Abstract
Objective: This study aimed to explore the association between D-dimer levels and presence of portal venous system thrombosis (PVST) in liver cirrhosis. Methods: All consecutive patients with a diagnosis of liver cirrhosis who underwent D-dimer test were retrospectively enrolled. Normal reference range of D-dimer level was 0-0.3 µg/mL. PVST was diagnosed on the basis of contrast-enhanced computed tomography and/or magnetic resonance imaging scans. Results: Of the 66 included patients, 24 were diagnosed with PVST. Mean D-dimer level was 0.51±0.72 µg/mL (range: 0.10-3.44). Mean D-dimer level was not significantly different between PVST and non-PVST groups (0.68±0.93 µg/mL versus 0.41±0.56 µg/mL, P=0.146). Area under the receiver operating curve for D-dimer level for predicting the presence of PVT was 0.606 (95% confidence interval: 0.478-0.724, P=0.1393). The optimal cut-off value for D-dimer was 0.22 with a sensitivity of 58.3% and a specificity of 69.0%. The subgroup analyses of patients without splenectomy or those with different Child-Pugh classes demonstrated no significant difference in the D-dimer level between PVST and non-PVST groups. Conclusion: D-dimer might not be useful to identify the presence of PVST in liver cirrhosis. However, given the retrospective nature of this study, further well-designed prospective study should be necessary to confirm this finding.
Keywords: Portal vein thrombosis, liver cirrhosis, D-dimer, fibrinolysis, pathogenesis
Introduction
Liver cirrhosis is a worldwide public health problem with a high morbidity and mortality [1]. Portal vein thrombosis (PVT) is a life-threatening complication of liver cirrhosis, which may aggravate the severity of liver dysfunction and risk of portal hypertension [2-5]. Except for a decreased portal vein flow as the most important local risk factor for the occurrence of PVT in liver cirrhosis [6], coagulation disorders may be considered as systemic thrombotic risk factors. However, the levels of anticoagulant proteins (i.e., antithrombin, protein C, and protein S) might not be associated with the development of PVT in liver cirrhosis [7]. Recently, some studies have explored whether or not fibrinolytic abnormalities might increase the risk of PVT in liver cirrhosis. Zhang et al. found that D-dimer level was significantly higher in cirrhotic patients with PVT than in those without PVT, but tissue type plasminogen activator and plasminogen activator inhibitor-1 levels were not significantly different between the two groups [8]. This finding suggested an increased risk of PVT in liver cirrhosis with hyperfibrinolysis. By comparison, Rossetto et al. showed that cirrhotic patients with PVT had higher levels of thrombin activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 than those without PVT [9]. This finding suggested a positive correlation between hypofibrinolytic condition and an increased risk of PVT in liver cirrhosis.
More recently, our published meta-analysis suggested that cirrhotic patients with PVT might have higher D-dimer concentrations than those without PVT (standardized mean difference: 1.249, 95% confidence interval: 0.740-1.758, P < 0.0001) [10]. However, it should be noted that the heterogeneity among these included studies was significant. To date, the role of fibrinolytic conditions in the occurrence of PVT in liver cirrhosis remains unclear. Herein, we conducted a retrospective observational study to further clarify whether or not D-dimer levels could predict the presence of portal venous system thrombosis (PVST) in cirrhotic patients.
Patients and methods
Study population
This study was approved by the ethics committee of the General Hospital of Shenyang Military Region. The number was K (2014) 22. The informed consent for every patient was waived due to the retrospective observational nature of this study. All cirrhotic patients who were admitted to our hospital and underwent D-dimer tests between July 2011 and June 2014 were enrolled in this study. Eligibility criteria were as follows: 1) a diagnosis of liver cirrhosis based on the clinical presentations, laboratory tests, imaging tests, and liver biopsy if necessary; 2) no malignancy, especially hepatocellular carcinoma; 3) D-dimer tests; and 4) contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) to evaluate the vessel patency within the portal venous system, including intrahepatic portal vein branches, main portal vein, superior mesenteric vein, and splenic vein [11].
Data collection
Clinical records from all patients with liver cirrhosis admitted during the period were retrospectively reviewed. The primary data collected were demographic data (age and sex), history of diagnosis of liver cirrhosis and prior splenectomy, clinical presentations, laboratory tests (regular blood test, liver function, and renal function), D-dimer levels, and Child-Pugh score and class. Two investigators were responsible for collecting the data (JD and YP), and one investigator was responsible for checking the data (XQ). PVST was diagnosed according to the findings from the contrast-enhanced CT and/or MRI scans. One investigator was also responsible for reading the CT and MRI scans for the presence of PVST (XQ). In the disagreement or uncertainty, these investigators would consult with two chief doctors (HL and XG).
D-dimer test
Fresh blood sample from peripheral veins 3 mL was collected in a sodium citrate anticoagulant tube and was centrifuged with a speed of 3000 r/min. NycoCard D-dimer test kits were employed. Normal reference range of D-dimer level was 0-0.3 µg/mL.
Statistical analysis
Continuous data were presented with mean ± standard deviation (range) and were compared by using the independent sample t tests. Categorical data were expressed as frequency and were compared by using the Chi-square tests. Receiver operating curve (ROC) was employed to evaluate the specificity and sensitivity of D-dimer for predicting the presence of PVST. An optimal cut-off value was defined as the value of specificity plus sensitivity was maximal. Areas under ROC (AUROC) with 95% confidence interval (CI) were calculated. P value < 0.05 was of statistically significant. All statistical analyses were performed by using the MedCalc software.
Results
During the enrollment period, a total of 66 cirrhotic patients without any malignancy underwent D-dimer tests and CT and/MRI scans at their admissions. Among them, 53 patients had contrast-enhanced CT scans alone, 11 patients had contrast-enhanced MRI scans alone, and 2 patients had both contrast-enhanced CT and MRI scans. Of them, 36% (24/66) were diagnosed with PVST. Location of PVST was the left portal vein branch (n=15), right portal vein branch (n=16), main portal vein (n=9), superior mesenteric vein (n=11), and splenic vein (n=6). Two patients had cavernous transformation of the portal vein.
Baseline characteristics of these included patients were shown in Table 1. Among them, 26, 28, and 12 patients had a Child-Pugh class A, B, and C, respectively. The etiology of liver cirrhosis was chronic hepatitis B virus (HBV) infection (n=24), hepatitis C virus (HCV) infection (n=5), HBV plus HCV (n=1), alcohol abuse (n=12), HBV infection plus alcohol abuse (n=3), 1 HCV plus alcohol abuse, autoimmune hepatitis (n=3), Wilson’s disease (n=1), and unknown (n=16). Seven patients had a history of splenectomy.
Table 1.
Baseline characteristics in 66 patients
| Variables | Values |
|---|---|
| Age (years) | 53.88±12.91 (22-83) |
| Sex (Male/Female)-n | 43/23 |
| History of liver cirrhosis (years) | 3.18±5.60 (0-30) |
| Prior splenectomy-n | 7 |
| Hepatic encephalopathy-n | 2 |
| Splenomegaly-n | 55 |
| Ascites-n | 39 |
| Upper gastrointestinal bleeding-n | 29 |
| Hemoglobin (g/L) | 96.21±31.84 (1.6-164.0) |
| White blood cell (109/L) | 5.16±4.34 (1.2-24.6) |
| Platelets count (109/L) | 96.71±74.49 (12-341) |
| Total bilirubin (µmol/L) | 35.61±54.30 (7.4-371.6) |
| Albumin (g/L) | 33.42±6.90 (17.8-50.4) |
| Alanine aminotransferase (U/L) | 46.88±54.66 (8-429) |
| Asparate aminotransferase (U/L) | 71.03±111.37 (14-889) |
| Alkaline phosphate (U/L) | 114.22±85.56 (11-531) |
| Creatinine (µmol/L) | 59.84±20.91 (28.6-164.0) |
| Prothrombin time (seconds) | 17.02±6.73 (11.5-62.8) |
| International normalized ratio | 1.43±0.91 (0.83-7.96) |
| D-dimer levels | 0.51±0.72 (0.10-3.44) |
| Child-Pugh score | 7.3±2.09 (5-15) |
Overall analysis
Mean D-dimer level was 0.51±0.72 (range: 0.10-3.44). Mean D-dimer level was not significantly different between PVST and non-PVST groups (0.68±0.93 versus 0.41±0.56, P=0.146). In addition, 32% (21/66) of patients had an elevated D-dimer level. The prevalence of an elevated D-dimer level was not significantly different between PVST and non-PVST groups (37.5% [9/24] versus 28.6% [12/42], P=0.225).
In the ROC analysis, AUROC for D-dimer level for predicting the presence of PVST was 0.606 (95% CI: 0.478-0.724, P=0.1393). The optimal cut-off value for D-dimer was 0.22 with a sensitivity of 58.3% and a specificity of 69.0% (Figure 1).
Figure 1.
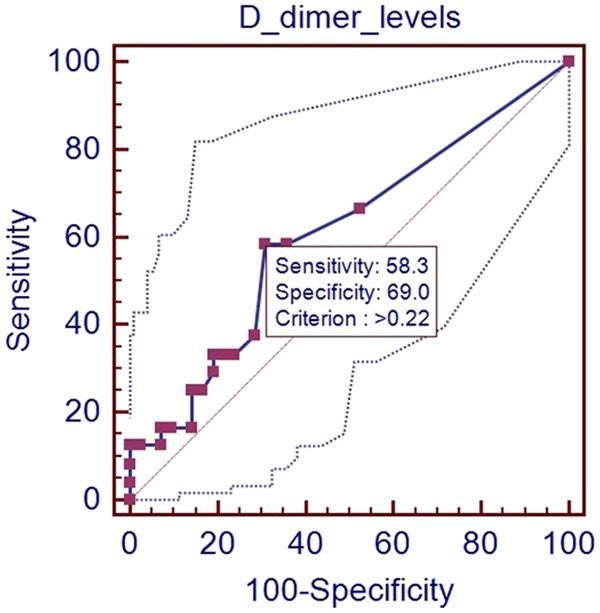
ROC analysis of D-dimer level for predicting the presence of PVST in all patients.
Subgroup analysis in patients without splenectomy
Mean D-dimer level was 0.4703±0.6815 (range: 0.10-3.44). In addition, 30.5% (18/59) of patients had an elevated D-dimer level.
In the ROC analysis, AUROC for D-dimer level for predicting the presence of PVST was 0.564 (95% CI: 0.429-0.693, P=0.4267). The optimal cut-off value for D-dimer was 0.22 with a sensitivity of 55.6% and a specificity of 68.3% (Figure 2).
Figure 2.
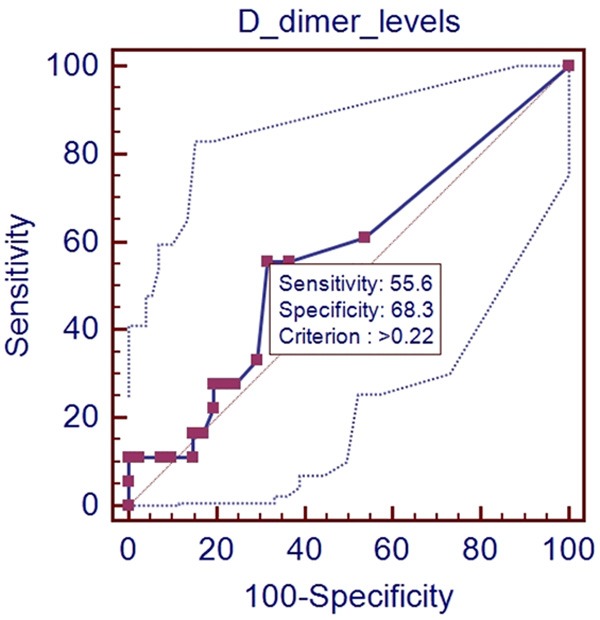
ROC analysis of D-dimer level for predicting the presence of PVST in patients without splenectomy.
Subgroup analysis in patients with Child-Pugh class A
Mean D-dimer level was 0.1865±0.1723 (range: 0.10-0.90). In addition, 7.7% (2/26) of patients had an elevated D-dimer level.
In the ROC analysis, AUROC for D-dimer level for predicting the presence of PVST was 0.61 (95% CI: 0.400-0.793, P=0.4483). The optimal cut-off value for D-dimer was 0.1 with a sensitivity of 60.0% and a specificity of 61.9% (Figure 3).
Figure 3.
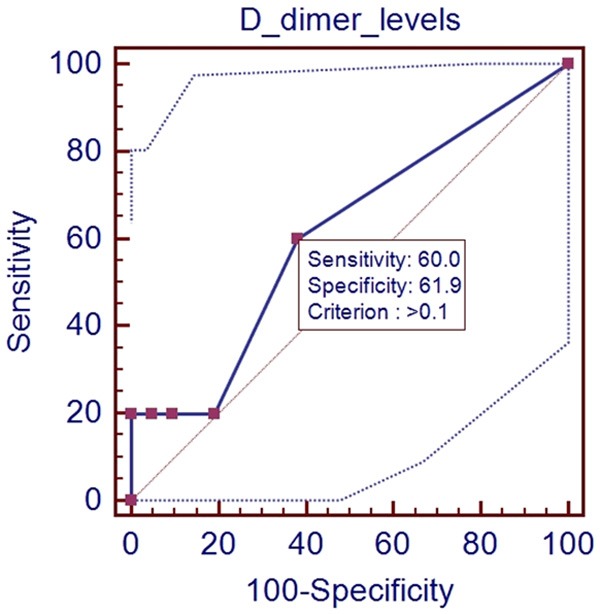
ROC analysis of D-dimer level for predicting the presence of PVST in patients with Child-Pugh class A.
Subgroup analysis in patients with Child-Pugh class B
Mean D-dimer level was 0.7483±0.9370 (range: 0.10-3.44). In addition, 46.4% (13/28) of patients had an elevated D-dimer level.
In the ROC analysis, AUROC for D-dimer level for predicting the presence of PVST was 0.562 (95% CI: 0.367-0.745, P=0.5744). The optimal cut-off value for D-dimer was 2.16 with a sensitivity of 23.1% and a specificity of 100% (Figure 4).
Figure 4.
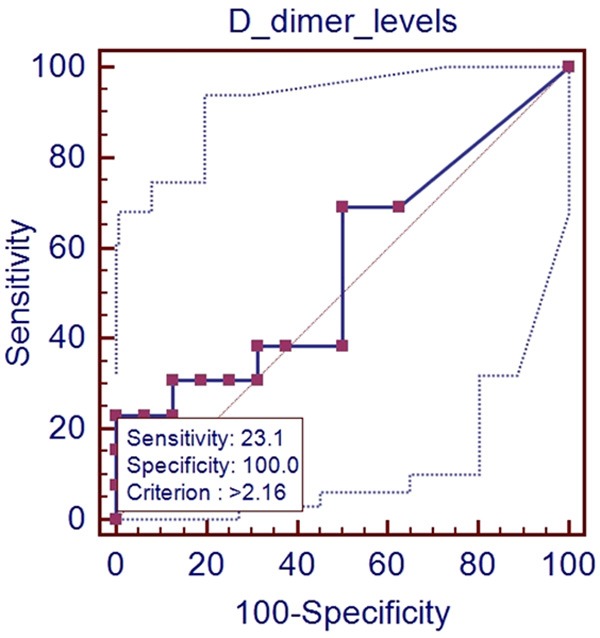
ROC analysis of D-dimer level for predicting the presence of PVST in patients with Child-Pugh class B.
Subgroup analysis in patients with Child-Pugh class C
Mean D-dimer level was 0.7583±0.6868 (range: 0.10-1.90). In addition, 58.3% (7/12) of patients had an elevated D-dimer level.
In the ROC analysis, AUROC for D-dimer level for predicting the presence of PVST was 0.750 (95% CI: 0.428-0.945, P=0.1368). The optimal cut-off value for D-dimer was 0.9 with a sensitivity of 100% and a specificity of 66.7% (Figure 5).
Figure 5.
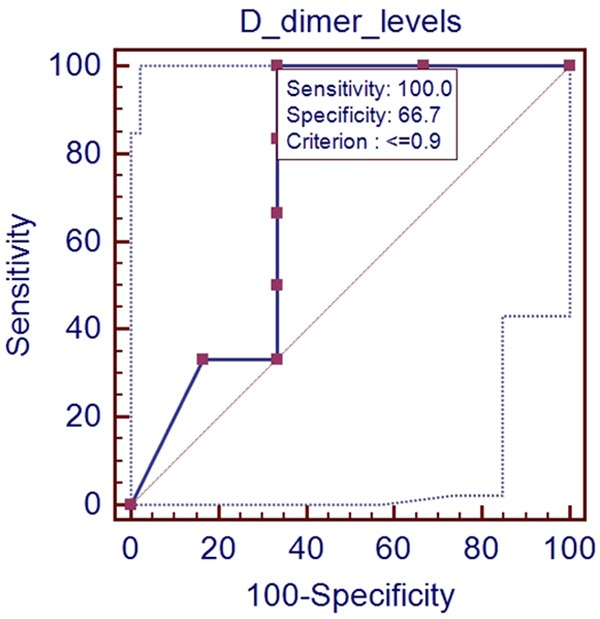
ROC analysis of D-dimer level for predicting the presence of PVST in patients with Child-Pugh class C.
Discussion
D-dimer test has been widely employed to establish a diagnosis of venous thromboembolism (i.e., deep vein thrombosis and pulmonary embolism), to predict the occurrence and recurrence of venous thromboembolism, and to guide the duration of anticoagulants in patients with venous thromboembolism [12-16]. It appears to be rational that D-dimer levels may be associated with the development of PVST in liver cirrhosis. Our study did not find any significant difference in the D-dimer levels between cirrhotic patients with and without PVST. Considering that splenectomy might increase the risk of PVST [17], a subgroup analysis was performed in patients without splenectomy. In addition, three subgroup analyses were performed in patients with Child-Pugh class A, B, and C, because the degree of liver dysfunction influences the development of PVST in liver cirrhosis. However, these subgroup results were the same to the overall result. These findings suggested that abnormal D-dimer levels might not be associated with the presence of PVST in liver cirrhosis.
A similar study by Zhang et al. also explored the association between D-dimer level and PVT in liver cirrhosis [8]. In contrast to our study, they found that D-dimer was significantly higher in PVT group than in non-PVT group, and that the significant difference remained regardless of Child-Pugh classes. Notably, compared with our study, the patients included in their studies had worse liver function (Child-Pugh class A, n=21; class B, n=49; class C, n=46) and higher D-dimer levels. But the information might not be adequate to explain the reasons why the conclusions were different between the two studies. Repeated studies were needed to further confirm their relationship in a larger number of patients.
About one third of our patients had a D-dimer level of beyond the upper limit of normal reference range. In agreement with previous studies, D-dimer levels might reflect the severity of liver cirrhosis. Compared with healthy individuals, the patients with liver diseases had significantly elevated D-dimer levels [18]. An increased degree of D-dimer level was influenced by the progression of liver disease and status of cirrhosis (cirrhosis or not, compensated or decompensated cirrhosis, Child-Pugh class, and liver failure or not) [18]. Studies also suggest that D-dimer levels at baseline should be associated with the presence of ascites, esophageal variceal bleeding, and hepatocellular carcinoma in cirrhotic patients [19,20]. Additionally, an elevated D-dimer plasma level can predict an increased risk of gastrointestinal hemorrhage and a worse outcome of esophageal variceal bleeding during follow-up in cirrhotic patients [21,22].
The major strength of our study should be the strict eligibility criteria. All included patients had contrast-enhanced CT and/or MRI scans. The imaging data were re-checked to identify the presence of PVST. However, a major limitation should be emphasized that all patients were retrospectively enrolled in this study. Thus, the potential bias of patient selection should be acknowledged. Although our study had a large number of patients who underwent D-dimer tests, only a small proportion of them (12%, 66/554) had adequate imaging data for evaluating the portal vein patency or occlusion.
In conclusion, our study demonstrated that D-dimer levels might not predict the presence of PVST in liver cirrhosis. The contribution of fibrinolytic conditions to the development of PVST in liver cirrhosis should be further analyzed.
Acknowledgements
This study was partially supported by the grant from the National Natural Science Foundation of China (no. 81500474) and Natural Science Foundation of Liaoning Province (no. 2015020409).
Disclosure of conflict of interest
None.
References
- 1.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi X, Han G, Fan D. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol. 2014;11:435–46. doi: 10.1038/nrgastro.2014.36. [DOI] [PubMed] [Google Scholar]
- 3.Qi X, Su C, Ren W, Yang M, Jia J, Dai J, Xu W, Guo X. Association between portal vein thrombosis and risk of bleeding in liver cirrhosis: A systematic review of the literature. Clin Res Hepatol Gastroenterol. 2015 doi: 10.1016/j.clinre.2015.02.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Qi X, Dai J, Jia J, Ren W, Yang M, Li H, Fan D, Guo X. Association between portal vein thrombosis and survival of liver transplant recipients: a systematic review and meta-analysis of observational studies. J Gastrointestin Liver Dis. 2015;24:51–9. doi: 10.15403/jgld.2014.1121.qix. [DOI] [PubMed] [Google Scholar]
- 5.Qi X, Dai J, Yang M, Ren W, Jia J, Guo X. Association between Portal Vein Thrombosis and Survival in Non-Liver-Transplant Patients with Liver Cirrhosis: A Systematic Review of the Literature. Gastroenterol Res Pract. 2015;2015:480842. doi: 10.1155/2015/480842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, Riccardi L, Lancellotti S, Santoliquido A, Flore R, Pompili M, Rapaccini GL, Tondi P, Gasbarrini GB, Landolfi R, Gasbarrini A. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–9. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Qi X, Chen H, Han G. Effect of antithrombin, protein C and protein S on portal vein thrombosis in liver cirrhosis: a meta-analysis. Am J Med Sci. 2013;346:38–44. doi: 10.1097/MAJ.0b013e31826485fc. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol. 2010;25:116–21. doi: 10.1111/j.1440-1746.2009.05921.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossetto V, Spiezia L, Senzolo M, Rodriguez-Castro KI, Gavasso S, Woodhams B, Simioni P. Does decreased fibrinolysis have a role to play in the development of non-neoplastic portal vein thrombosis in patients with hepatic cirrhosis? Intern Emerg Med. 2014;9:397–403. doi: 10.1007/s11739-013-0929-7. [DOI] [PubMed] [Google Scholar]
- 10.Dai J, Qi X, Li H, Guo X. Role of D-dimer in the development of portal vein thrombosis in liver cirrhosis: A meta-analysis. Saudi J Gastroenterol. 2015;21:43–52. doi: 10.4103/1319-3767.157567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi X, Han G, He C, Yin Z, Guo W, Niu J, Fan D. CT features of non-malignant portal vein thrombosis: a pictorial review. Clin Res Hepatol Gastroenterol. 2012;36:561–8. doi: 10.1016/j.clinre.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Cosmi B, Legnani C, Tosetto A, Pengo V, Ghirarduzzi A, Alatri A, Prisco D, Poli D, Tripodi A, Palareti G. Use of D-dimer testing to determine duration of anticoagulation, risk of cardiovascular events and occult cancer after a first episode of idiopathic venous thromboembolism: the extended follow-up of the PROLONG study. J Thromb Thrombolysis. 2009;28:381–8. doi: 10.1007/s11239-009-0315-5. [DOI] [PubMed] [Google Scholar]
- 13.Cosmi B, Legnani C, Tosetto A, Pengo V, Ghirarduzzi A, Testa S, Prisco D, Poli D, Tripodi A, Marongiu F, Palareti G PROLONG Investigators (on behalf of Italian Federation of Anticoagulation Clinics) Usefulness of repeated D-dimer testing after stopping anticoagulation for a first episode of unprovoked venous thromboembolism: the PROLONG II prospective study. Blood. 2010;115:481–8. doi: 10.1182/blood-2009-08-237354. [DOI] [PubMed] [Google Scholar]
- 14.Fabia Valls MJ, van der Hulle T, den Exter PL, Mos IC, Huisman MV, Klok FA. Performance of a diagnostic algorithm based on a prediction rule, D-dimer and CT-scan for pulmonary embolism in patients with previous venous thromboembolism. A systematic review and metaanalysis. Thromb Haemost. 2015;113:406–13. doi: 10.1160/TH14-06-0488. [DOI] [PubMed] [Google Scholar]
- 15.Geersing GJ, Janssen KJ, Oudega R, Bax L, Hoes AW, Reitsma JB, Moons KG. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ. 2009;339:b2990. doi: 10.1136/bmj.b2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, Pengo V, Ghirarduzzi A, Pattacini C, Testa S, Lensing AW, Tripodi A PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780–9. doi: 10.1056/NEJMoa054444. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Bai M, Guo X, Fan D. Pharmacologic prophylaxis of portal venous system thrombosis after splenectomy: a meta-analysis. Gastroenterol Res Pract. 2014;2014:292689. doi: 10.1155/2014/292689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gram J, Duscha H, Zurborn KH, Bruhn HD. Increased levels of fibrinolysis reaction products (D-dimer) in patients with decompensated alcoholic liver cirrhosis. Scand J Gastroenterol. 1991;26:1173–8. doi: 10.3109/00365529108998610. [DOI] [PubMed] [Google Scholar]
- 19.Toschi V, Rocchini GM, Motta A, Fiorini GF, Cimminiello C, Violi F, Castelli C, Sironi D, Gibelli A. The hyperfibrinolytic state of liver cirrhosis: possible pathogenetic role of ascites. Biomed Pharmacother. 1993;47:345–52. doi: 10.1016/0753-3322(93)90084-x. [DOI] [PubMed] [Google Scholar]
- 20.Spadaro A, Tortorella V, Morace C, Fortiguerra A, Composto P, Bonfiglio C, Alibrandi A, Luigiano C, De Caro G, Ajello A, Ferrau O, Freni MA. High circulating D-dimers are associated with ascites and hepatocellular carcinoma in liver cirrhosis. World J Gastroenterol. 2008;14:1549–52. doi: 10.3748/wjg.14.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Violi F, Ferro D, Basili S, Quintarelli C, Musca A, Cordova C, Balsano F. Hyperfibrinolysis resulting from clotting activation in patients with different degrees of cirrhosis. The CALC Group. Coagulation Abnormalities in Liver Cirrhosis. Hepatology. 1993;17:78–83. [PubMed] [Google Scholar]
- 22.Primignani M, Dell’Era A, Bucciarelli P, Bottasso B, Bajetta MT, de Franchis R, Cattaneo M. High-D-dimer plasma levels predict poor outcome in esophageal variceal bleeding. Dig Liver Dis. 2008;40:874–81. doi: 10.1016/j.dld.2008.01.010. [DOI] [PubMed] [Google Scholar]


