Abstract
Keloids are benign skin tumors characterized by collagen accumulation and hyperproliferation of fibroblasts. The receptor for activated C-kinase 1 (RACK1) was involved in liver fibrosis. However, the role of RACK1 in dermal fibrosis keloids is still unclear. Therefore, in this study, we investigated the effects of RACK1 on keloid fibroblasts (KFs) and transforming growth factor-β1 (TGF-β1)-induced collagen expression and explored the underlying mechanism. We found that RACK1 was decreased in KFs, overexpression of RACK1 significantly inhibited TGF-β1-induced KFs proliferation. RACK1 also obviously inhibited the expression of TGF-β1-induced TGF-β receptor I, II, type I collagen and α-smooth muscle actin (α-SMA) in human KFs. In addition, RACK1 suppressed the expression of TGF-β1-induced Smad2 and Smad3 phosphorylation in human KFs. Taken together, our study suggested that RACK1 inhibits collagen synthesis in KFs via inhibition the TGF-β1/Smad signaling pathway, and RACK1 is a potential target for treatment of the keloid disease.
Keywords: RACK1, keloid fibroblasts (KFs), collagen synthesis, TGF-β1/Smad signaling pathway
Introduction
Keloid disease is a dermal fibroproliferative tumor of unknown aetiology, which represents an abnormal pathological response to cutaneous wound healing. Keloids are characterized by substantial deposition of extracellular matrix (ECM) and invasiveness beyond the original boundary of the insult [1]. Keloid formation severely impairs quality of life by causing cosmetic and functional deformities, discomfort, and psychological stress [2]. Currently, although a wide array of therapeutic options including surgical excision, corticosteroid injections, compression therapy and laser therapy is available for treating keloids [3], there is no single effective therapeutic treatment for keloids. Therefore, there is an urgent need for a better understanding of keloid pathogenesis in order to develop better prevention and treatment approaches.
Transforming growth factor b (TGF-β) family cytokine regulates cell proliferation, migration, differentiation and plays a key role in development, tissue turnover and repair [4]. TGF-β1 classically transmits intracellular signaling via Smad proteins, then regulate the transcription of target genes, including those important in fibrosis [5]. Therefore, targeting the TGF-β/Smad signaling pathway is a promising approach for treating keloids.
The receptor for activated C-kinase 1 (RACK1) was originally identified as an intracellular receptor for protein kinase C (PKC) [6]. Previous studies have shown that RACK1 was involved in diverse cellular processes, including signal transduction, immune response, as well as cell growth, migration and differentiation [7]. Recently, it has been reported that RACK1 promotes TGF-β1 and platelet-derived growth factor (PDGF)-mediated activation of pro-fibrogenic pathways as well as the differentiation, proliferation and migration of hepatic stellate cells (HSCs), and depletion of RACK1 suppresses the progression of thioacetamide-induced liver fibrosis in vivo [8]. However, the role of RACK1 in dermal fibrosis formation is still unclear. Therefore, in this study, we investigated the effects of RACK1 on keloid fibroblasts (KFs) and TGF-β1-induced collagen expression and explored the underlying mechanism.
Materials and methods
Keloid-derived and normal dermal fibroblast origin and cell culture
Six keloid tissue samples used in this study were obtained from six Chinese patients [four males and two males, age range 17-43 (mean 28.3) years] after undergoing surgical excision. Normal skin tissue samples were obtained from six different Chinese volunteers. All experiments were performed after obtaining approval of the ethics committee of the First Hospital of Zibo City, and in accordance with the Declaration of Helsinki Principles. Written informed consent was obtained from each individual.
After specimen collection, the KFs were isolated and cultured as described previously [9]. The cells were grown to confluence and only low-passage cultures (passages 4-6) were analyzed for further experiments.
Plasmids and transfection assays
The cDNAs encoding green fluorescent protein (GFP) and GFP-RACK1 were sub-cloned to pAdTrack-cytomegalovirus (CMV). Then, the recombinant shuttle plasmids pAdTrack-CMV and pAdEasy-1 were homologous in Escherichia coli strain BJ5183. The obtained recombinant plasmids were transfected into 293 cells to generate recombinant adenovirus. The virus was amplified and purified, and titers were determined using the p24 ELISA kit (Cell Biolabs, Inc., San Diego, CA, USA). For in vitro transfection, human KFs were seeded in each well of 24-well microplates, grown for 24 h to reach 50% confluence, and transfected with overexpression of RACK1 using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocols.
Cell proliferation assay
Cell proliferation was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After achieving 50% confluence, KFs were starved for 24 h with α-MEM and transfected with overexpression of RACK1 for 96 h, then 5 mg/ml of MTT (Sigma, St. Louis, MO, USA) was added to the wells and incubated at 37°C for 4 h. The MTT medium was carefully removed and dimethyl sulphoxide (Sigma, St. Louis, MO, USA) was added to each well. After 10 minutes, the absorbance was measured at 550 nm on an enzyme-linked immunosorbent assay plate reader (Bio-tek, Winooski, VT, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
KFs were collected in cell lysis buffer. Total RNA was extracted from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the supplier’s instructions. Reverse transcription of 5 μg of the total RNA into cDNA was performed by using M-MLV reverse transcriptase (Clon-tech, Palo Alto, CA, USA). The specific primers for RACK1 were sense, 5’-TTCTCCTCTGACAACCGGCA-3’, and antisense, 5’-GCCATCCTTGCCTCCAGAA-3’; and for β-actin were sense, 5’-AAATCGTGCGTGACATCAAAGA-3’ and antisense, 5’-GGCCAT CTCCTGCTCGAA-3’. The primers were all synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The cycling conditions were as follows: initial denaturation at 94°C for 5 min; 30 cycles of denaturation at 94°C for 20 s, annealing at 55°C for 30 s and extension at 72°C for 20 s; and melt curve from 65 to 95°C. β-actin was used as the internal reference gene. Then, the relative quantification of the gene of interest was determined by using the comparative DCt method.
Western blot analysis
The protein in cell lysis was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by transference to a nitrocellulose membrane (Amersham, Little Chalfont, UK). The membrane was then blocked with 2.5% nonfat dry milk for 1 h. Primary antibodies (anti-RACK1, anti-TGF-β RI, anti-TGF-β RII, anti-α-smooth muscle actin (α-SMA), anti-collagen I, anti-Smad2, anti-Smad3, anti-p-Smad2, anti-p-Smad3 and anti-GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added and incubated overnight at 4°C. After incubation with the corresponding horseradish peroxidase-conjugated secondary antibody (Boster Corporation, Wuhan, China), the target protein was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Statistical analysis
Data were expressed as the means ± SD. The statistical significance was determined by using the Student t test for differences between two groups and one-way ANOVA for differences among multiple groups. A P value of less than 0.05 was considered statistically significant.
Results
RACK1 is decreased in human KFs
RACK1 has been reported in other fibroblasts, and fibroblasts are thought to be the predominant effector cell in skin fibrosis, therefore, we investigated the expression of RACK1 in normal skin fibroblasts (NFs) and KFs. As shown in Figure 1A, the mRNA expression of RACK1 in KFs was obviously decreased compared to that of NFs (P<0.05). Similarly, the protein expression of RACK1 in KFs was also significantly decreased compared to that of NFs (P<0.05) (Figure 1B). These data implied that RACK1 expression was inhibited in KFs progression.
Figure 1.
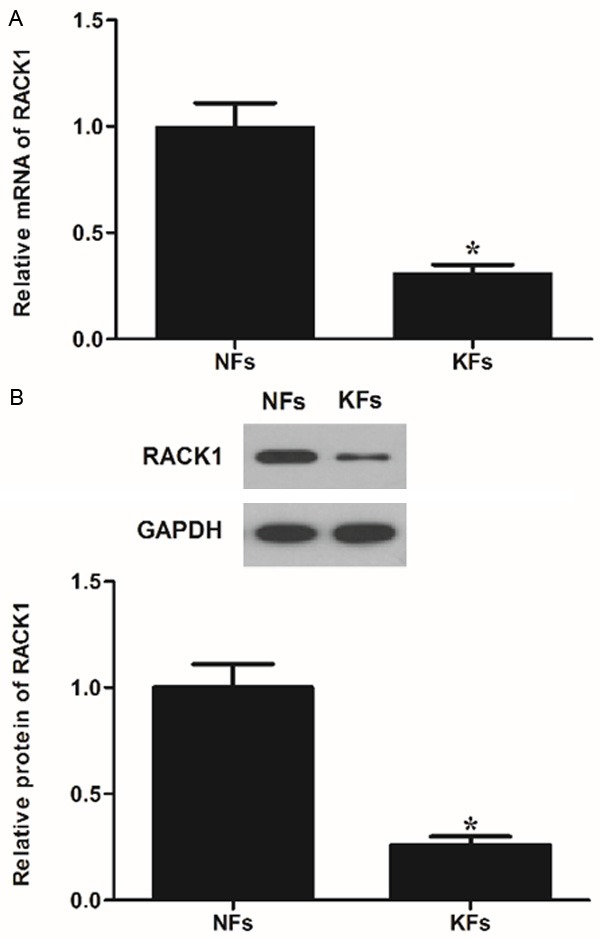
Expression of RACK1 in keloid fibroblasts. (A) RT-PCR and (B) Western blot images for RACK1 expression in keloid fibroblasts (KFs). A representative result of three independent experiments is shown. Normal dermal fibroblasts (NFs) were the positive control of RACK1 expression. Data were expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 compared with the NFs group.
Overexpression of RACK1 inhibits TGF-β1-induced KFs proliferation
To investigate whether overexpression of RACK1 alleviates KFs, we constructed recombinant adenovirus expressing RACK1. The expression of RACK1 in KFs was analyzed. As shown in Figure 2A, RACK1 mRNA expression was significantly increased after transfection of recombinant adenovirus expressing RACK1. Consistent with the results of RT-PCR, western blot analysis showed that RACK1 protein expression was also increased by Ad-RACK1 (Figure 2B). Moreover, we evaluated the effect of overexpression of RACK1 on KFs proliferation. As shown in Figure 2C, overexpression of RACK1 significantly inhibited the proliferation of KFs, which was induced by TGF-β1. These results suggested that RACK1 had a regulatory effect on TGF-β1-induced KFs proliferation.
Figure 2.
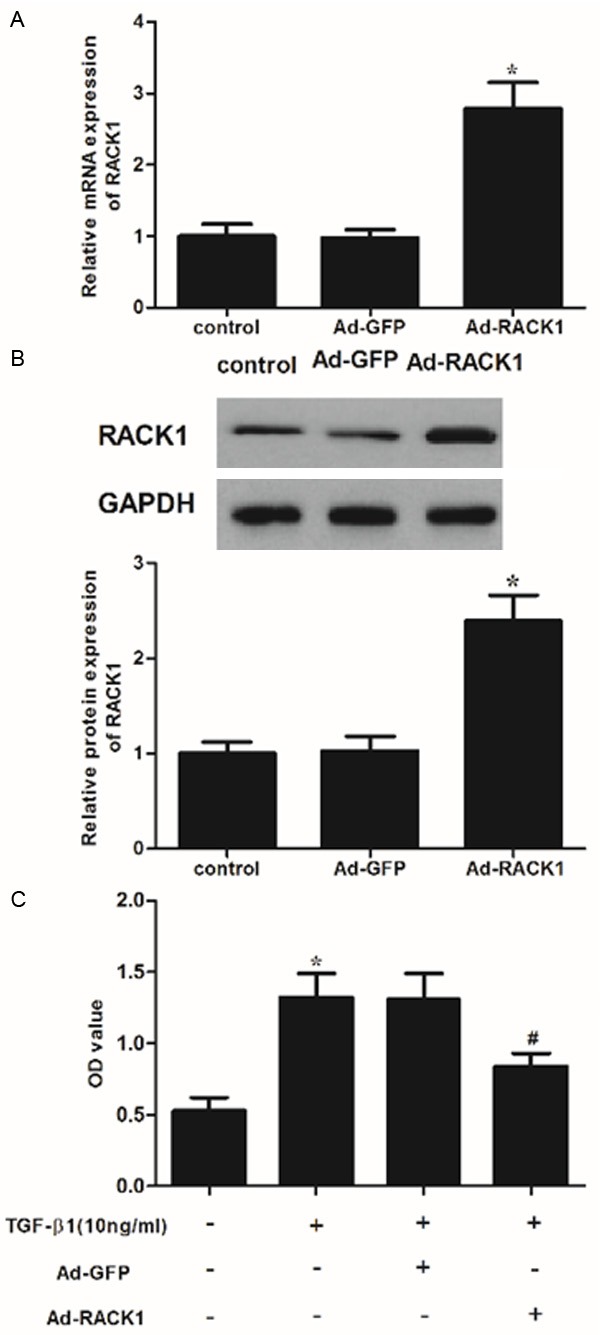
RACK1 inhibited transforming growth factor (TGF)-β1-induced KF proliferation. A. RACK1 mRNA expression was detected by qRT-PCR 48 h after transfection with over-expression of RACK1. *P<0.05 compared with the control group. B. Western blot analysis of RACK1 48 h after transfection with overexpression of RACK1. GAPDH was used as a loading control. All experiments were repeated at least three times and all data are reported as mean ± SD; C. KFs were incubated with overexpression of RACK1 or control for 96 h. Then cells were treated with TGF-β1 (10 ng/ml). The effect of RACK1 on the viability of KFs. Cell proliferation was estimated using the MTT assay. Data were expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 compared with the control group. #P<0.05 compared with the TGF-β1 group.
Overexpression of RACK1 inhibits the expression of TGF-β1-induced a-SMA and type I collagen in human KFs
Keloids, characterized by excess ECM deposition of fibroblasts, occur as a result of abnormal wound healing after injury to the skin [10]. Therefore, we examined the effect of RACK1 on α-SMA and collagen I expression levels in TGF-β1-stimulated KFs. As shown in Figure 3, TGF-β1 treatment increased the expression of α-SMA and collagen I in KFs, however, RACK1 significantly suppressed TGF-β1-induced the expression levels of α-SMA and collagen I.
Figure 3.
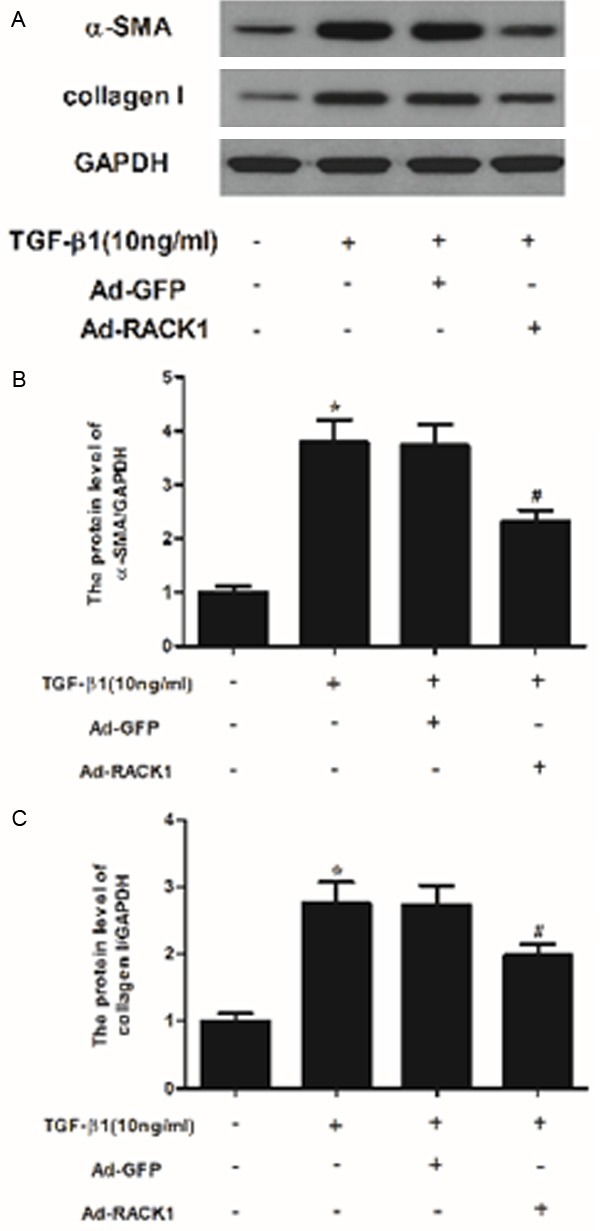
RACK1 inhibited the expression of α-SMA and collagen type I in TGF-β1-induced KFs. (A) Western blot analysis was performed to detect the protein expression levels of α-SMA and collagen type I using the antibodies indicated. The relative protein expression levels of α-SMA (B) and collagen type I (C) were quantified using Image-Pro Plus 6.0 software and normalized to GAPDH. Data were expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 compared with the control group. #P<0.05 compared with the TGF-β1 group.
Overexpression of RACK1 inhibits the expression of TGF-β1-induced TGF-β receptor I and II in human KFs
Because TGF-β/Smad signaling pathways perform a significant role in keloid formation and TGF-β receptor binding initiates the signaling cascade [11,12], therefore, we examined the effect of RACK1 on TGF-β receptor I (TGF-β RI) and II expression levels in TGF-β1-stimulated KFs. As shown in Figure 4, TGF-β1 significantly increased TGF-β RI and TGF-β RII expression in KFs. However, RACK1 treatments dramatically suppressed the TGF-β1-enhanced TGF-β RI and TGF-β RII expression in KFs.
Figure 4.
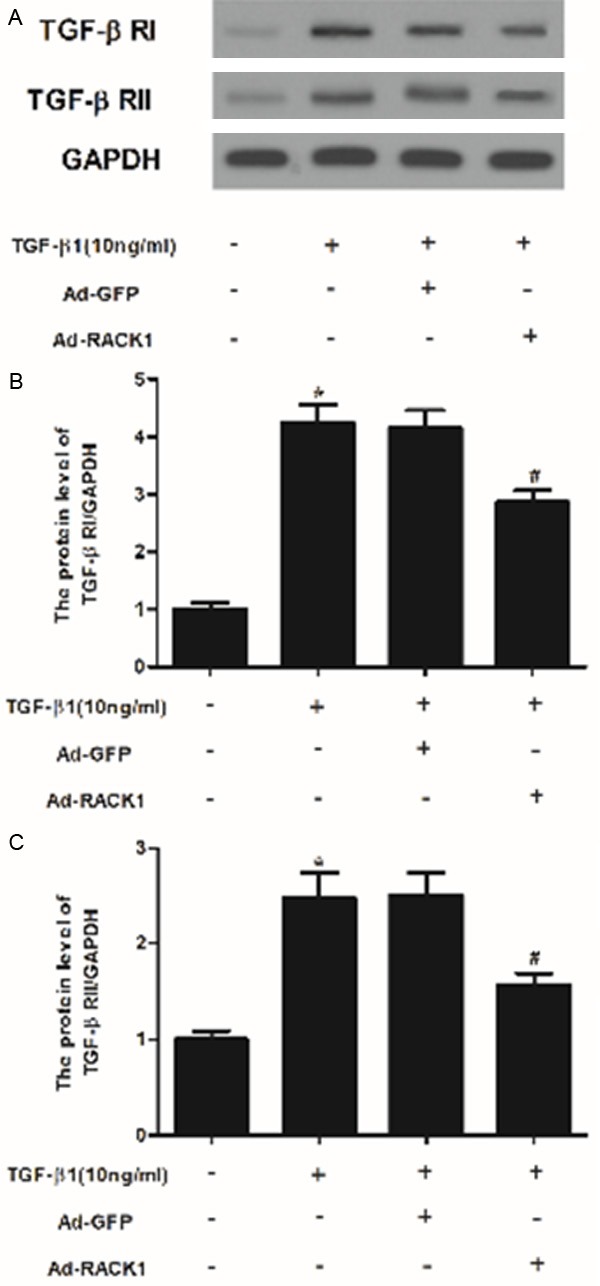
RACK1 inhibited the expression of TGF-β receptor I and II in TGF-β1-induced KFs. (A) Western blot analysis was performed to detect the protein expression levels of TGF-β RI and II using the antibodies indicated. The relative protein expression levels of TGF-β RI (B) and II (C) were quantified using Image-Pro Plus 6.0 software and normalized to GAPDH. Data were expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 compared with the control group. #P<0.05 compared with the TGF-β1 group.
Overexpression of RACK1 inhibits the expression of TGF-β1-induced Smad2, Smad3 and Smad2/3 phosphorylation in human KFs
TGF-β plays a central role in the pathogenesis of fibrosis by inducing and sustaining activation of KFs [13,14]. Therefore, to evaluate the potential mechanism of the effects of RACK1 on TGF-β RI and TGF-β RII, we investigated the effect of RACK1 on the expression of TGF-β1-induced Smad2 and Smad3 phosphorylation in KFs. As shown in Figure 5, TGF-β1 treatment stimulated phosphorylation of Smad2 and Smad3, however, RACK1 prevented TGF-β1-induced phosphorylation of Smad2 and Smad3 in KFs.
Figure 5.
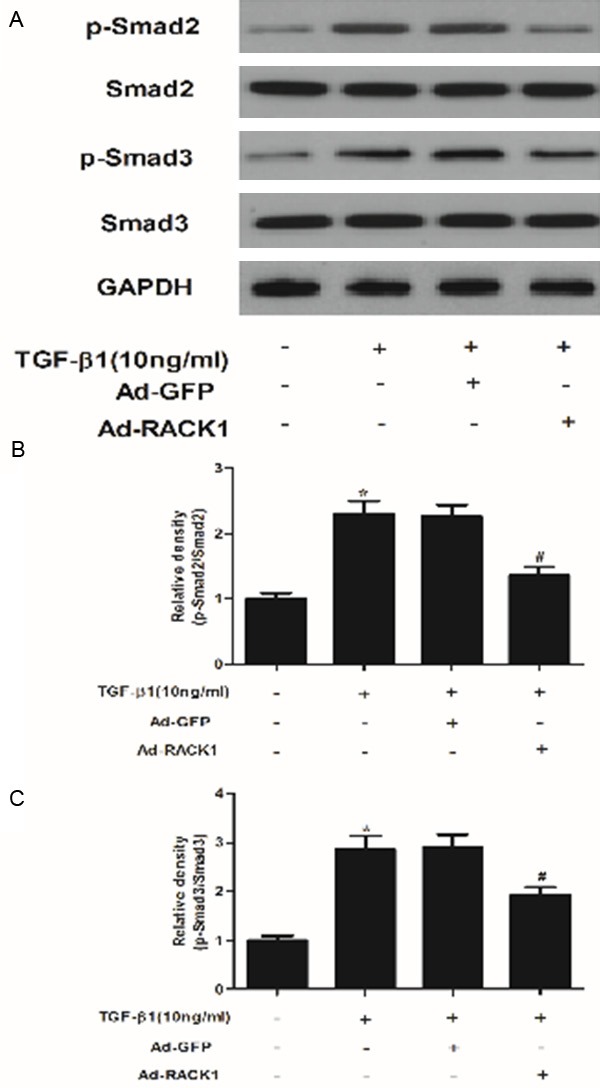
RACK1 is involved in the regulation of TGF-β1-mediated signaling pathways in KFs. (A) KFs were incubated with overexpression of RACK1 or control for 48 h. Then cells were treated with TGF-β1 (10 ng/ml) for 24 h and subjected to western blot analysis using the antibodies indicated. Quantitative analysis of protein expression levels of p-Smad2 (B) and p-Smad3 (C) using Image-Pro Plus 6.0 software and normalized to GAPDH. Data were expressed as means ± SD. Experiments were performed in triplicate. *P<0.05 compared with the control group. #P<0.05 compared with the TGF-β1 group.
Discussion
Keloids occur as a result of a pathological wound-healing process, characterized by excessive production of ECM protein. In the present, we found that RACK1 was decreased in KFs, overexpression of RACK1 inhibited TGF-β1-induced KFs proliferation. RACK1 also inhibits the expression of TGF-β1-induced TGF-β RI, II, collagen I and α-SMA in human KFs. In addition, RACK1 suppresses the expression of TGF-β1-induced Smad2 and Smad3 phosphorylation in human KFs.
RACK1 has been reported to be dysregulated in several cancer types, including gastric cancer, colon cancer and breast cancers [15-17]. However, the expression pattern and the function of RACK1 in dermal fibrosis remained unknown. Most recently, Satish et al. demonstrated that the expression of RACK1 was decreased in both adult skin and mucosal wounds relative to unwounded controls [18]. Consistently, we found that the expression of RACK1 was significantly decreased in KFs, and RACK1 inhibited TGF-β1-induced KFs proliferation, which suggested that RACK1 may inhibit the development of keloids. However, one study demonstrated that the expression of RACK1 is increased in activated HSCs, and up-regulation of RACK1 induces TGF-β1 and PDGF-mediated pro-fibrogenic pathways as well as the differentiation, migration and proliferation of HSCs [8]. These findings suggest that RACK1 performs a pro-fibrogenic or anti-fibrogenic function depends on the cell type or context.
A number of evidence have revealed that the excessive deposition of type I collagen and α-SMA in ECM is another clinical characteristic of keloid disease [19-21]. In this study, we found that RACK1 inhibited the expression of TGF-β1-induced collagen I and α-SMA in KFs. These results suggest that RACK1 exhibits a suppressive effect on collagen I and α-SMA expression in KFs in the presence of TGF-β1, and thereby inhibits the development of keloids.
TGF-β signals through two types of membrane-bound serine/threonine kinase receptors and intra-cellular Smad proteins. Ligand binding results in the formation of the TGF-β type I/II receptor heteromeric complex. Then the Smad2/Smad3/Smad4 complex translocates into the nucleus binding target gene promoters and activating transcription [22,23]. Increasing evidence has demonstrated that blocking the TGF-β/Smad signaling pathway plays an important role in inhibiting KF proliferation and collagen synthesis, thereby preventing the development of keloids [24-26]. It has been reported that the expression levels of TGF-β RI and TGF-β RII in KFs were higher than that of in normal dermal fibroblasts [27]. Moreover, within the R-Smad family, Smad3 mainly mediates collagen production in dermal fibroblasts stimulated by TGF-β. Wang et al. reported that inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts [28]. Phan et al. demonstrated that when normal fibroblasts were co-cultured with keloid-derived keratinocytes, Smad2 and Smad3 phosphorylation was up-regulated, and the formation of the Smad2/3/4 protein complex was also enhanced [29]. In agreement with previous studies, in the present study, we found that TGF-β1 increased the expression of TGF-β RI and TGF-β RII and activated phosphorylation of Smad2 and Smad3 in KFs, however, RACK1 treatments dramatically suppressed the TGF-β1-enhanced TGF-β RI and TGF-β RII expression, and prevented phosphorylation of Smad2 and Smad3. These results suggest that RACK1 inhibits KF proliferation and collagen synthesis in KFs via inhibition the TGF-β1/Smad signaling pathway.
In conclusion, these results suggest that RACK1 inhibits collagen synthesis in KFs via inhibition the TGF-β1/Smad signaling pathway, and RACK1 is a potential target for treatment and/or prevention of the keloid disease.
Disclosure of conflict of interest
None.
References
- 1.Jagadeesan J, Bayat A. Transforming growth factor beta (TGFβ) and keloid disease. Int J Surg. 2007;5:278–285. doi: 10.1016/j.ijsu.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Seifert O, Mrowietz U. Keloid scarring: bench and bedside. Arch Dermatol Res. 2009;301:259–272. doi: 10.1007/s00403-009-0952-8. [DOI] [PubMed] [Google Scholar]
- 3.Wolfram D, Tzankov A, Pülzl P, PIZA-KATZER H. Hypertrophic scars and keloids-a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 4.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaing and gene regulation: consequence for extracellular matrix remodelig and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Branton MH, Kopp JB. TGF-β and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 6.Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 7.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 8.Jia D, Duan F, Peng P, Sun L, Liu X, Wang L, Wu W, Ruan Y, Gu J. Up-regulation of RACK1 by TGF-β1 promotes hepatic fibrosis in mice. PLoS One. 2013;8:e60115. doi: 10.1371/journal.pone.0060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang GY, Yi CG, Li X, Ma B, Li ZJ, Chen XL, Guo SZ, Gao WY. Troglitazone suppresses transforming growth factor-β1-induced collagen type I expression in keloid fibroblasts. Brit J Dermatol. 2009;160:762–770. doi: 10.1111/j.1365-2133.2008.08989.x. [DOI] [PubMed] [Google Scholar]
- 10.Bettinger DA, Yager DR, Diegelmann RF, Cohen KI. The Effect of TGF-[beta] on Keloid Fibroblast Proliferation and Collagen Synthesis. Plast Reconstr Surg. 1996;98:827–833. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Tang B, Zhu B, Liang Y, Bi L, Hu Z, Chen B, Zhang K, Zhu J. Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res. 2011;303:563–572. doi: 10.1007/s00403-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 12.Bran GM, Sommer UJ, Goessler UR, Hoermann K, Riedel F, Sadick H. TGF-β1 antisense impacts the SMAD signalling system in fibroblasts from keloid scars. Anticancer Res. 2010;30:3459–3463. [PubMed] [Google Scholar]
- 13.Zhang G, Cheng T, Luan Q, Liao T, Nie C, Zheng X, Xie X, Gao W. Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Brit J Dermatol. 2011;164:729–737. [Google Scholar]
- 14.Mun JH, Kim YM, Kim BS, Kim JH, Kim MB, Ko HC. Simvastatin inhibits transforming growth factor-β1-induced expression of type I collagen, CTGF, and α-SMA in keloid fibroblasts. Wound Repair Regen. 2014;22:125–133. doi: 10.1111/wrr.12136. [DOI] [PubMed] [Google Scholar]
- 15.Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY, Li G, Ji XD, Shi S, Guan DX. RACK1 suppresses gastric tumorigenesis by stabilizing the β-catenin destruction complex. Gastroenterology. 2012;142:812–823. e815. doi: 10.1053/j.gastro.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Subauste MC, Ventura-Holman T, Du L, Subauste JS, Chan SL, Yu VC, Maher JF. RACK1 down-regulates levels of the pro-apoptotic protein Fem1b in apoptosis-resistant colon cancer cells. Cancer Biol Ther. 2009;8:2297–305. doi: 10.4161/cbt.8.23.10262. [DOI] [PubMed] [Google Scholar]
- 17.Cao XX, Xu JD, Xu JW, Liu XL, Cheng YY, Wang WJ, Li QQ, Chen Q, Xu ZD, Liu XP. RACK1 promotes breast carcinoma proliferation and invasion/metastasis in vitro and in vivo. Breast Cancer Res Treat. 2010;123:375–386. doi: 10.1007/s10549-009-0657-x. [DOI] [PubMed] [Google Scholar]
- 18.Satish L, Hogg J, Johnson S, Oswald D, Post JC, Ehrlich GD, Kathju S. Expression of receptor for activated C kinase 1 in healing skin and mucosal wounds. Ann Plast Surg. 2010;64:238–241. doi: 10.1097/SAP.0b013e31819537fc. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal C, Britton ZT, Alaseirlis DA, Li Y, Wang JHC. Healing and normal fibroblasts exhibit differential proliferation, collagen production, α-SMA expression, and contraction. Ann Biomed Eng. 2006;34:653–659. doi: 10.1007/s10439-006-9090-z. [DOI] [PubMed] [Google Scholar]
- 20.Friedman DW, Boyd CD, Mackenzie JW, Norton P, Olson RM, Deak SB. Regulation of collagen gene expression in keloids and hypertrophic scars. J Surg Res. 1993;55:214–222. doi: 10.1006/jsre.1993.1132. [DOI] [PubMed] [Google Scholar]
- 21.Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Jap J Pharmacol. 1992;60:91–96. doi: 10.1254/jjp.60.91. [DOI] [PubMed] [Google Scholar]
- 22.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 23.Massagué J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST, Longaker MT. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: implications for the treatment of excessive scars. J Trauma. 2004;57:1032–1037. doi: 10.1097/01.ta.0000114087.46566.eb. [DOI] [PubMed] [Google Scholar]
- 25.He S, Liu X, Yang Y, Huang W, Xu S, Yang S, Zhang X, Roberts M. Mechanisms of transforming growth factor β1/Smad signalling mediated by mitogenactivated protein kinase pathways in keloid fibroblasts. Brit J Dermatol. 2010;162:538–546. doi: 10.1111/j.1365-2133.2009.09511.x. [DOI] [PubMed] [Google Scholar]
- 26.Tang LX, He RH, Yang G, Tan JJ, Zhou L, Meng XM, Huang XR, Lan HY. Asiatic acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS One. 2012;7:e31350. doi: 10.1371/journal.pone.0031350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin GS, Liu W, Peled Z, Lee TY, Steinbrech DS, Hsu M, Longaker MT. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423–429. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang H, Hou T, Wang Q, Yuan X, Zhu X, Wu H, Jin Y. Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthet Surg. 2007;60:1193–1199. doi: 10.1016/j.bjps.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Phan T, Lim I, Aalami O, Lorget F, Khoo A, Tan E, Mukhopadhyay A, Longaker M. Smad3 signalling plays an important role in keloid pathogenesis via epithelial-mesenchymal interactions. J Pathol. 2005;207:232–242. doi: 10.1002/path.1826. [DOI] [PubMed] [Google Scholar]


