Abstract
Objective: To systematic review and estimate the accuracy of Interleukin 6 assay in the diagnosis of sepsis by meta-analysis. Methods: With the aim to confirm this correlation, this paper performed a meta-analysis of 6 studies and the Sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) with corresponding 95% confidence intervals (CI) of each study were calculated and the pooled sensitivity was calculate using Random Effects Model and Summary receiver operating characteristic curves were constructed. Results: The pooled sensitivity for the diagnosis of sepsis was 80% (95% CI, 77% to 83%) and the specificity of 85% (95% CI, 81% to 88%). For sepsis versus health or infection, the area under the curve was 0.868. In neonate subgroup, IL-6 had a pooled sensitivity of 77.0% (95% CI, 73.0% to 81.0%) and specificity of 91.0% (95% CI, 86.0% to 94.0%) for sepsis diagnosis. In adult, IL-6 had a pooled sensitivity of 85.0% (95% CI, 80.0% to 88.0%) and specificity of 62.0% (95% CI, 55.0% to 68.0%) to identify sepsis. The AUC was 81.0%, and Q was 0.74. Conclusions: IL6 is a highly accurate diagnostic modality for the identification of sepsis, with promise for integration into routine imaging protocols for thyroid nodules.
Keywords: Sepsis, IL-6, meta-analysis, diagnosis, SROC
Introduction
Sepsis is a potentially life-threatening complication of an infection [1] and it causes millions of deaths globally each year [2], and more than 200 000 deaths each year in the United States [3]. Sepsis occurs when chemicals released into the bloodstream to fight the infection trigger inflammatory responses throughout the body [4]. This inflammation can trigger a cascade of changes that can damage multiple organ systems, causing them to fail. As a severe disease, sepsis not only lowers patient’s living quality, but also increases the mortality significantly.
Cytokines such as tumor necrosis factor, interleukin 1, and interleukin 6 can activate procoagulation factors in the cells lining blood vessels and lead to endothelial damage. The damaged endothelial surface inhibits anticoagulant properties as well as increases anti-fibrinolysis, which can lead to a systemic inflammatory response syndrome (SIRS), sepsis shock, disseminated intravascular coagulation (DIC), and multiple organ dysfunction syndrome (MODS) [5]. Now, those cytokines have been researched as potential biological markers of Sepsis Diagnosis or assessment [4,6,7]. But the clinical values of the biomarkers are still uncertain or controversial in diagnosis and evaluation of sepsis.
Up to now, several studies has investigated validity of Interleukin-6 (IL-6) validity for early Sepsis diagnosis. However, large sample multi-center study or meta-analysis on interleukin-6 on sepsis diagnosis is still lacking. So we performed this systematic review and meta-analysis to assess the validity of IL-6 test for early sepsis diagnosis systematically and quantitatively.
Methods
Data sources and search
Relevant studies without language restrictions were systematically searched by using the NCBI, Medline, Web of Science and Embase databases by two authors independently. The last retrieval date was August 14, 2014 and the search terms were “sepsis” “infected” and “interleukin-6” or “IL-6”. When more publications with duplicate samples, only the newest study was used in this research. The flow chart of the study including process was shown in Figure 1.
Figure 1.
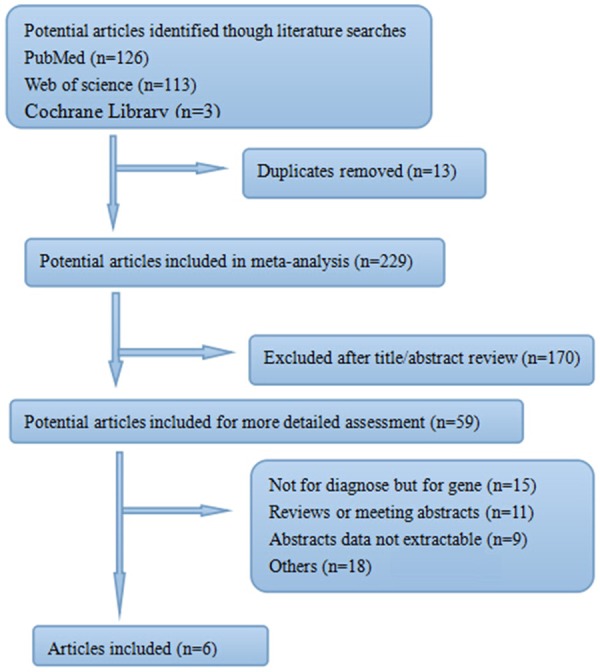
Flow diagram of study selection.
Inclusion and exclusion criteria
The inclusion criteria were: (1). studies which assessed the diagnostic accuracy of the IL-6 test on sepsis; (2). case-control study; (3). studies sensitivity and specificity were both provided and (4). articles that IL-6 levels were evaluated in study. The exclusion criteria were: (1). animal studies; (2). the reported data did not meet this study needed and (3). the reported data was not adaptable for our pooled study.
Study selection and data extraction
Two reviewers independently evaluated, extracted and integrated all of the studies retrieved based on pre-specified selection criteria and disagreements were resolved by consensus. Information of each papers, such as first author, year of publication, study method, region, measure method of IL-8, diagnostic cut-off point and time, sample size, cutoff (pg/mL), Sensitivity (%), Specificity (%), positive predictive value (%), negative predictive value (%), area under the curve were all exacted and rearrangement.
Statistical analysis
Meta-DiSc version 1.4 and RevMan 5.0.21 statistical software were applied to statistical analysis and publication bias. Sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) with corresponding 95% confidence intervals (CI) of each study were calculated and the pooled sensitivity was calculate using Random Effects Model. Statistical heterogeneity was measured using the I2-statistic and Q-statistic (P ≤ 0.05 was considered to be representative of statistically significant heterogeneity). Summary receiver operating characteristic curve (SROC) was constructed using data from all thresholds with the use of the Littenberg and Moses method, which showed the relationship between sensitivity and specificity (proportion of false positives). Q value was defined where the SROC curve crosses the anti-diagonal from (0; 1) to (1; 0) of the SROC space; hence TPR = 1-FPR at Q, and so the probability of an incorrect result from the test is the same for cases and non-cases. Meanwhile, the area under SROC curve was also calculated to show the diagnostic accuracy of IL-6 test.
Results
Literature search and study selection
The search identified a total of 242 citations, of which 59 were potentially relevant after initial evaluation. From these studies, 53 full articles were excluded. Six relevant studies with 695 Spesis and 613 adults subjects were involved in this meta-analysis based on preliminary arrangement and were analyzed (Figure 1).
Study characteristics
Table 1 showed information of the main characteristics of the selected studies. In the 6 studies analyzed, 3 evaluated the accuracy of IL-6 in neonatal sepsis diagnosis, and 3 evaluated in adults.
Table 1.
Characteristics and data extracted of the studies included in the systematic review
| Author/year of publication | Country | Age (mean ± SD or range) | Patients no. | Cut off (pg/mL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Spesis | Control | |||||||||
| Celik et al [8]/2013 | Turkey | Neonate | 206 | 98 | 18.9 | 82 (76-87) | 93 (86-97) | 97 | 67 | 91 |
| Tsalik et al [9]/2012 | USA | 52 (38-65) | 247 | 89 | 40 | 90.2 (86-94) | 33.9 (24-45) | 26.9 | 92.8 | 70 |
| Jekarl et al [10]/2013 | South Korea | 51.5 ± 22.4 | 78 | 99 | 145 | 66.7 (55-77) | 80.3 (71-87) | 29.4 (15.1-47.5) | 95.1 | 75.8 |
| Gouel-Cheron et al [11]/2012 | France | 37 ± 17 | 37 | 63 | 67.1 | 84.6 (68-94) | 72.5 (60-83) | 58 | 90 | 75 (64-84) |
| Dilli et al [12] 2010 | Turkey | Newborns | 35 | 74 | 24.9 | 80 (63-92) | 91.8 (83-97) | 82.3 | 90.6 | 89 |
| Celik et al [13]/2010 | Turkey | Newborns | 232 | 50 | 24.65 | 72 (66-78) | 84 (71-93) | 95 | 42 | |
Diagnostic performance of IL-6
As Figure 2 shown, IL-6 had a pooled sensitivity of 80.0% (95% CI, 77% to 83%) and specificity of 85% (95% CI, 81% to 88%) to detect sepsis respectively. As Figure 3 shown, the Q value and AUC of SROC for sensitivity and specificity were 0.798 and 0.868 respectively in this meta-analysis.
Figure 2.
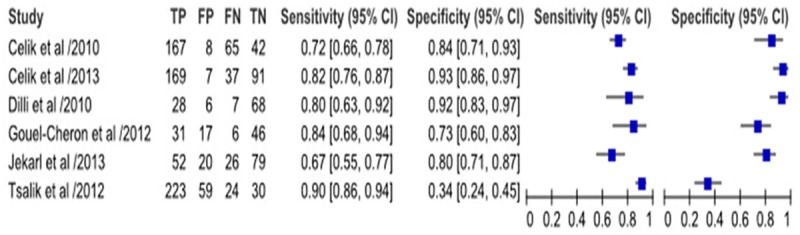
Results of the included studies.
Figure 3.
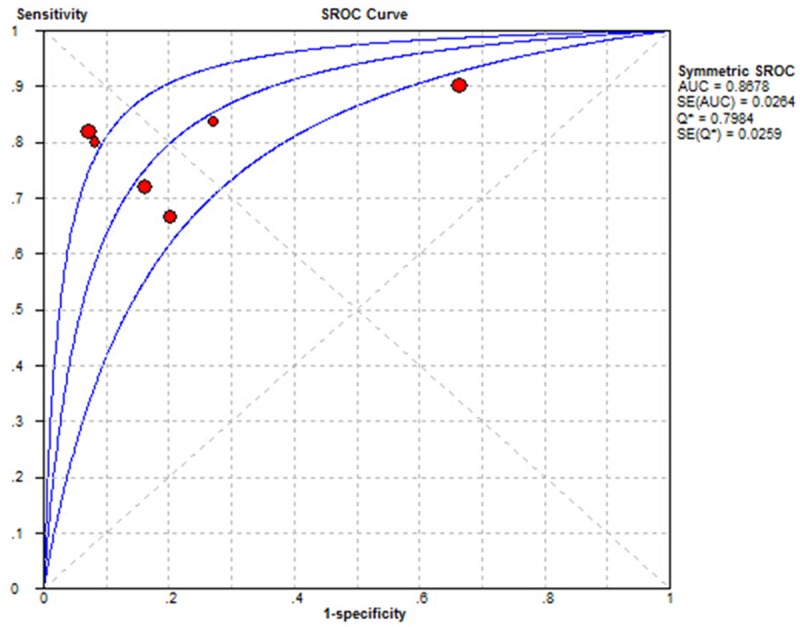
The ROC curve of the systematic review.
Sensitivity analysis was performed in 2 subgroups
We divided those 6 papers into 2 subgroups based on age. In neonate subgroup, IL-6 had a pooled sensitivity of 77.0% (95% CI, 73.0% to 81.0%) and specificity of 91.0% (95% CI, 86.0% to 94.0%) for sepsis diagnosis (Figure 4). In adult, IL-6 had a pooled sensitivity of 85.0% (95% CI, 80.0% to 88.0%) and specificity of 62.0% (95% CI, 55.0% to 68.0%) to identify sepsis. The AUC was 81.0%, and Q was 0.74 (Figure 5).
Figure 4.
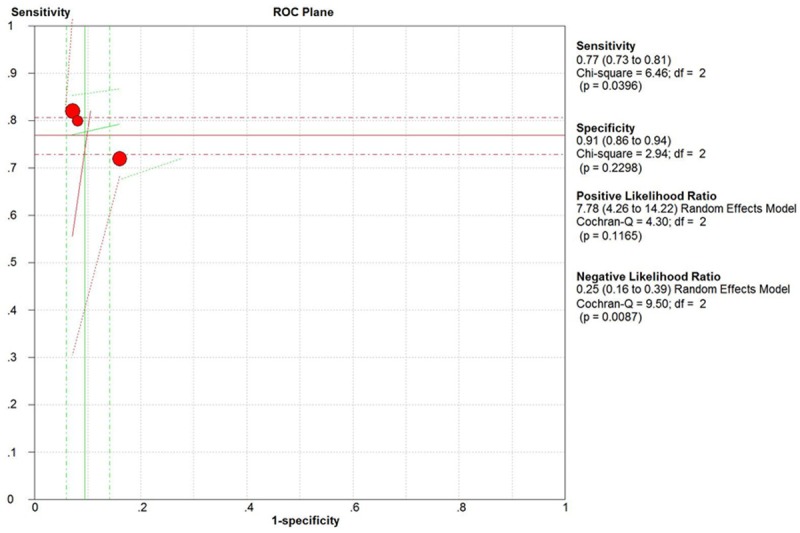
The ROC curve of neonate subgroup.
Figure 5.
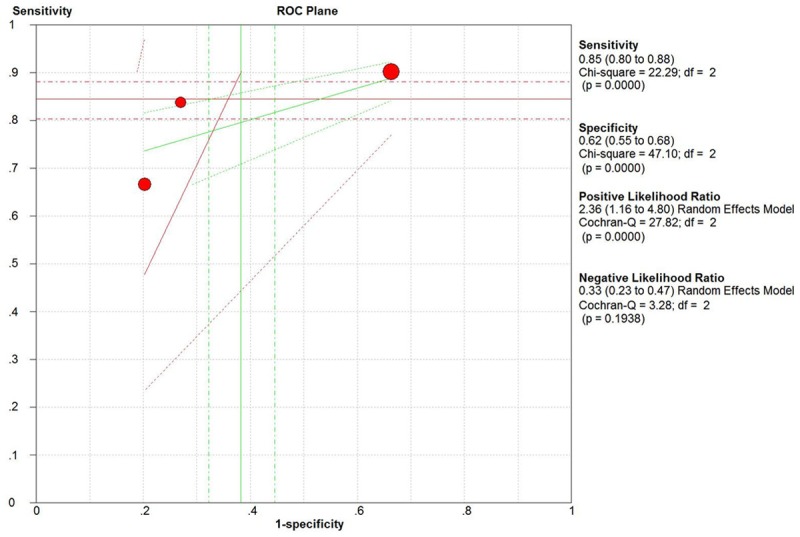
The ROC curve of adult subgroup.
Heterogeneity test
Great heterogeneity in specificity was found among studies, but not in sensitivity. In this meta-analysis, when we deleted Tsalik’s report, the value of chi-square for heterogeneity reduced from 106.30 to 16.87, indicating the heterogeneity of specificity might come from Tsalik’s report.
Discussion
Sepsis is a complex, heterogeneous disorder that is frequently misdiagnosed with significant clinical consequences [8,9]. The research on accurate and timely diagnosis or exclude of suspected sepsis is vital to patient, which can reduce morbidity, reduces cost, and improves patient outcome. To date, multiple sepsis biomarkers have been investigated for distinguish of sepsis from infectious to non-infectious processes, but most have fallen short due to poor specificity and their results remain elusive [10].
The difficult of diagnosis of bacterial infection arose from 3 aspects: 1). routine laboratory tests is lack of sensitivity and specificity; 2). the results of confirmatory microbiologic studies can’t be applied immediately; 3). atypical clinical manifestations or even absence of elderly, pediatric, and immunosuppressed infected patients [11].
IL-6 is potent inflammatory mediator and its plasma concentration has been tested as prognostic factors in several studies [12-14]. So the aim of this study is to evaluate the accuracy of biomarker IL-6 in the serum to identify sepsis by meta-analysis. Our results show that the IL-6 has a sensitivity of 80.0%, a specificity of 75.0%, and an AUC of 0.868 for the detection of sepsis or early sepsis. Subgroup comparisons revealed that no matter neonates or adults, IL-6 always have a high sensitivity and specificity to identified sepsis. It implies that IL-6 could be extensively tested for detecting sepsis in clinic. The results of this meta-analysis are similar to the previous researches, which show IL-6 and CRP are frequently elevated in non-infectious illness and can serve as useful prognostic tools in this undifferentiated population consisting of both infected and non-infected critically ill patients [15-17].
Previously studies have demonstrated that serum concentrations of IL-6 is significant increased during sepsis, which can increase incidence of poor outcome and occurrence of shock and death [18,19]. And Reinhart et al. recently demonstrated in septic patients that patients with IL-6 over 1000 pg/ml had a mortality of 56% compared to 40% of those below this IL-6 level [20].
Because of relatively small sample size of included studies and heterogeneity existed, further intensive researches with big sample size should be conducted, and to reduce heterogeneity for IL-6 as a diagnostic biomarker on sepsis diagnosis to make meta-analysis more convenient.
Conclusion
In a conclusion, IL6 is a highly accurate diagnostic modality for the identification of sepsis, with promise for integration into routine imaging protocols for thyroid nodules. Due to insufficient testing data, the experiment results need continuous re-evaluation and clinical validation.
Acknowledgements
We thank the support of Guangdong General Hospital, Guangdong Academy of Medical Sciences and Guangzhou Hospital of TCM.
Disclosure of conflict of interest
None.
References
- 1.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou Q, Wen W, Zhang XC. Presepsin as a novel sepsis biomarker. World J Emerg Med. 2014;5:16–19. doi: 10.5847/wjem.j.issn.1920-8642.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJ. Prognostic value of midregional pro-atrial natriuretic peptide in ventilator-associated pneumonia. Intensive Care Med. 2008;34:2084–2091. doi: 10.1007/s00134-008-1173-x. [DOI] [PubMed] [Google Scholar]
- 5.Nimah M, Brilli RJ. Coagulation dysfunction in sepsis and multiple organ system failure. Crit Care Clin. 2003;19:441–458. doi: 10.1016/s0749-0704(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 6.Guignant C, Voirin N, Venet F, Poitevin F, Malcus C, Bohe J, Lepape A, Monneret G. Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med. 2009;35:1859–1867. doi: 10.1007/s00134-009-1610-5. [DOI] [PubMed] [Google Scholar]
- 7.Wu HP, Chen CK, Chung K, Jiang BY, Yu TJ, Chuang DY. Plasma transforming growth factor-beta1 level in patients with severe community-acquired pneumonia and association with disease severity. J Formos Med Assoc. 2009;108:20–27. doi: 10.1016/S0929-6646(09)60028-0. [DOI] [PubMed] [Google Scholar]
- 8.de Kruif MD, Limper M, Gerritsen H, Spek CA, Brandjes DP, ten Cate H, Bossuyt PM, Reitsma PH, van Gorp EC. Additional value of procalcitonin for diagnosis of infection in patients with fever at the emergency department. Crit Care Med. 2010;38:457–463. doi: 10.1097/CCM.0b013e3181b9ec33. [DOI] [PubMed] [Google Scholar]
- 9.Glickman SW, Cairns CB, Otero RM, Woods CW, Tsalik EL, Langley RJ, van Velkinburgh JC, Park LP, Glickman LT, Fowler VG Jr, Kingsmore SF, Rivers EP. Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med. 2010;17:383–390. doi: 10.1111/j.1553-2712.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsalik EL, Woods CW. Sepsis redefined: the search for surrogate markers. Int J Antimicrob Agents. 2009;34(Suppl 4):S16–20. doi: 10.1016/S0924-8579(09)70560-6. [DOI] [PubMed] [Google Scholar]
- 11.Chan YL, Tseng CP, Tsay PK, Chang SS, Chiu TF, Chen JC. Procalcitonin as a marker of bacterial infection in the emergency department: an observational study. Crit Care. 2004;8:R12–20. doi: 10.1186/cc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, Guyomarch S, Tardy B, Bertrand JC. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000;26:1082–1088. doi: 10.1007/s001340051321. [DOI] [PubMed] [Google Scholar]
- 13.Gardlund B, Sjolin J, Nilsson A, Roll M, Wickerts CJ, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 14.Patel RT, Deen KI, Youngs D, Warwick J, Keighley MR. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg. 1994;81:1306–1308. doi: 10.1002/bjs.1800810914. [DOI] [PubMed] [Google Scholar]
- 15.Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, Fowler VG, Cairns CB, Kingsmore SF, Woods CW. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emerg Med. 2012;43:97–106. doi: 10.1016/j.jemermed.2011.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haasper C, Kalmbach M, Dikos GD, Meller R, Muller C, Krettek C, Hildebrand F, Frink M. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of multi organ dysfunction syndrome (MODS) or sepsis. Technol Health Care. 2010;18:89–100. doi: 10.3233/THC-2010-0571. [DOI] [PubMed] [Google Scholar]
- 17.Mei YQ, Ji Q, Liu H, Wang X, Feng J, Long C, Cheng B, Xing Y, Li J, Hu D. Study on the relationship of APACHE III and levels of cytokines in patients with systemic inflammatory response syndrome after coronary artery bypass grafting. Biol Pharm Bull. 2007;30:410–414. doi: 10.1248/bpb.30.410. [DOI] [PubMed] [Google Scholar]
- 18.Pettila V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcito nin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28:1220–1225. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- 19.Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996;24:163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Oberhoffer M, Karzai W, Meier-Hellmann A, Bogel D, Fassbinder J, Reinhart K. Sensitivity and specificity of various markers of inflammation for the prediction of tumor necrosis factor-alpha and interleukin-6 in patients with sepsis. Crit Care Med. 1999;27:1814–1818. doi: 10.1097/00003246-199909000-00018. [DOI] [PubMed] [Google Scholar]


