Abstract
Objective: Modified Glasgow prognostic score (mGPS) had been reported to associate with the prognosis ofgastric cancer (GC), butits significance in gastric cancer patients has not been studied fully. Methods: PubMed; EMBASE; Web of Science and CNKI data base were searched to identify studies using the mGPS in gastric cancer patients. Outcome measures that were evaluated included overall survival (OS), lymphatic invasion and venous invasion inpatients with gastric cancer. Results: A total of seven studies comprising 3206 patients were included in the meta-analysisof which all used OS as an outcome measure, three studies reported lymphatic invasionand three evaluated venous invasion. The results show that OS was worse in patients with an mGPS=1 and 2 (odds ratio [OR]=2.54, 95% [CI]: 1.62-3.98 and OR=12.02, 95% [CI]: 6.79-21.28, respectively) compared with those with a score of 0 (both P<0.01). Furthermore, gastric cancer patients with mGPS≥1 have higher rates of lymphatic and venous invasion with ORs of 2.51 (95% CI: 1.80-3.51) and 2.63 (95% CI: 1.35-5.11) respectively (both P<0.01). Conclusions: Them GPS could be used as a prognosis predictorfor gastric cancer patients and associated lymphatic and venous invasion.
Keywords: mGPS, gastric cancer, prognostic factor, meta-analysis
Introduction
Gastric cancer (GC) is the fourth most common cancer andsecond leading cause of cancer-related mortalityin the world [1,2]. Although surgery and chemotherapy have improved treatment outcome, the survival rate of patients with GCremains unsatisfactory [3]. As treatment plans are becoming more individualized for each patient, it is important to assess disease progression in a timely manner and accuratelyevaluate the prognosis [4,5]. Tumorin flammatory markers are useful indicatorsof disease development as the inflammatory response is known to promote tumor growth, invasion, angiogenesis and metastasis [6]. Indeed, a close relationship between tumor prognosis and systemic inflammation has been established using markers detected in peripheral blood [7]. Chronic inflammation has also been associated with the progression of GC [8-10], though the exact mechanism for this requires further study.
The modified Glasgow Prognostic Scores (mGPS) provides an inflammation-based prognostic assessment of various tumor type [11,12]. Despite some studies that have reported the association between mGPS and GC patients, due to differences in inclusion criteriaof GC patients and limited sample sizes limiting its role. Its significance in patients with gastric cancer has not been studied fully. So, it is reasonable to hypothesize that mGPS is a good candidate for predicting the prognosis of GC. In order to more clearly evaluate this, a meta-analysis was conducted to determine whether the mGPS is a useful prognostic factor in GC patients and to assess the relationship between mGPS and clinico pathologic parameters.
Materials and methods
Data sources and searches
The following databases were searched for relevant articles published up until December 2014: PubMed; EMBASE; Web of Science and the China National Knowledge Infrastructure. Search terms included “gastriccancer”, “prognostic”, “mGPS” and “modified Glasgow Prognostic Score”. Two reviewers manually searched the reference lists of identified studies for potential related articles. Only literature published in peer-reviewed journals was included.
Inclusion and exclusion criteria
For inclusion in the meta-analysis, relevant studies were required to include: 1) pathologic examination for diagnosis of GC; 2) pretreatment C-reactive protein (CRP) and albuminlevelsmeasured from peripheral blood samples, mGPS evaluation criteria are formulated by their own laboratories; 3) multivariate analysis for estimation of the hazard ratio (HR). Patients who had other inflammatory diseases causing serum elevations of CRP and albumin were excludedfrom the study. Nonhuman GC studies, duplicatearticles, abstracts and letters were excluded from the analysis. Two reviewers evaluated all candidate literature and resolved any disagreement by discussion.
Data extraction
The following data were extracted from relevant identified: author’s first name, year ofpublication, country and size of the population studied, Tumor-node metastasis stage of GC; treatment, the number of patients with mGPS=0, 1 or 2; follow-up period, lymphatic and venous invasion, and overall survival (OS) rate. Some studies do not provide exhaustive OS, we calculate the number of overall survival patients based on overall survivalfigure in the studies.
Statistical analysis
Analysis was conducted using RevMan 5.2 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Associations between mGPS and clinical or pathologic parameters were performed using odds ratios (OR) and 95% confidence intervals (CI). If several estimates were reported with in the same article, the strongest value was selected. The estimates of ORs were weighted and pooled using the Mantel-Haenszelfixe deffects model. If I2≥ 50%, the random-effects model was appliedto calculate the pooled OR and 95% CI. Statistical heterogeneity was assessed using the Cochran’s Q and I2 statistics, Publication bias was assessed by visual inspection of the funnel plot. All statistical tests were two-sided, and statistical significance was defined as P≤0.05.
Results
Study selection
A flow chart depicting the search and study selectionis showed in Figure 1. The initial search identified 84 studies, of which seven studiescomprising 3206 patients that were published between 2011 and 2014 were finally included for the meta-analysis [12-18]. Study characteristics are presented in Table 1.
Figure 1.
Flow diagram for inclusion of studies included in the meta-analysis.
Table 1.
Baseline characteristics of the studiesincluded in the meta-analysis
| Ref. | Study region | Samples (n) | Treatment | Outcome | Clinical stage | Survival analysis | Number of mGPS=0/1/2 |
|---|---|---|---|---|---|---|---|
| Tadahiro et al., 2011 | Japan | 232 | Gastrectomy and lymph nodedissection, no neoadjuvant therapy | OS | GC | Multivariate analysis | 140/64/28 |
| Jae-Heon et al., 2012 | Korea | 104 | Palliative chemotherapy | OS | Advanced GC | Multivariate analysis | 58/29/17 |
| Shinsuke et al., 2014 | Japan | 552 | Curative gastrectomy with lymphnode dissection, adjuvant chemotherapy | OS | GC | Multivariate analysis | 494/24/34 |
| Kotaro et al., 2014 | Japan | 294 | Gastrectomy and lymphnode dissection | OS | GC | Multivariate analysis | 174/84/36 |
| Aurello et al., 2014 | Italy | 102 | Gastrectomy and lymph node dissection | OS | GC | Multivariate analysis | 49/25/28 |
| Jiang et al., 2012 | Japan | 1710 | Curative or palliative gastrectomy | OS | GC | Multivariate analysis | 1565/78/67 |
| Zhang et al., 2014 | China | 212 | Curative or palliative gastrectomy, chemotherapy | OS | Stage III-IV GC | Multivariate analysis | 136/45/31 |
GC: gastric cancer; OS: overall survival; DFS: disease-free survival; PFS: progression-free survival.
OS
There was significant heterogeneity (I2≥ 50%) among these studies with regard to mGPS and OS, and thus a random-effects model was applied to calculate the pooled OR and 95% CI (Figure 2). The results show that patients with a mGPS=1 or 2 have a shorter OS than those with a score of 0 (both P=0.02).
Figure 2.
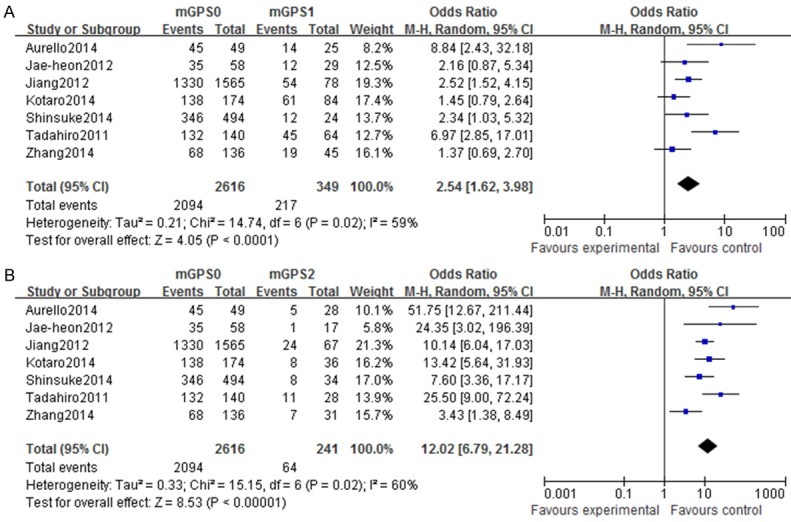
Forest plots of studies evaluating overall survival and modified Glasgow Prognostic Score (mGPS). Overall survival in gastric cancer patients with (A) a mGPS score of 1 and (B) a mGPS score of 2 compared with patients with a mGPS score of 0. CI: confidence interval.
mGPS and lymphatic invasion
Three studies compared mGPS and lymphatic invasion in GC patients. The analysis show thatpatients with an mGPS≥1 havea significantly higher positive lymphatic invasionrate (P<0.01) (Figure 3).
Figure 3.
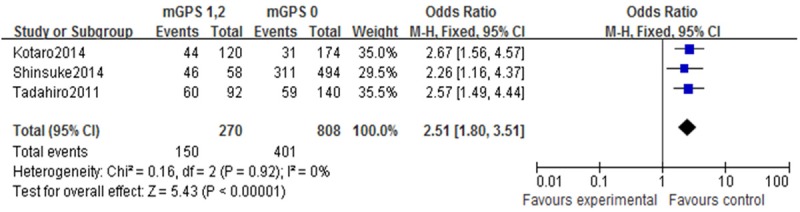
Forest plots of studies evaluating lymphatic invasion and modified Glasgow Prognostic Score (mGPS). Lymphatic invasionin gastric cancer patients with an mGPS score ≥1 compared with patients with a mGPS score of 0. CI: confidence interval.
mGPS and venous invasion
Three studies compared mGPS and venous invasion in GC patients. Arandom-effects model was applied to deal with heterogeneity in this section. The results show that patients with a mGPS≥1 have a significantly higher positivevenous invasion rate (P<0.01) (Figure 4).
Figure 4.
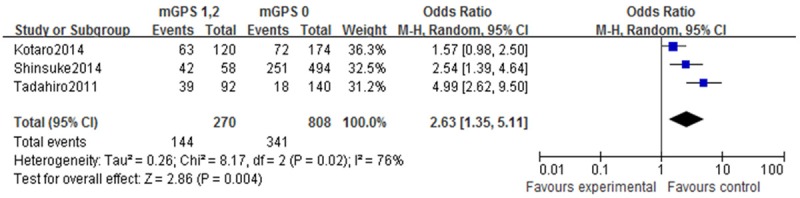
Forest plots of studies evaluating venous invasion and modified Glasgow Prognostic Score (mGPS). Venous invasionin gastric cancer patients with a mGPS score ≥1 compared with patients with a mGPS score of 0. CI: confidence interval.
Publication bias
A funnel plot was used to assess the included studies foroverall publication bias showed symmetry for OS rate (Figure 5).
Figure 5.
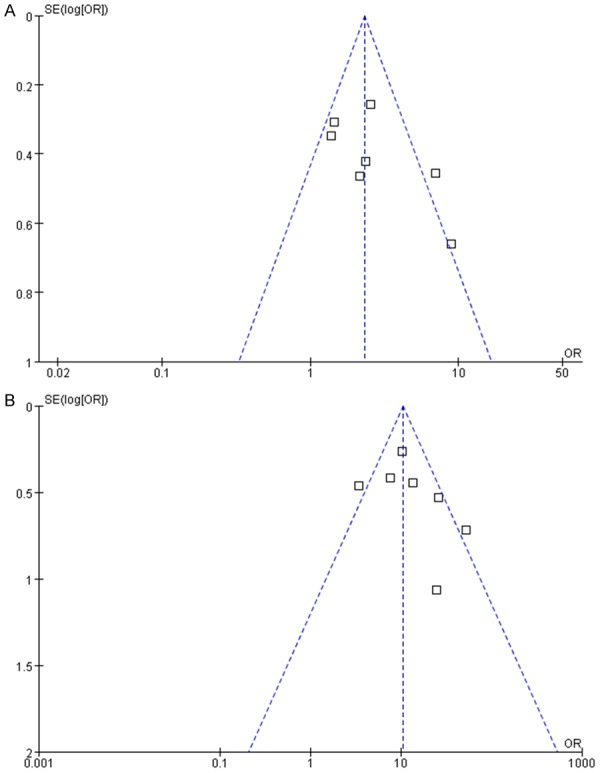
Funnel plot for evaluation OS of publication bias. mGPS 0 and mGPS1 (A) and mGPS 0 and mGPS2 (B). OR: odds ratio.
Discussion
Thehost inflammatory response influences the progression of cancerand recent studies indicate that these responses and cancer immune-editing playimportant roles inpromoting the response and immunity of tumors [19-21]. Inflammatory cells provide tumors with nutritional factors, as well as adhesion molecules and chemokines which aid in metastasis [22]. Some inflammatory cytokine increase svascular permeability and promotes tumor metastasis [23].
There are several markers that can be used to assess the systemic inflammatory response, including serum CRP levels and hypoal buminemia. Hypoalbuminemiais thought tobe aconsequence of the inflammatory response associated with elevated CRP levels [24] and has been considered as a prognostic indicator for gastroin testinal tumors [25,26] colorectal [27,28], esophageal [29], and pancreaticcancers [30]. The mGPS is basedon evaluation of CRP levels and hypoal buminemia, andhasrecently been associated with the prognosis of patients with digestive tract cancer [31,32].
Interleukin 1, interleukin 6, tumor necrosis factor and other proin flammatory cytokines can cause serum C-reactive protein elevated in patients with gastric cancer. These cytokines can promote gastric cancer cell proliferation, anti-apoptosis and angiogenesis by activating the downstream transcription factor, such as STAT3 and so on, which is significantly associatedwith inflammation, immunity, and oncogenesis [33,34] and promotes lymph node metastasis and vascular metastasis [35,36]. So constitutive activation of STAT3 have a poor prognosisin gastric cancer patients associated with mGPS [37-39]. Thus, mGPS have a close relationship with tumor metastasis in gastric cancer patients. The results of this meta-analysis show that the mGPS can also be used as a prognostic indicator for GC.
In addition to a reduced OS, GC patients with a higher mGPS are more likely to show lymphatic and venous invasion have a worse prognosis. These findings are consistent with previous studies showing thatnode metastasisand angiogenicmetastasis which affect the prognosis of GC [40,41].
In summary, the results of this meta-analysis indicate that GC patients with a mGPS≥1 have a worse prognosis than patients with a mGPS=0, thus the preoperative mGPS could serve as a prognostic factor to evaluate the survival of these patients. However, the limited number of eligible studiesand different laboratories has different evaluation criteria about mGPSincluded in the meta-analysis necessitates further verification to confirm these results.
Disclosure of conflict of interest
None.
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities indifferent geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: asystematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 5.Pasini F, Fraccon AP, DE Manzoni G. The role of chemotherapy in metastatic gastric cancer. Anticancer Res. 2011;31:3543–3554. [PubMed] [Google Scholar]
- 6.Mantovani A, Marchesi F, Porta C, Sica A, Allavena P. Inflammation and cancer: breast cancer as a prototype. Breast. 2007;16(Suppl 2):S27–33. doi: 10.1016/j.breast.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, Zhou J, Wang XC, Gong JF, Gao J, Li J, Li Y, Shen L. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–950. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 8.Lee DY, Hong SW, Chang YG, Lee WY, Lee B. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer. 2013;13:111–116. doi: 10.5230/jgc.2013.13.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba T, Marusawa H, Ushijima T. Inflammationassociated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550–563. doi: 10.1053/j.gastro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 11.Elahi MM, McMillan DC, McArdle CS, Angerson WJ, Sattar N. Score based on hypoalbuminemia and elevated C-reactive predicts survival in patients with advanced gastrointestinal cancer. Nutr Cancer. 2004;48:171–173. doi: 10.1207/s15327914nc4802_6. [DOI] [PubMed] [Google Scholar]
- 12.Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, Ezaki T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg. 2011;201:186–191. doi: 10.1016/j.amjsurg.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, Kim YR. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83:292–299. doi: 10.1159/000342376. [DOI] [PubMed] [Google Scholar]
- 14.Takeno S, Hashimoto T, Shibata R, Maki K, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87:205–214. doi: 10.1159/000362601. [DOI] [PubMed] [Google Scholar]
- 15.Kotaro H, Masayuki W, Hironobu S, Imamura Y, Ida S, Iwatsuki M, Ishimoto T, Iwagami S, Baba Y, Baba H. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49:1040–1046. doi: 10.1007/s00535-013-0855-5. [DOI] [PubMed] [Google Scholar]
- 16.Aurello P, Tierno SM, Berardi G, Tomassini F, Magistri P, D’Angelo F, Ramacciato G. Value of preoperative inflammation-based prognostic scores in predicting overall survival and disease-free survival in patients with gastric cancer. Ann Surg Oncol. 2014;21:1998–2004. doi: 10.1245/s10434-014-3533-9. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Hiki N, Nunobe S, Kumagai K, Kubota T, Aikou S, Sano T, Yamaguchi T. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer. 2012;107:275–279. doi: 10.1038/bjc.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YP, Wei J, Yang Y, Shen J, Liu BR. Multivariate analysis of prognosis in patients with advanced gastric cancer. Chin Clin Oncol. 2014;19:524–529. [Google Scholar]
- 19.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41:64–69. doi: 10.1080/01635581.2001.9680613. [DOI] [PubMed] [Google Scholar]
- 20.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shaiba R, McMillan DC, Angerson WJ, Leen E, McArdle CS, Horgan P. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer. 2004;91:205–207. doi: 10.1038/sj.bjc.6601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, Huang MH, Huang BS. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8:1041–1048. doi: 10.1016/j.gassur.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, Cervera E, Mohar-Betancourt A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381–389. doi: 10.1245/s10434-006-9093-x. [DOI] [PubMed] [Google Scholar]
- 27.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer. 2008;7:331–337. doi: 10.3816/CCC.2008.n.044. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi T, Teruya M, Kishiki T, Endo D, Takenaka Y, Tanaka H, Miki K, Kobayashi K, Morita K. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144:729–735. doi: 10.1016/j.surg.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Glen P, Jamieson NB, McMillan DC, Carter R, Imrie CW, McKay CJ. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology. 2006;6:450–453. doi: 10.1159/000094562. [DOI] [PubMed] [Google Scholar]
- 31.Richards CH, Leitch EF, Horgan PG, Anderson JH, McKee RF, McMillan DC. The relationship between patient physiology, the systemicinflammatory response and survival in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2010;103:1356–1361. doi: 10.1038/sj.bjc.6605919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizuka M, Nagata H, Takagi K, Kubota K. Influence of inflammation based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal. Ann Surg. 2009;250:268–272. doi: 10.1097/SLA.0b013e3181b16e24. [DOI] [PubMed] [Google Scholar]
- 33.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4:250–255. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, Mao F, Wang M, Zhu W, Qian H, Xu W. The IL-6-STAT3 axis mediates a reciprocal crosstalkbetween cancer-derived mesenchymal stem cells andneutrophils to synergistically prompt gastric cancerprogression. Cell Death Dis. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 38.Xiong H, Du W, Wang JL, Wang YC, Tang JT, Fang JY. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl) 2012;90:1037–1046. doi: 10.1007/s00109-012-0869-0. [DOI] [PubMed] [Google Scholar]
- 39.Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646–651. doi: 10.1111/j.1440-1746.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- 40.Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380–384. doi: 10.1080/00365520310008629. [DOI] [PubMed] [Google Scholar]
- 41.Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Kosaka T, Ono HA, Otsuka Y, Akiyama H, Ichikawa Y, Shimada H. Tumor diameter as a prognostic factor in patients with gastric cancer. Ann Surg Oncol. 2008;15:1959–1967. doi: 10.1245/s10434-008-9884-3. [DOI] [PubMed] [Google Scholar]


