Abstract
Objectives: The aim of this study was to investigate the intracellular mechanism involved in the anti-apoptotic effect of ghrelin on human umbilical vein endothelial cells (HUVECs). Methods: HUVECs were pretreated with ghrelin before exposure to 200 μg/ml advanced glycation end products (AGEs)-BSA for 48 h. Cell viability and apoptosis were determined by MTT assay and Annexin V/PI staining. Intracellular cGMP levels evaluation and cGMP analogs were employed to explore possible mechanisms. Results: The inhibitory effect on AGEs induced HUVECs apoptosis could be exerted by ghrelin and co-incubation with growth hormone secretagogue receptor (GHSR)-1a antagonist [D-Lys3]-GHRP-6 abolished this inhibition. Decreased cGMP level in AGEs induced HUVECs apoptosis was restored by ghrelin pretreatment and abolished by [D-Lys3]-GHRP-6 co-incubation. cGMP analogs (8 Br-cGMP and DB-cGMP) pretreatment also exhibited inhibitory effect on AGEs induced HUVECs apoptosis. Conclusions: Our results demonstrated that ghrelin produces a protective effect on HUVECs through GHS-R1a and cGMP/NO signaling pathway mediates the effect of ghrelin. These observations suggest a novel intracellular mechanism in the process of AGEs induced HUVECs apoptosis.
Keywords: Ghrelin, apoptosis, cGMP/NO signaling, HUVECs, GHS-R1a
Introduction
Hyperglycemia is defined as a condition in which an excessive amount of glucose circulates in the plasma [1]. Hyperglycemia is considered as a classic symptom in patients with diabetes mellitus (DM) [2]. Advanced glycation end products (AGEs) could be formed because of the reaction between blood glucose and amino group of proteins [3]. It is reported that AGEs play an important role in regulating the development of vascular lesion, inducing endothelial dysfunction and accelerating vascular complications in DM patients [4,5]. However, the pathophysiological process of vascular remodeling induced by AGEs remains elusive. Ghrelin, a 28-amino acid peptide hormone secreted by stomach, has been shown to be involved in several physiological processes, including growth hormone (GH) release stimulation, energy balance induction and vascular tone regulation [6-8]. Zhao et al proved that ghrelin could regulate vascular endothelial cell function through inhibition of vascular endothelial cell apoptosis [9]. However, the accurate molecular mechanism has not fully explored yet.
Here, we presented a study to investigate the intracellular mechanism involved in the protective effect of ghrelin in AGE induced human umbilical vein endothelial cells (HUVECs) apoptosis. Our results demonstrated that a novel cGMP/NO signaling pathway mediates the effect exerted by ghrelin.
Materials and methods
Cell culture
Human umbilical venous endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) , and maintained in Medium 200 (Life technology, Carlsbad, CA, USA) containing low serum growth supplement (LSGS; Life technology, Carlsbad, CA, USA), 1% penicillin-streptomycin at 37°C in a 5% CO2 incubator. HUVECs used in the experiments were between passage 2 and 6.
Cell treatment
HUVECs were seeded equally into cell culture plated and allowed to grow until 90% confluence before the medium was changed to Medium 200. To determine the anti-apoptosis effect of ghrelin and its relationship with growth hormone secretagogue receptor (GHSR)-1a, cells were pretreated with pre-incubated with vehicle or [D-Lys3]-GHRP-6 (100 μM) for 1 h and the treated with vehicle or ghrelin (1 μM; Phoenix Pharmaceuticals Inc., Burlingame, CA, USA) for 24 h followed by exposure to AGE-BSA (200 μg/ml; Biovision, Milpitas, CA USA)) for 48 h. To assess the effect of cGMP analogs pretreatment, cells were pretreated with 8-Br-cGMP (0.5 μM; Calbiochem, San Diego, CA, USA) and DB-cGMP (0.5 μM; Calbiochem) for 2 h followed by exposure to AGE-BSA (200 μg/ml) for 48 h.
MTT assay
HUVECs (5 × 103 cells/well) were seeded in 96-well plate in quadruplicate overnight and were processed different treatment described above. Cell viability was determined by MTT assay at 492 nm wavelength and calculated as follows: Cell viability (%) = (OD value of experimental group/OD value of control group) × 100%.
Apoptosis assay
HUVECs cells (2 × 105/well) grown were in 6-well plate were treated according to above description. After the treatment, HUVECs cells were harvested and analyzed for cell apoptosis by Annexin-V and propidium iodide (PI) staining, using FITC Annexin-V apoptosis detection kit (Life technology) according to the manufacturer’s instructions with a flow cytometry (Beckman Coulter, Miami, FL, USA).
Western-blot
HUVECs were processed according to above described treatment. After the treatment, cells were collected and counted. Approximate 1 × 106 Cells were washed with ice-cold phosphate buffered saline (PBS) and lysed with lysis buffer (Cell signaling Technology, Danvers, MA, USA), and the following protease and phosphatase inhibitors: aprotinin (10 mg/mL), leupeptin (10 mg/mL) (ICN Biomedicals, Asse-Relegem, Belgium), phenylmethylsulfonyl fluoride (1.72 mM), NaF (100 mM), NaVO3 (500 mM), and Na4P2O7 (500 mg/mL) (Sigma-Aldrich, St.Louis, MO, USA). The protein concentration was determined by BCA assay (Pierce, Rockford, IL USA). Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto PVDF. Immunostaining of the blots was performed using the primary antibodies, followed by secondary antibodies conjugated to horseradish peroxidase and detection by enhanced chemiluminescence reagent (Pierce, US). The primary antibodies used were anti-Caspase-3 (total and cleaved; Cell Signaling Technology), anti-Bax (Cell Signaling Technology), anti-Bcl2 (Cell Signaling Technology) and anti-beta-actin (Sigma-Aldrich). The secondary antibodies were purchased from Amersham Biosciences (Piscataway, NJ, USA).
cGMP level measurement
HUVECs (1 × 104 cells/well) were seeded in 96-well plate in quadruplicate overnight and were processed different treatment described above. To measure intracellular cGMP levels, a cGMP enzyme immunoassay (EIA) system (Amersham Biosciences) was used. The Briefly, 200 ml of lysis reagent 1 (0.5% dodecyltrimethylammonium bromide) was added to each well and the plates were shaken for 10 min at RT, then 20 ml of acetylation reagent [acetic anhydride : triethylamine (1:2 v/v)] was added, and the plates incubated for 5 min at RT to acetylate the intracellular cGMP before 100 ml of anti-cGMP antiserum was added to each well and incubated for 10 min. A sample (50 ml) of the mixture was then transferred to a well of a 96-well EIA plate coated with donkey anti-rabbit IgG antibody. After incubation for 2 h at 48°C, 100 ml of peroxidase (PO)-conjugated cGMP was added to the well, and the plate incubated for 1 h at 48C, then non-bound PO-cGMP was removed by washing with 0.05% TweenTM 20/PBS, and 200 ml of the substrate, TMB (3, 3’, 5, 5’-tetramethyl benzidine/hydrogen peroxide in 20% dimethyl formamide) was added, and the plate incubated at RT for 30 min. The cGMP concentration was then measured the absorption at 630 nm using n microplate reader (Molecular Device, Sunnyvale, CA, USA).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using one way ANOVA and an unpaired Student’s t-test. A p-value of 0.05 or less was considered statistically significant. Statistical calculations were performed using SPSS software for the MS Windows operating system (Version 18.0; SPSS Inc, Chicago, IL, USA).
Results
Ghrelin protected HUVECs from AGEs induced cell apoptosis
We first examined the effect of ghrelin treatment on AGEs induced HUVECs apoptosis. As shown in Figure 1, ghrelin pretreatment could significantly increase cell viability (78% ±10% vs. 50% ± 8%, P < 0.05) and decrease cell apoptosis (8% ± 3% vs. 21% ± 5%, P < 0.05) compared with AGEs treated cells (Figures 1, 5A-C).
Figure 1.
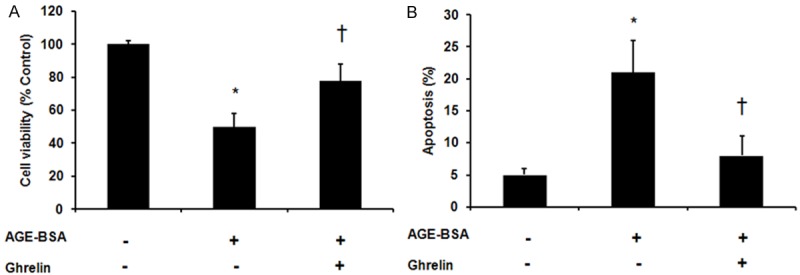
Effect of ghrelin on advanced glycation end products (AGEs) induced apoptosis in HUVECs. HUVECs were pre-incubated with vehicle control or ghrelin (1 μM) for 24 h followed by exposure to AGE-BSA for 48 h. A. Cell viability analysis by MTT assay. B. Flow cytometry analysis of apoptotic cells using Annexin-V and propidium iodide staining. *P < 0.05 when compared with vehicle control. †P < 0.05 when compared with AGEs treated cells.
Figure 5.
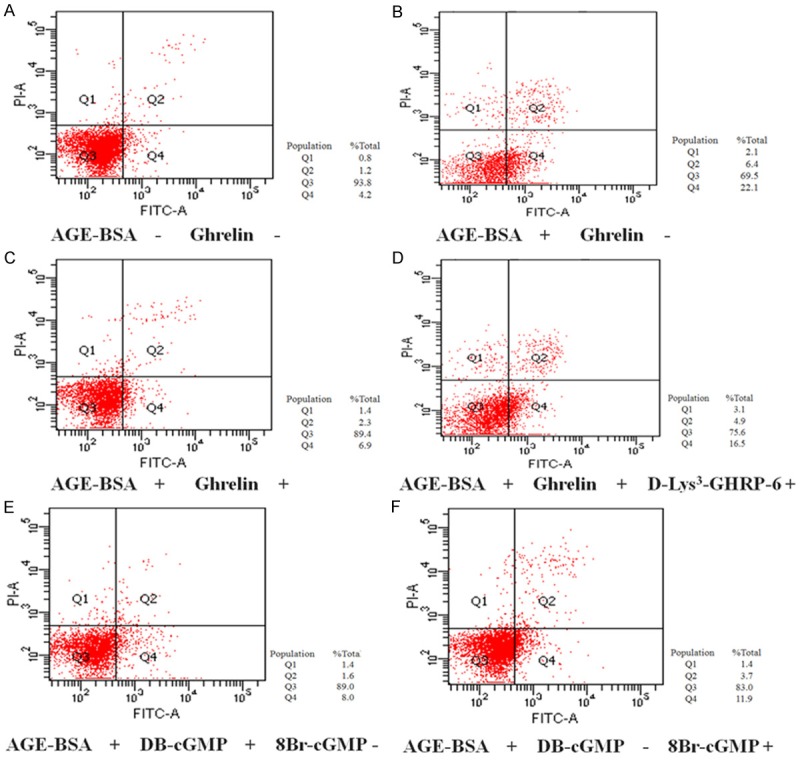
Apoptosis of HUVECs was analyzed by flow cytometry. A. Apoptosis of AGE-BSA (-) and Ghrelin (-) HUVECs; B. Apoptosis of AGE-BSA (+) and Ghrelin (-) HUVECs; C. Apoptosis of AGE-BSA (+) and Ghrelin (+) HUVECs; D. Apoptosis of AGE-BSA (+), Ghrelin (+) and D-Lys3-GHRP-6 (+) HUVECs. E. Apoptosis of AGE-BSA (+), DB-cGMP (+) and 8-Br-cGMP (-) HUVECs; F. Apoptosis of AGE-BSA (+), DB-cGMP (-) and 8-Br-cGMP (+) HUVECs.
Ghrelin protected HUVECs from AGEs induced cell apoptosis via GHSR-1a
After observation of the protection effect exerted by ghrelin, we examined the expression of its receptor GHSR-1a on HUVECs. The Western blot analysis revealed GHS-R1a expression in HUVECs (Figure 2A). Then we further tested whether there is an interaction between GHSR-1a and ghrelin, therefore GHSR-1a antagonist [D-Lys3]-GHRP-6 were employed. The MTT assay showed that the protection effect on cell viability was abolished after the pre-incubation with [D-Lys3]-GHRP-6 (Figures 2B, 5D) while the flow cytometry analysis revealed the protection effect on cell apoptosis was also diminished after the pre-incubation with [D-Lys3]-GHRP-6 (Figures 2C, 5D) . In addition, we also evaluated the expression level of molecules in apoptosis pathway. A consistent trend was found on cleaved Caspase 3 (Figure 2D), Bcl-2 and Bax (Figure 2E).
Figure 2.
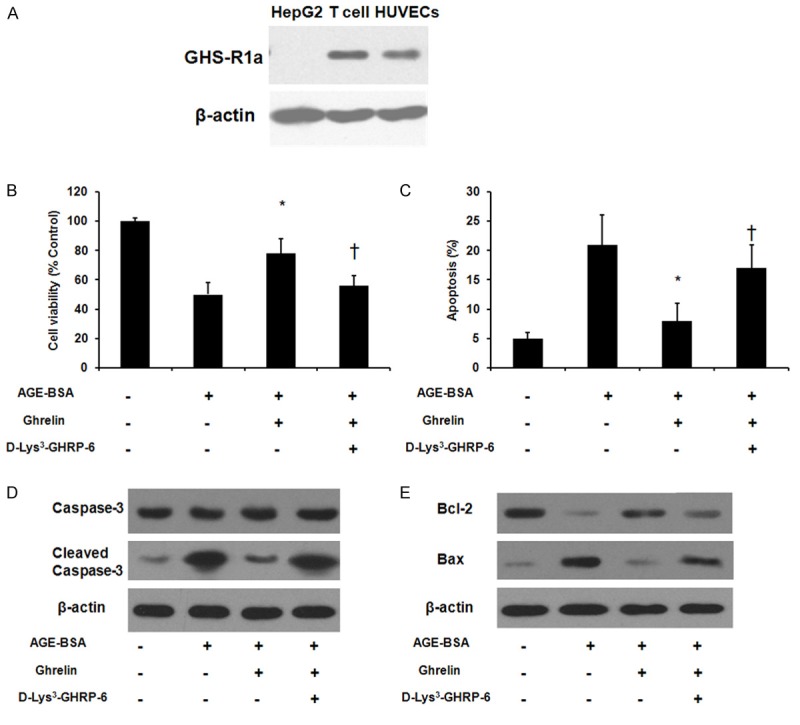
Growth hormone secretagogue receptor (GHS-R)-1a mediated the protective effect exerted by ghrelin. A. GHS-R-1a protein expression assessed by Western blot analysis. Total protein extracted from human T cells and HepG2 cells was included as a positive control and a negative control, respectively. HUVECs were pre-incubated with vehicle or [D-Lys3]-GHRP-6 (100 μM) for 1 h and the treated with vehicle or ghrelin (1 μM) for 24 h followed by exposure to AGE-BSA for 48 h. B. Cell viability analysis by MTT assay. C. Flow cytometry analysis of apoptotic cells using Annexin-V and propidium iodide staining. D. Cleaved caspase 3 protein level was assessed by Western blot. E. Bcl-2 and Bax protein level were evaluated by Western blot. *P < 0.05 when compared with AGE-BSA treated cells. †P < 0.05 when compared with ghrelin treated group.
Involvement of cGMP/NO signaling pathway in the protection effect exerted by ghrelin
To determine the molecular mechanisms, we examined by the cGMP level in HUVECs treated with AGE and ghrelin. The cGMP level was significantly decreased after AGE treatment [(4 ± 3.75) fM vs. (25.5 ± 2.8) fM, P < 0.05]. Ghrelin pretreatment significantly increased the cGMP level when compared with AGE treated cells [(18.6 ± 3.54) fM vs. (4 ± 3.75) fM, P < 0.05] while [D-Lys3]-GHRP-6 pretreatment significantly decreased cGMP level when compared with ghrelin treated cells [(8 ± 4.08) fM vs. (18.6 ± 3.54) fM, P < 0.05] (Figure 3).
Figure 3.
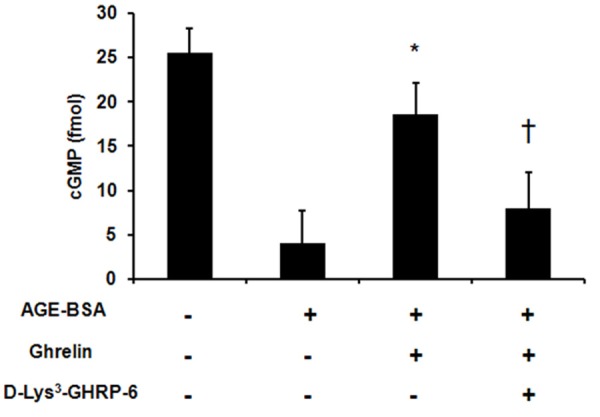
Ghrelin prevents the decrease in cGMP level advanced glycation end products (AGEs) induced apoptosis in HUVECs. HUVECs were pre-incubated with vehicle control or ghrelin (1 μM) for 24 h followed by exposure to AGE-BSA for 48 h. cGMP level was determined by enzyme immunoassay. *P < 0.05 when compared with AGE-BSA treated cells. †P < 0.05 when compared with ghrelin treated group.
Treatment with cGMP analogs exhibit similar protection effect as ghrelin
To identify whether there is protection effect exerted via cGMP/NO signaling pathway, we employed cGMP analogs pretreatment followed by AGE induced cell apoptosis. The results showed that pretreatment with cGMP analogs 8-Br-cGMP and DB-cGMP could effectively increase the cell viability and decrease cell apoptosis in AGE-induced cell apoptosis (Figures 4, 5E, 5F).
Figure 4.
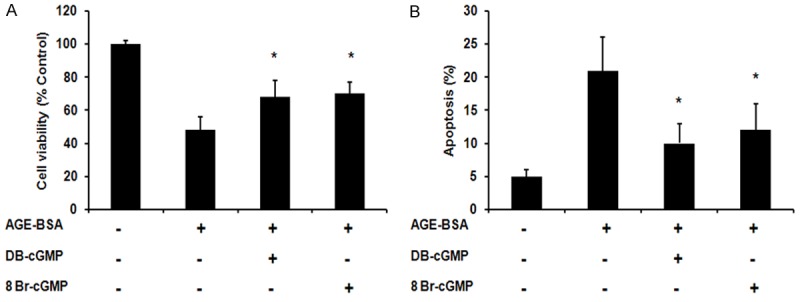
cGMP analog 8-Br-cGMP (0.5 μM) and DB-cGMP (0.5 μM) protected HUVECs from advanced glycation end products (AGEs) induced apoptosis. A. Cell viability analysis by MTT assay. B. Flow cytometry analysis of apoptotic cells using Annexin-V and propidium iodide staining. *P < 0.05 when compared with AGE-BSA treated cells.
Discussion
In present study, we investigated the effects of ghrelin on AGEs induced apoptosis in HUVECs and explored its underlying molecular mechanism. Our results showed that ghrelin exerted a protective effect on AGEs induced apoptosis. The inhibitory effect of ghrelin was exerted through interaction with GHSR-1a and affecting the apoptosis signaling molecules including Caspase 3, Bcl-2 and Bax. Moreover, we found elevated cGMP level was also account for the protective effect of ghrelin and involvement of cGMP/NO signaling pathway was confirmed by using the cGMP analogs to rescue the cell apoptosis. To the best of our knowledge, this is the first study to claim the involvement of cGMP/NO signaling pathway in the protective effect of ghrelin in AGE induced apoptosis.
Previous studies have shown that the biological actions of ghrelin are initiated through binding its receptor GHSR-1a [10,11]. GHSR-1a, a G-protein coupled transmembrane receptor, is highly expressed in endothelial cells [12]. Here, we confirmed the expression of GHSR-1a on HUVECs by Western blot. Moreover, we used GHSR-1a selective antagonist [D-Lys3]-GHRP-6 to abolish the effect exerted by ghrelin and the results showed that [D-Lys3]-GHRP-6 pretreatment could reverse the protective effect of ghrelin, which is consistent to previous studies [13,14].
Xiang et al previous demonstrated that PI3K/Akt and ERK1/2 pathway were involved in the inhibitory effect of ghrelin in AGEs induced HUVECs apoptosis [15]. Recently, Zhu et al showed that mTOR/P70S6K signaling pathway was also involved in the protective effect of ghrelin [16]. However, the accurate signaling pathway regulation on ghrelin in AGEs induced HUVECs apoptosis has not been explored yet. Su et al [17] suggested PI3K/Akt dependent phosphorylation of endothelial nitric oxide synthase (eNOS) could result in elevated cGMP level in endothelial cells. Zhang et al [18] suggested that PI3K/Akt signaling pathway play a role in enhancement of the activity of eNOS in HUVECs. In other systems, cGMP was shown to exert the ability to activate p38 and ERK to initiate its action [19,20]. All these together gave us hints to test the cGMP level in AGE induced HUVEC apoptosis system. In the present study, we found that ghrelin caused an elevated cGMP level in HUVECs. We then examined the functional involvement of cGMP/NO signaling pathway in anti-apoptosis effect in HUVECs and did observe an improvement effect on cell viability and cell apoptosis after introducing the cGMP analog 8-Br-cGMP (0.5 μM) and DB-cGMP.
In conclusion, we demonstrated here that ghrelin protected against HUVECs apoptosis induced by AGEs via binding its receptor GHSR-1a. We also shown that ghrelin strongly increased cGMP level and protective effect ghrelin was mediated by the cGMP/NO signaling pathway in HUVECs. Our findings provide clear evidence for the potential therapeutic application of ghrelin and cGMP analogs in the prevention and treatment of endothelial injury induced by high glucose in patients with diabetes.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No 81100575) and the Natural Science Foundation of Heilongjiang Province (No D201069).
Disclosure of conflict of interest
None.
References
- 1.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- 2.Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 3.Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des. 2008;14:962–968. doi: 10.2174/138161208784139729. [DOI] [PubMed] [Google Scholar]
- 4.Tsuneki H, Sekizaki N, Suzuki T, Kobayashi S, Wada T, Okamoto T, Kimura I, Sasaoka T. Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur J Pharmacol. 2007;566:1–10. doi: 10.1016/j.ejphar.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhou YJ, Yang HW, Wang XG, Zhang H. Hepatocyte growth factor prevents advanced glycation end products-induced injury and oxidative stress through a PI3K/Akt-dependent pathway in human endothelial cells. Life Sci. 2009;85:670–677. doi: 10.1016/j.lfs.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP. Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovasc Res. 2006;69:227–235. doi: 10.1016/j.cardiores.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Peino R, Baldelli R, Rodriguez-Garcia J, Rodriguez-Segade S, Kojima M, Kangawa K, Arvat E, Ghigo E, Dieguez C, Casanueva FF. Ghrelin-induced growth hormone secretion in humans. Eur J Endocrinol. 2000;143:R11–4. doi: 10.1530/eje.0.143r011. [DOI] [PubMed] [Google Scholar]
- 8.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Liu G, Wang Q, Ding L, Cai H, Jiang H, Xin Z. Effect of ghrelin on human endothelial cells apoptosis induced by high glucose. Biochem Biophys Res Commun. 2007;362:677–681. doi: 10.1016/j.bbrc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Feng DD, Yang SK, Loudes C, Simon A, Al-Sarraf T, Culler M, Alvear-Perez R, Llorens-Cortes C, Chen C, Epelbaum J, Gardette R. Ghrelin and obestatin modulate growth hormone-releasing hormone release and synaptic inputs onto growth hormone-releasing hormone neurons. Eur J Neurosci. 2011;34:732–744. doi: 10.1111/j.1460-9568.2011.07787.x. [DOI] [PubMed] [Google Scholar]
- 11.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Chen Q, Li G, Ke D. Ghrelin stimulates angiogenesis via GHSR1a-dependent MEK/ERK and PI3K/Akt signal pathways in rat cardiac microvascular endothelial cells. Peptides. 2012;33:92–100. doi: 10.1016/j.peptides.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Delhanty PJ, van der Eerden BC, van der Velde M, Gauna C, Pols HA, Jahr H, Chiba H, van der Lely AJ, van Leeuwen JP. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol. 2006;188:37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- 14.Johansson I, Destefanis S, Aberg ND, Aberg MA, Blomgren K, Zhu C, Ghè C, Granata R, Ghigo E, Muccioli G, Eriksson PS, Isgaard J. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149:2191–2199. doi: 10.1210/en.2007-0733. [DOI] [PubMed] [Google Scholar]
- 15.Xiang Y, Li Q, Li M, Wang W, Cui C, Zhang J. Ghrelin inhibits AGEs-induced apoptosis in human endothelial cells involving ERK1/2 and PI3K/Akt pathways. Cell Biochem Funct. 2011;29:149–155. doi: 10.1002/cbf.1736. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Zheng C, Chen J, Luo J, Su B, Huang Y, Su W, Li Z, Cui T. Ghrelin protects human umbilical vein endothelial cells against high glucose-induced apoptosis via mTOR/P70S6K signaling pathway. Peptides. 2014;52:23–28. doi: 10.1016/j.peptides.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Su KH, Tsai JY, Kou YR, Chiang AN, Hsiao SH, Wu YL, Hou HH, Pan CC, Shyue SK, Lee TS. Valsartan regulates the interaction of angiotensin II type 1 receptor and endothelial nitric oxide synthase via Src/PI3K/Akt signalling. Cardiovasc Res. 2009;82:468–475. doi: 10.1093/cvr/cvp091. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wang SJ, Han ZH, Li YQ, Xue JH, Gao DF, Wu XS, Wang CX. PI3K/AKT signaling pathway plays a role in enhancement of eNOS activity by recombinant human angiotensin converting enzyme 2 in human umbilical vein endothelial cells. Int J Clin Exp Pathol. 2014;7:8112–8117. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMPdependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood. 2006;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J Biol Chem. 2001;276:42226–42232. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]


