Abstract
Purpose: Mild cognitive impairment is common in Parkinson’s disease, but the underlying pathological mechanism has not been fully understood. To examine the gray matter changes in patients with Parkinson’s disease and those with mild cognitive impairment (MCI) using voxel based Morphometry (VBM). Methods: Magnetic resonance images were obtained from 35 patients with PD and 20 age and sex-matched healthy control subjects. In the PD group, 14 subjects had no MCI and 21 had MCI. MRI 3D structural images were acquired and analyzed by means of the optimized VBM procedure with Statistical Parametric Mapping (SPM5). Results: Widespread areas of cortical atrophy were found in patients with PD compared with normal controls (in both temporal, occipital, parietal, frontal lobes and right limbic lobes, posterior lobes of the cerebellum and left caudate nucleus). Gray matter reductions were found in bilateral fusiform gyrus and lingual gyrus, left anterior cingulate cortex and insula, and right superior temporal gyrus, orbitofrontal cortex, central gyrus and precuneus in patients with PD with MCI compared with normal controls. Inpatients with PD with MCI, areas of reduced gray matter were found in both precentral gyrus and middle temporal gyrus, right cuneus, precuneus, and orbitofrontal cortex, and left fusiform gyrus compared with those without MCI. Conclusions: These findings suggest that PD is associated with the gray matter atrophy in the neocortical areas, and that cognitive impairment in patients with PD may be associated with gray matter changes in the parieto-occipital association cortex, right orbitofrontal cortex, and middle temporal gyrus.
Keywords: Parkinson’s disease, mild cognitive impairment, voxel-based morphometry, magnetic resonance imaging, gray matter
Introduction
Parkinson disease (PD) is among the most frequent chronic neurodegenerative diseases [1]. The estimated prevalence of PD with dementia (PDD) is approximately 30% [2] and the risk of dementia in patients with PD is almost 6 times higher than in the general elderly population [3]. Cognitive impairment of a lesser severity is also common in patients with PD without dementia, designated as mild cognitive impairment (MCI) of PD, or PD-MCI. Patients with PD with MCI have an increased risk of developing dementia, compared with those without [4-6]. These findings suggest that MCI in PD is an early manifestation of dementia. Study of the pathogenesis and the image manifestations of the patients with PD and MCI contribute to the application of the precautionary measures and suppress the dementia. However, to our knowledge, there are few studies of the pathogenesis of the patients with PD with MCI.
Voxel-based morphometry (VBM) is a classical fully automated quantitative magnetic resonance imaging (MRI) technique extensively employed to reveal in vivo neuropathological changes in the neurological brain and has been widely used in neuropsychiatric disorders and neurological disorders (including PD and PDD) [7]. Some studies have recently been published on the rates of atrophy in patients with Parkinson’s disease versus controls. Ramirez-Ruiz et al [8] found progressive decreases in the gray matter volume in limbic, paralimbic and temporo-occipital regions in patients with PD. Summerfield et al [9] showed that patients with PD had gray matter reductions compared with healthy controls in the right side of the hippocampus, left anterior cingulate gyrus, and left superior temporal gyrus. Beyer et al [10] reported that patients with PD had a cluster of reduced gray matter in the right superior temporal gyrus compared with normal controls. There has been no consensus on the gray matter (GM) in the cortical and subcortical regions in PD across studies. The divergence might be due to small and heterogeneous samples as well as the differences in the used imaging protocols among these published studies.
To our knowledge, few MRI studies of patients with Parkinson’s disease and MCI have yet been reported. Gray matter changes in patients with Parkinson’s disease and those with MCI have not been fully understood.
The aim of this study was to use VBM to examine more closely the structural brain changes responsible for PD and those with MCI. We, therefore, compared brain changes in patients with PD, in patients with PD with and without MCI, and in healthy age and sex-matched control subjects. We hypothesized that patients with PD and those with MCI would have more pronounced cortical atrophy than controls, and that those with Parkinson’s disease with MCI would show more atrophy than those with Parkinson’s disease with no MCI.
Materials and methods
Participants
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Second Hospital of Lanzhou University. Written informed consent was obtained from all subjects.
Thirty-five patients with Parkinson disease (19 men and 16 women; age range, 38-77 years; mean age, 61.86±8.98 years;) and 20 age- and sex-matched control subjects (10 men and 10 women; age range, 38-75 years; mean age, 59.36±3.36 years;) were included in the study. The characteristic of patients with PD and healthy controls was given in Table 1. A diagnosis of PD was made by a neurologist using the criteria of the Parkinson’s Disease Society Brain Bank, London, England [11]. Patients were evaluated for the severity of the disease by using the Hoehn-Yahr scale [12]. All patients were right-handed according to the Edinburgh Handedness Inventory [13]. The duration of the disease was 0.6-13 years (mean ± SD, 4.81±3.43 years). Exclusion criterion were: 1) Patients with secondary parkinsonism; 2) Patients with parkinsonism-plus syndromes; 3) Patients with other internal medicine diseases apart from Parkinson’s disease or Parkinsonism which may affect the brain and cognition function evaluation; 4) Patients with a history of acute cerebrovascular disease within 3 months; 5) Patients with active epilepsy; 6) Patients with a history of mental disorder including delirium, depression and anxiety.
Table 1.
Characteristics of patients with Parkinson’s disease and normal controls
| Groups | N | Sex (Male/Female) | Age (Mean ± SD) | H & Y stage | Mean MMSE score | Mean education (years) | Mean duration of PD (years) |
|---|---|---|---|---|---|---|---|
| NC | 20 | 10/10 | 59.36+6.36 | NA | 28.90±0.96 | 9.50±2.74 | NA |
| PD | 35 | 19/16 | 61.86+8.98 | 2.0±0.84 | 28.94±1.05 | 8.54±3.25 | 4.81±3.43 |
| Non-MCI | 14 | 5/9 | 58.5±9.22 | 1.42±0.57 | 29.07±1.07 | 8.78±3.92 | 3.23±2.35 |
| MCI | 21 | 14/7 | 63.8±8.58 | 1.77±0.82 | 28.85±1.06 | 8.38±2.80 | 5.24±3.30 |
NC, normal controls; PD, Parkinson’s disease; MCI, mild cognitive impairment; NA, nonapplicable; H & Y stage, Hoehn and Yahr stage; MMSE, Mini-Mental State Examination.
Healthy controls were recruited from the healthy volunteers, and from relatives of patients with Parkinson’s disease. The controls had no active neurological or psychiatric disorder. They had no cognitive deficits, and were not taking drugs that could affect their cognition.
Mild cognitive impairment
MCI was defined according to the criteria proposed by Petersen et al [14]: impaired performance (i.e., 1.5 standard deviation or more below the mean of the control group) on one, two, or all three neuropsychological tests. In addition, information regarding memory problems or other subjective cognitive deficits was gathered by means of the care giver-based dementia interview and the mentation item from the mental subscale of Unified Parkinson Disease Rating Scale [15]. Cognitive impairment should not be severe enough to affect activities of daily living; thus, the criteria for dementia were not met [4].
Magnetic resonance imaging
All subjects underwent MRI examinations. No subjects had taken any medications on the day of the experiment. A 3.0-T MR scanner (Signa HDxt, GE Healthcare, Milwaukee, WI) with 8-channel head array coil was used in this study. Subjects were scanned in a supine, head-first position with symmetrically placed cushions on both sides of head to decrease motion.
The routine axial T2WI-PROPELLER and axial DWI sequences were used to exclude the intracranial hemorrhage, cerebral hemorrhage and organic brain disease. Axial T2-weighted images (turbo spin echo; TR 6000 ms, TE 15 ms) were obtained with a slice thickness of 6 mm, section gap of 1.5 mm, NEX of 1.5, FOV of 240 mm, and matrix of 352×352. Axial DWI was performed in the axial plane with the following parameters: TR 10000 ms, TE 80ms, FOV 240 mm, matrix 128×128, 6-mm slice thickness, 1.5 mm section gap, number of signal averages (NEX) 1.
In addition, aT1 weighted three-dimensional brain volume imaging (3D-BRAVO) sequences was used for structural data acquisition: TR/TE 8.4/3.3 ms, FOV 240 mm, slice thickness 1 mm, slice gap 0 mm, matrix 228×256, NEX 1, flip angle (FA) 13, and voxel volume 0.47×0.47×1 mm3.
Voxel-based morphometry with T1-Weighted MR imaging
High-resolution T1-weighted images were processed according to the method for VBM described by Good et al [16] with SPM5 software (Welcome Department of Imaging Neuroscience, London, UK; http//www.fil.ion.ucl.ac.uk/spm) and Matlab7.0 software (Math-Works, Natick, Massachusetts). Briefly, this method involved an initial segmentation of T1-weighted MR images into gray matter (GM) and white matter (WM) images in native space, followed by a normalization of the GM and WM images to templates in stereotactic space to obtain optimized normalization parameters. During normalization, images were interpolated to isotropic 1×1×1 mm voxels. The normalization parameters were then re-applied to the original whole-brain structural images before a second segmentation. Finally, the modulated volumes were smoothed with a Gaussian kernel of 8 mm full width at half maximum (FWHM).
Statistical analysis
Two-sample t tests were applied to compare the gray matter results: (1) between the patients with PD and normal controls; (2) between the patients with PD-MCI and normal controls; (3) between the patients with PD-MCI and normal controls; and (4) between the patients with PD-nMCI and PD-MCI. When subgroup analysis (PD-nMCI versus PD-MCI) was performed, age was included as nuisance covariate to control for the possible influences of the factor on the results. The resulting statistical map was set at a combined threshold of P<0.005 and a minimum cluster size of 200 voxels. Statistical analysis were performed with the software SPSS, version 13.0 (SPSS, Chicago, IL, USA). Categorical data were analyzed by using the chi-square test and P<0.05 were considered statistically significant.
Results
A total of 55 participants were included: PD-nMCI (n=14), PD-MCI (n=21) and normal controls (n=20). Table 1 presents the demographic and clinical characteristics. We found no significant differences in age, and sex between the three groups.
Gray matter changes in Patients with PD compared with normal controls
Compared with normal controls, patients with Parkinson’s disease had reduced cortical gray matter density in the limbic lobes (right hippocampus and parahippocampal gyrus) and both temporal ,occipital, parietal, frontal lobes. On the left side, there was also reduced gray matter density in the caudate nucleus. On the right side, there was reduced gray matter density in the posterior lobes of the cerebellum (Figure 1; Table 2).
Figure 1.
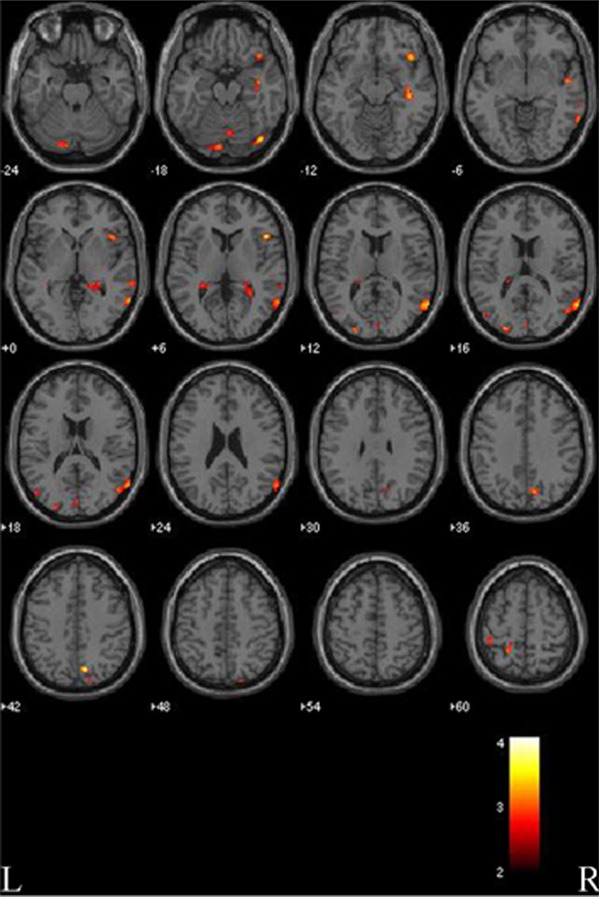
Gray matter reductions in patients with Parkinson’s disease (PD) compared to normal controls (NC). VBM analysis revealed significant changes are found in the temporal, occipital, parietal, frontal lobes (both sides), hippocampus, parahippocampal gyrus and posterior lobe of the cerebellum (right side), and caudate nucleus (left side). L, left side; R, right side. The color bar represents the T score. Yellow presents a high T score.
Table 2.
Anatomical location of areas of reduced grey matter in patients with PD with NC
| Voxel level | Anatomical location | Cluster size | x | y | z | T score |
|---|---|---|---|---|---|---|
| R | Cuneus and precuneus | 695 | 11 | -70 | 41 | 4.09 |
| R | Inferior frontal lobes | 1448 | 49 | 20 | 5 | 3.91 |
| R | Orbitofrontal cortex | 312 | 43 | 22 | -14 | 3.76 |
| L | Cuneus | 291 | -26 | -89 | 23 | 3.73 |
| R | Lingual gyrus and fusiform gyrus | 450 | 42 | -78 | -16 | 3.65 |
| R | Superior temporal gyrus | 2982 | 64 | -62 | 15 | 3.63 |
| R | Middle and inferior temporal gyrus | 2708 | 65 | -57 | 0 | 3.36 |
| L | Lingual gyrus and fusiform gyrus | 1012 | -19 | -87 | -21 | 3.53 |
| R | Temporal lobes and hippocampus | 844 | 41 | -23 | -13 | 3.51 |
| L | PARC | 368 | -20 | -48 | 59 | 3.37 |
| L | Central gyrus | 249 | -17 | -38 | 61 | 2.91 |
| L | Central gyrus | 237 | -42 | -34 | 57 | 3.11 |
| R | Hippocampus and parahippocampal gyrus | 979 | 17 | -39 | 1 | 3.11 |
| L | MTG | 214 | -47 | -74 | 18 | 3.05 |
| R | Posterior lobes of the cerebellum | 245 | 5 | -71 | -18 | 3 |
| R | Cuneus | 342 | 2 | -89 | 11 | 2.78 |
| L | CN | 351 | -21 | -34 | 16 | 2.89 |
PARC, paracentral lobule; MTG, middle temporal gyrus; CN, caudate nucleus.
MRI changes in Patients with PD-nMCI compared with normal controls
In patients with PD-nMCI, there were areas of marked gray matter reduction mainly in the right temporal lobe including middle temporal gyrus, inferior temporal gyrus, hippocampus and anterior cingulate cortex (Figure 2; Table 3).
Figure 2.

Gray matter comparison between patients with Parkinson disease with no mild cognitive impairment (PD-nMCI) and normal controls (NC). Clusters of density differences are observed in the temporal lobe including middle, superior temporal gyrus, hippocampus, and anterior cingulate regions (right side). L, left side; R, right side. The color bar represents the T score. Yellow presents a high T score.
Table 3.
Anatomical location of areas of reduced gray matter in patients with PD with no MCI compared with NC (after adjustment for age)
| Voxel level | Anatomical location | Cluster size | x | y | z | T score |
|---|---|---|---|---|---|---|
| R | Middle temporal gyrus | 466 | 50 | -47 | 6 | 6.33 |
| R | Inferior middle temporal gyrus | 5998 | 57 | -54 | -20 | 4.84 |
| R | Hippocampus | 1970 | 40 | -22 | -14 | 4.74 |
| R | ACC | 761 | 17 | 14 | 33 | 4.44 |
| R | Inferior temporal gyrus | 202 | 60 | -29 | -26 | 3.32 |
R, right; ACC, anterior cingulate cortex; MCI, mild cognitive impairment; NC, normal controls; the coordinates x, y and z refer to the anatomical location.
Gray matter changes in Patients with PD-MCI compared with normal controls
Patients with PD with MCI had reductions in gray matter concentration both fusiform gyrus and lingual gyrus, anterior cingulate and insular cortex (left side), and right superior temporal gyrus, orbitofrontal cortex, central gyrus and precuneus, compared with the controls (Figure 3; Table 4). Patients with Parkinson’s disease with MCI did not have any areas of more cortical gray matter density than normal controls.
Figure 3.
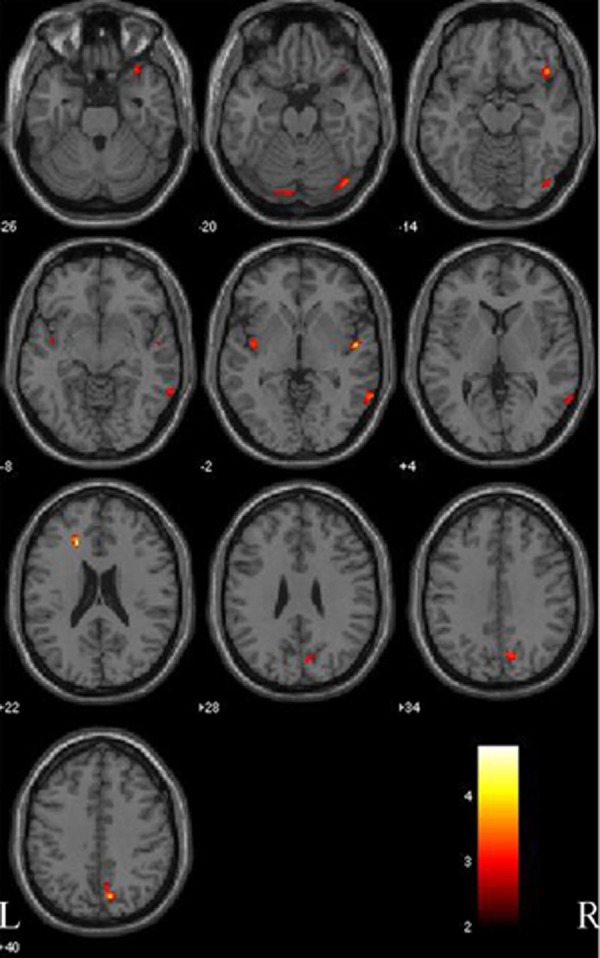
Gray matter comparison between patients with Parkinson disease with mild cognitive impairment (PD-MCI) and normal controls (NC). Significant changes are found in the fusiform gyrus and lingual gyrus (both sides), anterior cingulate and insular cortex (left side), and right superior temporal gyrus, orbitofrontal cortex, central gyrus and precuneus (right side). L, left side; R, right side. The color bar represents the T score. Yellow presents a high T score.
Table 4.
Anatomical location of areas of reduced grey matter in patients with Parkinson’s disease with MCI compared with normal controls
| Voxel level | Anatomical location | Cluster size | x | y | z | T score |
|---|---|---|---|---|---|---|
| L | ACC | 216 | -20 | 30 | 21 | 4.77 |
| R | STG | 365 | 51 | -8 | -3 | 4.28 |
| R | Parietal precuneus, | 908 | 10 | -71 | 39 | 4.08 |
| R | precuneus, cuneus | 908 | 8 | -63 | 38 | 3.23 |
| R | Lingual gyrus and fusiform gyrus | 599 | 42 | -79 | -18 | 3.82 |
| R | Orbitofrontal cortex | 463 | 43 | 23 | -15 | 3.79 |
| R | Central gyrus | 237 | 10 | -50 | 70 | 3.53 |
| L | Insular cortex | 381 | -40 | -7 | -3 | 3.51 |
| L | Lingual gyrus and fusiform gyrus | 286 | -9 | -87 | -22 | 3.25 |
ACC, anterior cingulate cortex; STG, superior temporal gyrus.
Gray matter changes in Patients with PD with and without MCI
Patients with Parkinson’s disease with MCI had reduced cortical gray matter density compared with those with Parkinson’s disease without MCI in both precentral gyrus and middle temporal gyrus, right cuneus, precuneus, and orbitofrontal cortex, and left fusiform gyrus (Figure 4; Table 5). Patients with Parkinson’s disease with MCI did not have any areas of more cortical gray matter density than patients with Parkinson’s disease without MCI.
Figure 4.
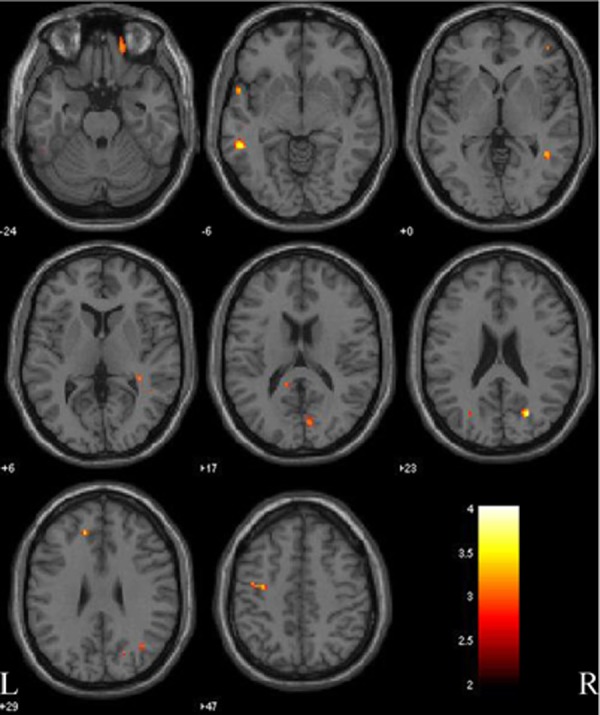
Gray matter comparison between patients with Parkinson disease with and without mild cognitive impairment (PD-MCI versus PD-nMCI). Significant changes are observed in precentral gyrus and middle temporal gyrus (both side), cuneus, precuneus, and orbitofrontal cortex (right side), and fusiform gyrus (left side). L, left side; R, right side. The color bar represents the T score. Yellow presents a high T score.
Table 5.
Anatomical location of areas of reduced grey matter in patients with Parkinson’s disease with MCI compared with no MCI
| Voxel level | Anatomical location | Cluster size | x | y | z | T score |
|---|---|---|---|---|---|---|
| R | precuneus, cuneus | 350 | 20 | -69 | 26 | 2.88 |
| R | precentral gyrus | 260 | -31 | -11 | 41 | 2.83 |
| L | precentral gyrus | -39 | -9 | 48 | 2.82 | |
| R | Orbitofrontal cortex | 452 | 15 | 48 | -26 | 2.82 |
| L | middle temporal gyrus | 271 | -52 | -42 | -8 | 2.82 |
| L | fusiform gyrus | 255 | -51 | -41 | -25 | 2.82 |
| R | middle temporal gyrus | 319 | 46 | -57 | 5 | 2.82 |
Discussion
In the present study, we studied gray matter changes in patients with Parkinson’s disease and those with MCI using structural MRI and VBM. The main finding was the widespread reduced density of cortical gray matter in patients with PD compared with controls, which confirms the results from previous studies [17,18]. Temporal, occipital, parietal, frontal lobes were affected in patients with PD, in accordance with our hypotheses. They also had reduced gray matter concentration in the hippocampus, parahippocampal gyrus and cerebellar posterior lobes (right side) and caudate nuclei (left side) when compared with normal controls.
In previous studies, patients with PD showed frontal [19-21], temporal [10,21], parietal [22], occipital and limbic lobes [8,9] gray matter volume loss relative to controls. Nagano-Saito et al [19] reported that patients with PD had cortical atrophy mainly in frontal lobe, and limbic and paralimbic system. Burton et al [20] suggested that patients with parkinson’s disease without dementia (PDND) showed frontal gray matter changes, including superior, middle and inferior frontal gyrus in the right side compared to the normal controls. Cordato et al [22] found that patients with PD had reductions in parietal gray matter density, compared with controls. Ramírez-Ruiz [8] showed a progressive gray matter volume decrease in patients with PD without dementia in limbic, paralimbic and neocortical associative temporo-occipital regions. Brenneis [23] demonstrated that in comparison to controls, significant cortical atrophy was only observed in PD patients in left caudate nuclei. Benninger et al [24] demonstrated for the first time that gray matter volume decrease in the right quadrangular lobe and declive of the cerebellum in PD with tremor compared to those without. Although significant differences are across studies, the similarity was the widespread morphological change in the brain of the PD patients. This is in accordance with the pathogenesis of the PD multisystemic involvement. Neuropathological tests have shown that with the progress of the PD, neocortex including limbic system, paralimbic system, and frontal, temporal, parietal, and occipital lobes were gradually involved, leading to the local neuronal loss and cortical atrophy. Our results are similar to but not identical with, those reported in previous studies. There are several possible explanations for these differences. The patients with PD in the previous study had a markedly shorter disease duration compared with our cohort (range, 0.5-13 years). Thus, that cohort had a later age at onset of Parkinson’s disease, and an earlier and more rapid cognitive decline than our cohort. Another possible explanation is the lower statistical power in the previous study due to a smaller sample than in our study which is consistent with are cent report finding less widespread neocortical atrophy with an even smaller sample size [9]. Therefore, our results may reflect the nature of the disease more veritably.
In this study, patients with PD with or without MCI had cortical gray matter reduction compared with normal controls. In PD-nMCI group, gray matter density decreased mainly in the right temporal lobe and in the right hippocampus and anterior cingulate cortex. In PD-MCI group, gray matter reductions were more widespread, including right temporal lobe, precuneus, cuneus, lingual gyrus, fusiform gyrus, orbitofrontal cortex. Structural gray matter differences in the two groups showed MCI and nMCI were the two stages during the progress of the PD. This is consistent with the results reported by Caviness [25], which suggested that PD-MCI was intermediate between PD cognitively normal (PD-CogNL) and PD with dementia PD-D, and significantly different from both PD-CogNL and PD-D in terms of the mean duration of PD and MMSE scores. Brakket al [26] reported that limbic and paralimbic structures were involved at stage 4 and gradually increases and reached widespread neocortex at stages 5 and 6. In this study, patients with PD without cognitive impairment had gray matter reductions in right temporal lobe, hippocampus, and anterior cingulate cortex, whereas patients with PD with cognitive impairment mainly in parieto-occipital association cortex and frontal neocortex, which indicated that the lesion location in the two groups conforms to the staging of brain pathology related to PD.
We also found that patients with PD with MCI had cortical gray matter reduction in parieto-occipital association cortex, occipital lobes, bilateral precentral gyrus and middle temporal gyrus, and right orbitofrontal cortex compared with patients with Parkinson’s disease with normal cognition. These areas of reduced gray matter density may be associated with the cognitive impairment in PD. Our results were consistent with those reported in previous studies [27-29]. In a PET study, Flavio et al [27] observed that PD-MCI showed hypoperfusion in a left parietal region, mainly including precuneus and inferior parietal lobule, and in a right temporal-parietal-occipital region, including middle occipital and superior temporal gyri, and cuneus-precuneus, as compared to PD-nMCI. Abe et al [28] showed that the PD patients had significantly reduced regional cerebral blood flow in the bilateral occipital and posterior parietal cortices compared with normal controls. Besides, they also found that occipital hypoperfusion is likely to be associated with impairment of visual cognition. Based on event-related functional magnetic resonance imaging (fMRI), Lewis et al [29] demonstrated that significant signal intensity reductions during a working-memory paradigm in parietal-occipital association region and bilateral prefrontal lobe sites in patients with MCI compared with those patients who were not cognitively unimpaired.
Additionally, significantly reduced gray matter density was observed in primary motor cortex in patients with PD with MCI, compared to PD with no MCI. The authors consider that PD is a movement disorder and the lesion induced change in motor cortex should be more significant with the progress of the PD.
The main limitations of this study is the small sample size, particularly of patients with Parkinson’s disease with MCI, the possibility of selection bias, as a proportion of the patients with Parkinson’s disease were referrals to outpatient hospital clinics.
In conclusion, the findings of the present study suggest that Parkinson’s disease is associated with the structural neocortical atrophy in the brain, and that cognitive impairment in patients with PD may be associated with gray matter changes in the parieto-occipital association cortex, right orbitofrontal cortex, and middle temporal gyrus. Further imaging studies and clinicopathological studies with larger sample size are still needed to confirm these findings.
Acknowledgements
The study was supported by the Natural Science Foundation (No. 1010RJZA096) from the Science and Technology Office of Gansu Province in China.
Disclosure of conflict of interest
None.
References
- 1.De Rijk M, Breteler M, Graveland G, Ott A, Grobbee D, Van der Meche F, Hofman A. Prevalence of Parkinson’s disease in the elderly The Rotterdam Study. Neurology. 1995;45:2143–2146. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Tandberg E, Larsen JP, Cummings JL. Frequency of dementia in Parkinson disease. Arch Neurol. 1996;53:538–542. doi: 10.1001/archneur.1996.00550060082020. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Andersen K, Larsen J, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease A community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 4.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 5.Williams-Gray C, Foltynie T, Brayne C, Robbins T, Barker R. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 6.Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 7.Pan PL, Shi HC, Zhong JG, Xiao PR, Shen Y, Wu LJ, Song YY, He GX, Li HL. Gray matter atrophy in Parkinson’s disease with dementia: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34:613–619. doi: 10.1007/s10072-012-1250-3. [DOI] [PubMed] [Google Scholar]
- 8.Ramírez-Ruiz B, Martí MJ, Tolosa E, Bartrés-Faz D, Summerfield C, Salgado-Pineda P, Gómez-Ansón B, Junqué C. Longitudinal evaluation of cerebral morphological changes in Parkinson’s disease with and without dementia. J Neurol. 2005;252:1345–1352. doi: 10.1007/s00415-005-0864-2. [DOI] [PubMed] [Google Scholar]
- 9.Summerfield C, Junqué C, Tolosa E, Salgado-Pineda P, Gómez-Ansón B, Martí MJ, Pastor P, Ramírez-Ruíz B, Mercader J. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62:281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- 10.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel S, Lees A. Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. 1992;39:165–172. [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1998;50:318–318. doi: 10.1212/wnl.50.2.318. [DOI] [PubMed] [Google Scholar]
- 13.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Elton R, Marsden C, Calne D, Golstein M. Recent development in Parkinson’s disease. Floram Park, NJ Macmilian Health Care Information. 1987 [Google Scholar]
- 16.Good CD, Johnsrude IS, Ashburner J, Henson RN, Fristen K, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 17.Hu M, White S, Chaudhuri KR, Morris R, Bydder G, Brooks D. Correlating rates of cerebral atrophy in Parkinson’s disease with measures of cognitive decline. J Neural Transm. 2001;108:571–580. doi: 10.1007/s007020170057. [DOI] [PubMed] [Google Scholar]
- 18.Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Brain atrophy rates in Parkinson’s disease with and without dementia using serial magnetic resonance imaging. Mov Disord. 2005;20:1571–1576. doi: 10.1002/mds.20652. [DOI] [PubMed] [Google Scholar]
- 19.Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch J, Evans A, Dagher A, Ito K. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 20.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 21.RamírezírezMcKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebralola F and Junque C. Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur J Neurol. 2007;14:750–756. doi: 10.1111/j.1468-1331.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 22.Cordato N, Duggins A, Halliday G, Morris J, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain. 2005;128:1259–1266. doi: 10.1093/brain/awh508. [DOI] [PubMed] [Google Scholar]
- 23.Brenneis C, Seppi K, Schocke MF, Müller J, Luginger E, Bösch S, Löscher WN, Büchel C, Poewe W, Wenning GK. Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord. 2003;18:1132–1138. doi: 10.1002/mds.10502. [DOI] [PubMed] [Google Scholar]
- 24.Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson’s disease with and without rest tremor. J Neurol. 2009;256:256–263. doi: 10.1007/s00415-009-0092-2. [DOI] [PubMed] [Google Scholar]
- 25.Caviness JN, DriveriverKollias SS, Bassetti CL, Waldvogel D. Morphological dife VGH, Shill HA and Adler CH. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Tredici KD, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 27.Nobili F, Abbruzzese G, Morbelli S, Marchese R, Girtler N, Dessi B, Brugnolo A, Canepa C, Drosos GC, Sambuceti G. Amnestic mild cognitive impairment in Parkinson’s disease: a brain perfusion SPECT study. Mov Disord. 2009;24:414–421. doi: 10.1002/mds.22381. [DOI] [PubMed] [Google Scholar]
- 28.Abe Y, Kachi T, Kato T, Arahata Y, Yamada T, Washimi Y, Iwai K, Ito K, Yanagisawa N, Sobue G. Occipital hypoperfusion in Parkinson’s disease without dementia: correlation to impaired cortical visual processing. J Neurol Neurosurg Psychiatry. 2003;74:419–422. doi: 10.1136/jnnp.74.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


