Ly H, Thai K, Scholey JW. Effect of glucose on stress-activated protein kinase activity in mesangial cells and diabetic glomeruli. Kidney Int. 1999;55:2203–2214. doi: 10.1046/j.1523-1755.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakagami H, Morishita R, Yamamoto K, Yoshimura SI, Taniyama Y, Aoki M, Matsubara H, Kim S, Kaneda Y, Ogihara T. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes. 2001;50:1472–1481. doi: 10.2337/diabetes.50.6.1472. [DOI] [PubMed] [Google Scholar]
- 26.Pesce CG, Nogués G, Alonso CR, Baralle FE, Kornblihtt AR. Interaction between the- 170 CRE and the- 150 CCAAT box is necessary for efficient activation of the fibronectin gene promoter by cAMP and ATF-2. FEBS Lett. 1999;457:445–451. doi: 10.1016/s0014-5793(99)01091-1. [DOI] [PubMed] [Google Scholar]


