Abstract
Objectives: This work aims to assess the feasibility of perfusion CT in diagnosis of liver fibrosis in the early stage. Materials and Methods: Solutions of carbon tetrachloride (CCL4) were injected into the peritoneum of 45 rabbits to establish rabbit models of liver fibrosis. Perfusion CT were performed at 4-, 8-, 12- and 16- week after injection. The parametric perfusion indices of blood flow (BF), blood volume (BV), arterial liver perfusion (ALP), portal venous perfusion (PVP), and hepatic perfusion index (HPI) on perfusion maps were measured. Liver samples were scored as F0, F1, F2, F3, F4 for fibrosis. Results: In 50 rabbits, 23 rabbits survived. Of these survival rabbits, 5 rabbits were histopathologically scored as F0, 7 rabbits were F1, 8 rabbits were F2, and 3 rabbits were F3. For relatively small number of F3, multiple comparisons were made for F0 vs. F1, F1 vs. F2 and F0 vs. F2. A statistically significant difference was observed in PVP, BV, BF, ALP and HPI between F1 vs. F2 and F0 vs. F2, whereas a significant statistical difference was only achieved in PVP between F0 vs. F1. In the early stage of liver fibrosis PVP decreased with the progression of liver fibrosis, whereas HPI, ALP and BF increased with the progression of liver fibrosis. BV had no marked change. Conclusions: Perfusion CT is feasible in diagnosis of early stage of liver fibrosis. PVP appears to be the most promising parametric perfusion index.
Keywords: Liver fibrosis, animal model, perfusion CT
Introduction
Liver fibrosis is a consequence of sustained chronic injury from a variety of causes, including viral, drug-induced, autoimmune, cholestatic, and metabolic diseases [1]. Fibrosis is an important cause of liver dysfunction and portal hypertension [2]. There is increasing evidence that, unlike cirrhosis, fibrosis is treatable and reversible in its early stages [3-5]. Knowledge of the stage of fibrosis is crucial for patient care because patients with mild disease should be monitored and those with advanced disease must be treated [6,7].
The diagnosis of liver fibrosis is usually based on histological findings after liver biopsy. However, this procedure has inherent risks and it is prone to interobserver variability and sampling errors [8]. Therefore, there is obvious need for development of noninvasive assessment of liver fibrosis, with possibility of whole liver examination, eliminating sampling errors and reducing biopsy-related risks. Currently, most techniques including elastography, magnetic resonance diffusion weighted imaging and magnetic resonance spectroscopy could differentiate between cirrhosis or severe fibrosis and normal liver. However, accurate staging of fibrosis or diagnosis of mild fibrosis was often not achievable [9].
Perfusion imaging in liver fibrosis is based on the occurrence of substantial microcirculatory changes in this disease. It has been previously shown that perfusion changes occur early during fibrosis in chronic HCV infection and perfusion CT can differentiate patients with minimal fibrosis (F1) from those with intermediate fibrosis (F2 or F3) [10]. However, it is still unknown that whether perfusion CT could be used for differentiating early stage of liver fibrosis (F1 or F2) from normal liver (F0). The purpose of this study is to assess the feasibility of perfusion CT in diagnosis of liver fibrosis in early stage.
Materials and methods
Animal model
This study was approved by the animal care committee at our institute. Fifty adult New Zealand white rabbits, weighing 3.0 to 3.5 kg each, were used. Carbon tetrachloride (CCL4: Olive oil = 1:1) with dose of 0.2 ml per kg of body weight was injected into the peritoneum of 45 rabbits to establish rabbit models of liver fibrosis, and saline solution was injected into 5 rabbits as the control group. The time of injection was set at 8:00 am on Monday and Thursday each week.
Perfusion CT examination
For study liver fibrosis with different stages (F1, F2 or F3), perfusion CT were performed at 4-, 8-, 12- and 16-week after CCL4 injection. Five rabbits in model group and 1 to 2 rabbits in control group were randomly selected to receive CT scan on each time. After an overnight fast, the rabbits underwent CT on a 128-section multidetector CT scanner (Definition Flash, Siemens, Germany). Anesthesia was induced with intravenous katamine hydrochloride (50 mg per kg of body weight) and 2% xylazine (0.1 mL/kg).
Liver localization was performed by using unenhanced abdominal scanning. The CT scan ranged from the diaphragm to the bottom of the liver. The perfusion CT protocol parameters were as follows: 80 kVp; 100 mAs; matrix, 512 × 512; gantry rotation time, 0.5 second; reconstruction section thickness, 3 mm; field of view, 12 cm. DynMulti4D CT scanning was performed beginning 5 seconds before the intravenous bolus injection of 4 mL of contrast agent (Iohexol 350, GE Healthcare). The contrast material was injected at a rate of 1 mL/s through the auricular vein with a power injector, which was modified and optimized from previous study [11,12]. The total number of images per rabbit was 400, and the total acquisition time was 54.59 seconds.
Data analysis
The reconstructed image data were transferred to an imaging workstation for functional analysis using CT perfusion using the deconvolution algorithm. Pixels within the range of -200 to 120 HU values were chosen to exclude the bone, air, and iodine densities. The aorta was selected as the input artery and the portal vein was chosen as the input vein. Images between the beginning of contrast enhancement in the aorta and the end of contrast enhancement in the portal vein were automatically selected. Perfusion maps included maps of hepatic blood flow (BF, ml/min/100 mg body weight), hepatic blood volume (BV, ml/100 mg), arterial liver perfusion (ALP, ml/100 ml/min), portal vein liver perfusion (PVP, ml/100 ml/min), and hepatic perfusion index (HPI, %). The analysis package offered motion correction for in-plane movement.
Three senior radiologist (M.R., with 12 years experience in abdominal imaging) who was blinded to the histopathologic analysis measured values of BF, BV, ALP, PVP and HPI on perfusion maps. The regions of interest (ROI) were drawn in a representative transverse plane including the first hepatic hilar in each perfusion map (Figure 1). The same ROIs of each parametric perfusion indices were matched automatically. The ROIs ranged in size from 1.0 to 2.0 cm2. Six ROIs excluding major vessels were drawn in each representative transverse plane. The mean value of ROIs in each plane was used for statistical analysis.
Figure 1.
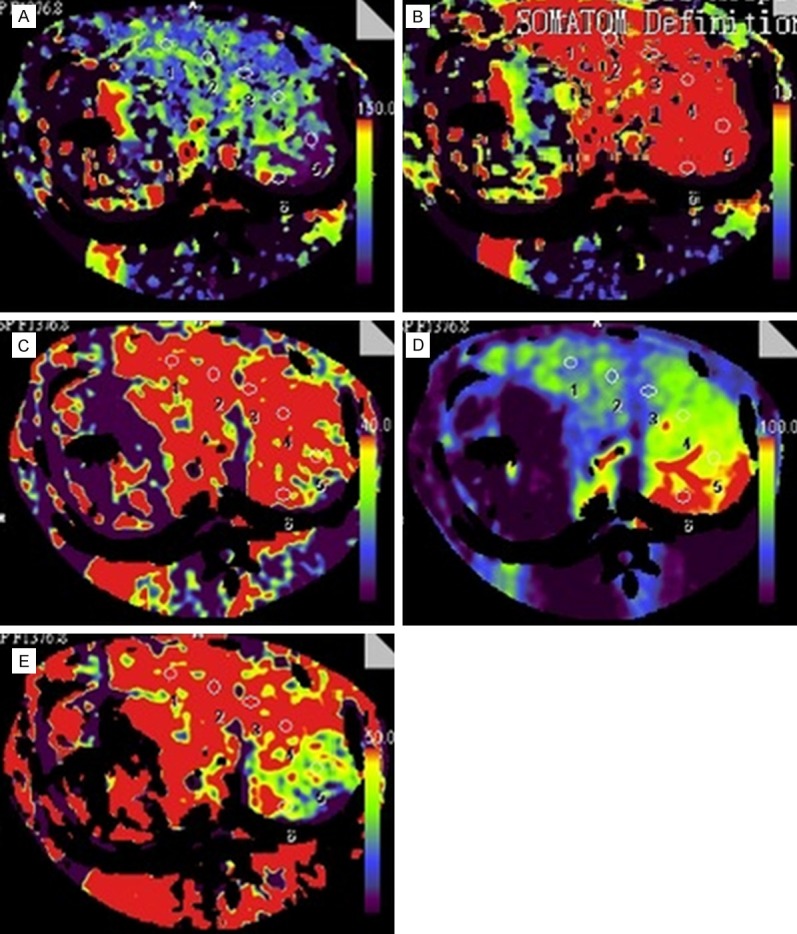
Transverse CT perfusion maps of BF (A), BV (B), ALP (C), PVP (D) and HPI (E) in a rabbit with F3 stage of liver fibrosis. Six ROIs were measured in the selected plane. The mean parametric indexes were as follow: BF, 69.26 ml min (-1) 100 ml (-1); BV, 24.09 ml min (-1) 100 ml (-1); ALP, 74.83 ml min (-1) 100 ml (-1); PVP, 68.19 ml min (-1) 100 ml (-1); HPI, 52.44%.
Histopathological evaluation
The rabbits were excised for liver samples within 24 hours following perfusion CT. The liver samples were fixed in formalin, embedded in paraffin, and stained with hematoxylin-eosin, picrosirius red, and Masson trichrome. They were analyzed by a pathologist (V.P., with 12 years experience in liver pathology) who was blinded to the results of the perfusion CT examinations. The size of the samples was measured in millimeters.
Fibrosis was assessed by using the Batts-Ludwig classification system [13]. This scoring system involves the use of a five-point scale for staging. Staging refers to the degree of fibrosis: stage 0 indicates no fibrosis; stage 1, portal fibrosis; stage 2, periportal fibrosis; stage 3, septal fibrosis; and stage 4, cirrhosis.
Statistical analysis
Data were analyzed with SPSS 16.0 software (SPSS, Chicago, IL). One-way analysis of variance (ANOVA) was used to compare group variables, followed by least-significant difference (LSD) post-hoc testing when indicated. One-way ANOVA with Games-Howell was performed for distributions where equal variances could not be assumed. Values are expressed as mean ± standard deviation. P < 0.05 indicated significance.
Results
In 50 rabbits, 23 rabbits survived. Of these survival rabbits, 5 rabbits were histopathologically scored as F0, 7 rabbits were F1 (Figure 2A), 8 rabbits were F2 (Figure 2B), and 3 rabbits were F3 (Figure 2C).
Figure 2.
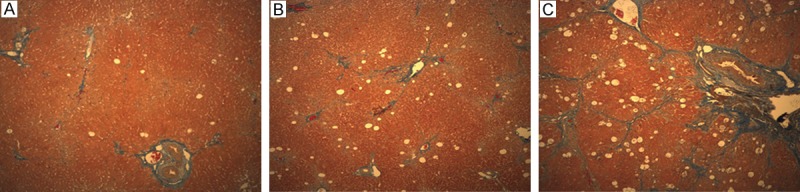
Histopathology of F1 (A), F2 (B), F3 (C) stage of liver fibrosis (Masson stain, magnification, 100 ×) revealed that portal fibrosis, periportal fibrosis and septal fibrosis were observed.
The parametric perfusion indices and histopathological stages of liver fibrosis were shown in Table 1.
Table 1.
parametric perfusion indices and histopathological stages of liver fibrosis
| Duration of CCL4 injection | Fibrosis stages (F) | Parametric perfusion indices | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| BF ml/min/100 mg | BV ml/100 mg | ALP ml/100 ml/min | PVP ml/100 ml/min | HPI % | ||
| Control group | F0 | 27.48 | 13.55 | 30.21 | 74.68 | 29.30 |
| F0 | 28.56 | 13.78 | 14.20 | 81.19 | 14.78 | |
| F0 | 27.30 | 14.57 | 31.61 | 73.85 | 30.00 | |
| F0 | 22.64 | 11.47 | 12.83 | 73.14 | 14.49 | |
| F0 | 25.73 | 14.67 | 28.18 | 73.02 | 28.67 | |
| 4 weeks | F1 | 26.71 | 12.53 | 21.10 | 53.71 | 28.23 |
| F1 | 23.41 | 11.86 | 19.99 | 48.69 | 30.06 | |
| F1 | 23.21 | 13.08 | 21.53 | 49.68 | 30.51 | |
| F1 | 21.13 | 11.78 | 21.48 | 41.39 | 34.29 | |
| F1 | 25.11 | 12.23 | 20.67 | 55.03 | 27.58 | |
| 8 weeks | F1 | 22.30 | 13.15 | 21.55 | 51.55 | 31.49 |
| F2 | 69.95 | 19.79 | 66.20 | 41.34 | 61.41 | |
| F2 | 62.83 | 15.59 | 73.31 | 42.47 | 63.20 | |
| F2 | 69.26 | 23.10 | 60.02 | 39.27 | 60.58 | |
| F3 | 69.30 | 19.79 | 66.20 | 41.34 | 61.41 | |
| 12 weeks | F1 | 21.58 | 12.01 | 20.73 | 52.38 | 28.85 |
| F2 | 61.45 | 14.05 | 61.35 | 40.01 | 61.38 | |
| F2 | 62.63 | 16.20 | 60.80 | 42.18 | 59.23 | |
| F2 | 64.50 | 20.30 | 63.50 | 38.28 | 64.51 | |
| F3 | 62.83 | 16.59 | 73.31 | 42.47 | 63.20 | |
| 16 weeks | F1 | 24.35 | 14.09 | 22.53 | 40.09 | 35.50 |
| F2 | 58.35 | 17.38 | 58.20 | 43.20 | 58.33 | |
| F3 | 69.26 | 24.09 | 74.83 | 68.19 | 52.44 | |
For relatively small number of F3, multivariable comparisons were made for F0 versus F1, F1 versus F2 and F0 versus F2 (Table 2). A statistically significant difference was observed in PVP, BV, BF, ALP and HPI between F1 vs. F2 and F0 vs. F2, whereas a significant statistical difference was only achieved in PVP between F0 vs. F1. No significant statistical differences were observed in BV between F0 vs. F1 and F1 vs. F2 and F0 vs. F2. In the early stage of liver fibrosis PVP decreased with the progression of liver fibrosis, whereas HPI, ALP and BF increased with the progression of liver fibrosis (Figures 3, 4). Changes of BV showed no marked trend in the early stage of liver fibrosis (Figure 5).
Table 2.
Multiple Comparisons of F0, F1 and F2 Fibrosis
| Variables | Fibrosis stages (F) | n | Mean ± Std | Sig. | ||
|---|---|---|---|---|---|---|
|
| ||||||
| F0 vs. F1 | F1 vs. F2 | F0 vs. F2 | ||||
| PVP | F0 | 5 | 75.3000 ± 3.9437 | .000 | .002 | .000 |
| F1 | 8 | 49.0650 ± 5.5330 | ||||
| F2 | 7 | 40.9643 ± 1.81986 | ||||
| ALP | F0 | 5 | 21.7050 ± 9.5765 | .994 | .000 | .003 |
| F1 | 8 | 21.1975 ± 0.7611 | ||||
| F2 | 7 | 63.3400 ± 5.0902 | ||||
| BF | F0 | 5 | 26.0575 ± 2.5556 | .186 | .000 | .000 |
| F1 | 8 | 23.4750 ± 1.8689 | ||||
| F2 | 7 | 64.1386 ± 4.1804 | ||||
| BV | F0 | 5 | 13.6225 ± 1.4892 | .470 | .008 | .029 |
| F1 | 8 | 12.5913 ± 0.7984 | ||||
| F2 | 7 | 18.0586 ± 3.1495 | ||||
| HPI | F0 | 5 | 21.9850 ± 8.5052 | .242 | .000 | .004 |
| F1 | 8 | 30.8138 ± 2.8323 | ||||
| F2 | 7 | 61.2343 ± 2.1419 | ||||
*The difference is significant at the 0.05 level.
Figure 3.
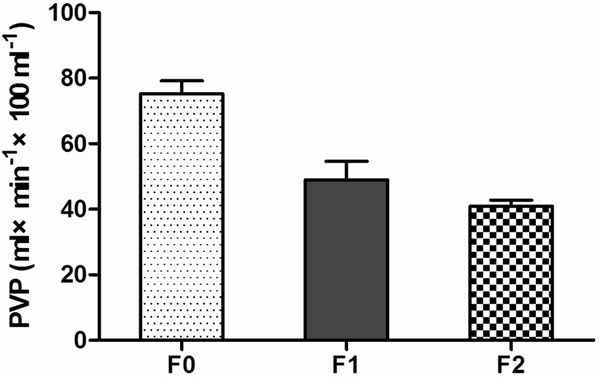
Distribution of PVP showed that PVP decreased gradually with the progression of liver fibrosis.
Figure 4.
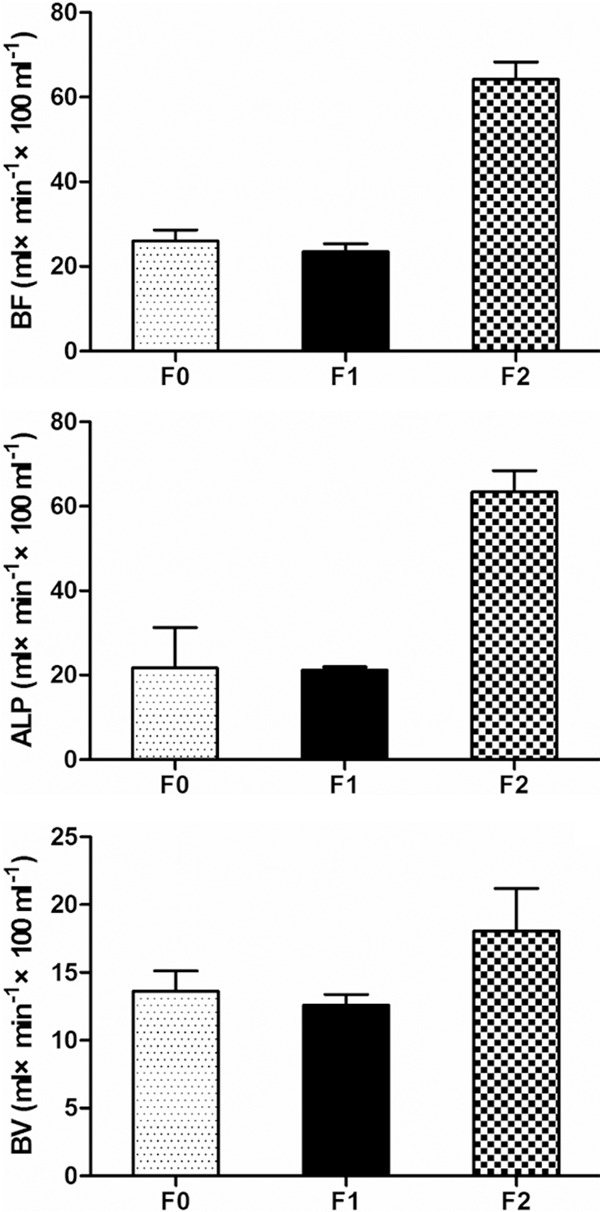
Distribution of BF, ALP and HPI showed that these parametric indices increased with the progression of liver fibrosis.
Figure 5.
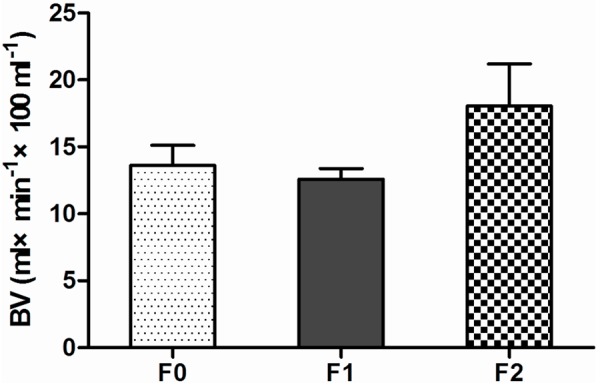
Distribution of BV showed that BV had no marked change trend in the early stage of liver fibrosis.
Discussion
Nowadays, the development of noninvasive procedures which could allow evaluation of whole liver, repetitive measurements for monitoring disease progression and treatment response is in major focus of clinical hepatology. It is important to accurately differentiate mild (F1) and moderate fibrosis (F2) stages from normal liver (F0) for liver fibrosis can be treatable and reversible in its early stages. Transient elastography can be reliable in the assessment of liver fibrosis [11]. However these results should be interpreted with caution as these studies were conducted in patients with low mean BMIs and small sample sizes [12]. Also the device of transient elastography is not commercially available. Although diffusion-weighted MRI enabled us to accurately differentiate mild (F1) and moderate fibrosis (F2) from advanced fibrosis stages (F3-F4), larger studies are needed to evaluate the influence of both diffusion and perfusion on ADC values in cirrhosis [13]. It was not possible to accurately discriminate normal, mild and moderate fibrosis due to significant overlap of corresponding ADC values [14].
Perfusion changes occur early in liver fibrosis [15]. In this study, we compared mild (F1) and moderate fibrosis (F2) with normal liver (F0) using perfusion CT. The results showed that PVP, BF, ALP and HPI in early stages (F1 and F2) of liver fibrosis were significantly different from normal liver (F0). Also this study showed PVP decreased with the progression of liver fibrosis, whereas HPI, ALP and BF increased with the progression of liver fibrosis. The results were promise in discrimination of normal, mild and moderate liver fibrosis. A possible explanation for these findings could be that the fibrotic tissues bounded the portal veins and hence reduced the portal venous perfusion when the liver was in F1 fibrosis stage (portal fibrosis) and F2 fibrosis stage (periportal fibrosis). It is well accepted that liver cirrhosis is associated with reduced liver perfusion: The increased arterial flow triggered by intrahepatic portal hypertension in liver fibrosis is insufficient to compensate for the reduced portal flow [16]. Therefore, the hepatic arterial perfusion increased in early stages of fibrosis [17].
Guan et al [18] induced liver diffuse lesions in rats with diethylniteosamine. They divided the processes of hepatic diffuse lesions into three stages of hepatitis, hepatic fibrosis, and cirrhosis. In the test group, HPI tended to increase gradually, BV and BF decreased at the same time. PVP increased gradually due to pathological changes. Hashimoto et al [19] reported that BF decreased with the severity of chronic liver disease. The HPI of the patients without liver disease was significantly lower than that of those with Child B and C liver disease. Ronot et al [10] observed that the portal venous and total liver perfusion significantly differed between patients with minimal fibrosis (F1) and those with intermediate fibrosis (F2 or F3). Different study parameters may have a certain discrepancy. Selection of patients, scan parameters and other factors may cause inconsistency [20]. From this study, perfusion changes occur early during liver fibrosis and perfusion CT can be used to diagnose early stages of liver fibrosis. PVP appears to be the most promising parametric perfusion index.
There are some limitations to our study. First, it suffered from the classic CT limitation: radiation. Radiation exposure delivered to each rabbit during perfusion CT scans was not a concern in our experimental study. However, protocols of perfusion CT scans have been improved to reduce the radiation dose when it was performed in humans. Meijerink et al [21] reported the optimal perfusion CT protocol for the human liver showed lower radiation exposure (12.0 mSv) than conventional-dose four-phase CT (20.7 mSv). Perfusion MR imaging may be a promising alternative to perfusion CT [22], but it is limited by low signal-to-noise ratio and absence of a linear relationship between signal intensity and concentration of the contrast mediums. Second, it has been known that xylazine-ketamine anesthesia may cause bradycardia which can affect perfusion values. Third, the motion during data acquisition may occur and this may lead to image misregistration. Fourth, the study number was relatively small. Future study needs large samples to verify the reliability of perfusion CT in assessment of early stages of liver fibrosis.
Disclosure of conflict of interest
None.
References
- 1.Friedman SL. Liver fibrosis--from bench to bedside. J Hepatol. 2003;38:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Roskams T, Baptista A, Bianchi L, Burt A, Callea F, Denk H, De Groote J, Desmet V, Hubscher S, Ishak K, MacSween R, Portmann B, Poulson H, Scheuer P, Terracciano L, Thaler H. Histopathology of portal hypertension: a practical guideline. Histopathology. 2003;42:2–13. doi: 10.1046/j.1365-2559.2003.01464.x. [DOI] [PubMed] [Google Scholar]
- 3.Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R, Serra G, Lai ME, Loy M, Caruso L, Desmet V, Purcell RH, Balestrieri A. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740–1749. doi: 10.1053/j.gastro.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Fowell AJ, Iredale JP. Emerging therapies for liver fibrosis. Dig Dis. 2006;24:174–183. doi: 10.1159/000090320. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL, Bansal MB. Reversal of hepatic fibrosis-fact or fantasy? Hepatology. 2006;43:S82–S88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 6.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 7.Marcellin P, Asselah T, Boyer N. Fibrosis and diseases progession in hepatitis C. Hepatology. 2002;36:S47–S56. doi: 10.1053/jhep.2002.36993. [DOI] [PubMed] [Google Scholar]
- 8.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneuous liver biopsy- a multicenter retrospective study on 68276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 9.Susanne B, Ihab K, Steven S, Jeanne C. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009;50:17–35. doi: 10.1016/j.jhep.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Ronot M, Asselah T, Paradis V, Michoux N, Dorvillius M, Baron G, Marcellin P, Van Beers BE, Vilgrain V. Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion. Radiology. 2010;256:135–142. doi: 10.1148/radiol.10091295. [DOI] [PubMed] [Google Scholar]
- 11.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 12.Castera L. Non-invasive diagnosis of steatosis and fibrosis. Diabetes Metab. 2008;34:674–679. doi: 10.1016/S1262-3636(08)74603-2. [DOI] [PubMed] [Google Scholar]
- 13.Kovač JD, Daković M, Stanisavljević D, Alempijević T, Ješić R, Seferović P, Maksimović R. Diffusion-weighted MRI versus transient elastography in quantification of liver fibrosis in patients with chronic cholestatic liver diseases. Eur J Radiol. 2011;81:2500–2506. doi: 10.1016/j.ejrad.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Koinuma M, Ohashi I, Hanafusa K, Shibuya H. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for eval-uation of hepatic fibrosis. J Magn Reson Imaging. 2005;22:80–85. doi: 10.1002/jmri.20344. [DOI] [PubMed] [Google Scholar]
- 15.Gülberg V, Haag K, Rössle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatology. 2002;35:630–634. doi: 10.1053/jhep.2002.31722. [DOI] [PubMed] [Google Scholar]
- 16.Moreno AH, Burchell AR, Rousselot LM, Panke WF, Slafsky F, Burke JH. Portal blood flow in cirrhosis of the liver. J Clin Invest. 1967;46:436–445. doi: 10.1172/JCI105545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen BB, Hsu CY, Yu CW, Wei SY, Kao JH, Lee HS, Shih TT. Dynamic contrast-enhanced magnetic resonance imaging with Gd-EOBDTPA for the evaluation of liver fibrosis in chronic hepatitis patients. Eur Radiol. 2012;22:171–180. doi: 10.1007/s00330-011-2249-5. [DOI] [PubMed] [Google Scholar]
- 18.Guan S, Zhao WD, Zhou KR, Peng WJ, Mao J, Tang F. CT perfusion at early stage of hepatic diffuse disease. World J Gastroenterol. 2005;11:3465–3467. doi: 10.3748/wjg.v11.i22.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto K, Murakami T, Dono K, Hori M, Kim T, Kudo M, Marubashi S, Miyamoto A, Takeda Y, Nagano H, Umeshita K, Nakamura H, Monden M. Assessment of the severity of liver disease and fibrotic change: the usefulness of hepatic CT perfusion imaging. Oncol Rep. 2006;16:677–683. [PubMed] [Google Scholar]
- 20.Zhong L, Wang WJ, Xu JR. Clinical application of hepatic CT perfusion. World J Gastroenterol. 2009;15:907–911. doi: 10.3748/wjg.15.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijerink MR, van Waesberghe JH, van der Weide L, van den Tol P, Meijer S, van Kuijk C. Total-liver-volume perfusion CT using 3-D image fusion to improve detection and characterization of liver metastases. Eur Radiol. 2008;18:2345–2354. doi: 10.1007/s00330-008-0996-8. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara M, Rusinek H, Lee VS, Losada M, Bannan MA, Krinsky GA, Taouli B. Advanced liver fibrosis: diagnosis with 3D whole-liver perfusion MR imaging-initial experience. Radiology. 2008;246:926–934. doi: 10.1148/radiol.2463070077. [DOI] [PubMed] [Google Scholar]


