Abstract
To explore the expression levels of T-type calcium channel receptors in spiral ganglion neurons of C57BL/6J mice and the effect of T-type calcium channel blockers on the spiral ganglion neurons of 42-44-W C57BL/6J mice. We first quantified the subunits of T-type calcium channel blockers in the spiral ganglion neurons of C57BL/6J mice in three groups (6-8 W, 24-26 W, 42-44 W) according to age via RT-PCR. Next, we administered three drugs (zonisamide, felodipine, saline) to the 42-44-W C57BL/6J mice by gavage for four weeks. We observed the changes in the hearing threshold of 42-44-W C57BL/6J mice after treatment. Meanwhile, we measured the expression of calcium-binding proteins of spiral ganglion neurons after treatment. Our results showed that three receptors were expressed in the spiral ganglion neurons of C57BL/6J mice. The expression level of α1H was stronger than that of α1G and α1I. The expression levels of three receptors especially for α1G and α1H significantly decreased with age. The hearing threshold at 24 kHz was significantly decreased after zonisamide administration. No significant difference in the expression level of calbindin in spiral ganglion neurons was noted. Interestingly, the expression level of calmodulin in spiral ganglion neurons was lower in the zonisamide-treated groups than in the felodipine- and saline-treated group. We concluded that the administration of T-type calcium channel blocker for four consecutive weeks can improve the hearing by ameliorating calcium overload on spiral ganglion neurons of 42-44-W C57BL/6J mice.
Keywords: T-type calcium channel, receptor, calcium channel blocker, spiral ganglion neurons, calcium-binding protein
Introduction
Presbycusis can significantly impact the quality of life of the elderly. Age-related hearing loss involves various parts of the ear and the nervous system. Loss of hair cells and spiral ganglion neurons (SGNs) is a major cause of presbycusis [1]. The molecular mechanisms underlying the age-related loss of hair cells and SGNs remain unclear. Mutations of mitochondrial DNA have been demonstrated to accumulate with aging in several types of neurons, including SGNs [2]. Mitochondrial damage is closely associated with intracellular calcium overload, which induces a cascade of responses. Excessive calcium activates phosphatase A2 and phosphatase C, promoting the hydrolysis of membrane phospholipids, the release of free fatty acids [3], and the peroxidation of lipids [4]. Calcium homeostasis is crucial for the regulation of a variety of physiological processes, including hearing [5,6].
Presently, there are no drugs that prevent or treat presbycusis, but calcium channel blockers may represent a potential therapeutic strategy. Voltage-gated calcium channels are classified into low-voltage-gated and high-voltage-gated channels. Low-voltage-gated calcium channels primarily include T-type calcium channels, whereas high-voltage-gated calcium channels include L-, P-/Q-, N-, and R-type calcium channels. T-type calcium channels are further categorized into three subtypes, Cav3.1, Cav3.2, and Cav3.3, based on their encoding genes. The receptor subtypes Cav3.1, Cav3.2, and Cav3.3 correspond to the α1G, α1H, and α1I subunits, respectively.
It has been suggested that T-type calcium channels contribute to the cell death of glia and neurons under ischemic conditions [7]. Inhibition of T-type calcium channels can protect neurons after stroke [8]. It has been reported that T-type calcium channel blockers can provide protection for the inner ear against cisplatin-mediated toxicity [9]. A T-type calcium channel blocker can ameliorate noise-induced hearing loss by preserving the outer hair cells [10]. However, few studies have explored the effect of T-type calcium channel blocker on auditory organ especially SGNs of aged mice. SGNs are the primary auditory neurons as the bridge connecting hair cells with cochlear nucleus. C57BL/6J mice are often used as a model of presbycusis because they exhibit marked progressive age-related hearing loss [11]. The hearing of C57BL/6J mice was profound loss at 42-44 weeks of age (42-44-W) in our previous study [12]. The objective of this study is to explore the effect on the SGNs of 42-26-W C57BL/6J mice by T-type calcium channel blockers. First, we quantified the subunits of T-type calcium channel blockers in the spiral ganglion neurons of C57BL/6J mice in three groups (6-8-W, 24-26-W, 42-44-W) according to age via RT-PCR. Next, we administered three drugs (zonisamide, felodipine, saline) to the 42-44-W C57BL/6J mice by gavage for four weeks. We observed the changes in the hearing threshold of 42-44-W C57BL/6J mice after treatment. Meanwhile, we measured the expression of calcium-binding proteins of spiral ganglion neurons after treatment.
Materials and methods
Animals and tissue preparation
Forty five C57BL/6J male mice consisted of three groups (6-8-W, 24-26-W, 42-44-W) according to age. Each group was separated into three subgroups (n=5 in each subgroup) to detect the three T-type calcium channel subunits (α1G, α1H, α1I) via RT-PCR. Another thirty 42-44-W C57BL/6J male mice were separated into three treatment groups (saline, zonisamide, and felodipine). Each group was subjected to an auditory brainstem response (ABR) test after treatment. Zonisamide and felodipine were obtained from Sigma Chemical Co. (St. Louis, USA). Preliminary experiments using mice led us to select dosages of 160 mg/d/kg for zonisamide and 10 mg/d/kg for felodipine. The drugs were administered to the animals via gavage for four consecutive weeks. All experiments were performed in compliance with Chinese legislation on the care and use of laboratory animals, and all studies were approved by the Soochow University Institutional Animal Care and Use Committee.
The mice were sacrificed via cervical dislocation after the ABR test. The SGNs were isolated using a dissecting microscope. The tissues used for in situ hybridization and immunohistochemistry were fixed using 4% paraformaldehyde and embedded in paraffin after complete fixation. The tissues used for RT-PCR were immersed in ice-cold RNA solution to prevent RNA degradation.
Real-time quantitative reverse transcriptase (RT)-polymerase chain reaction (PCR)
Total RNA was extracted using Trizol Reagent (Gibco, USA). The concentration and quality of the extracted RNA were determined by measuring the absorbance at 260 nm (A260) and 280 nm (A280). The A260/A280 ratio of each sample was 1.8 to 2.0. RT cDNA was synthesized using MMLV reverse transcriptase (Promega, Madison, USA), and PCR was performed according to the manufacturer’s instructions (ABI-7500, USA) using a total volume of 20 µL of a reaction mixture containing 4 µL of 5× RT buffer, 0.5 µL of Oligo (dT), 0.5 µL of dNTPs, 1 µL of MMLV reverse transcriptase, 10 µL of DEPC-treated water, and 4 µL of the RNA template. The reaction conditions for inactivating MMLV were 37°C for one hour followed by 95°C for 5 minutes.
PCR (SYBRGreen PCR kit, Thermo, USA) was performed using a total volume of 50 µL. The mixture contained 32.5 µL of SYBRGreen Mix, 0.5 µL of the upstream primer, 0.5 µL of the downstream primer, 14.5 µL of ddH2O, and 2 µL of the cDNA template. The PCR conditions were as follows: 95°C for 30 minutes, followed by 40 cycles of 95°C for 30 seconds, 58°C for 30 seconds, and 73°C for 90 seconds. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for the quantification of the PCR results. Relative quantification (RQ) was performed based on the number of PCR cycles necessary to obtain the threshold signal of fluorescence (Ct).
The DNA sequences of the primers (forward and reverse primers, respectively) were as follows: GAPDH (5-AACGGATTTGGTCGTATTG-3 and 5-GGAAGATGGTGATGGGATT-3), α1G subunit (5-ACCTGCCTGACACTCTGCAG-3 and 5-GCTGGCCTCAGCGCAGTCGG-3), α1H subunit (5-GGCGTGGTGGTGGAGAACTT-3 and 5-GATGATGGTGGGATTGAT-3), α1I subunit (5-ATCTGCTCCCTGTCGG-3 and 5-GAGAACTGGGTCGCTATG-3). The primers were synthesized using the Primer Premier 5.0 software program (Invitrogen, USA) at by the Shanghai Linyan Institute (Shanghai, China).
Auditory brainstem response (ABR)
Each mouse was anesthetized via intramuscular injection of a mixture of xylazine (25 mg/kg) and ketamine (100 mg/kg) and placed in a soundproof anechoic chamber containing a thermostabilized prone experimental platform that maintained the body temperature at 37°C. ABRs were recorded subcutaneously using needle electrodes placed at the vertex, the ipsilateral mastoid process and the contralateral mastoid process. The speaker was placed in the ear canal. Generation of stimulus signals and recording of evoked potentials were performed using an ABR workstation (Tucker-Davis Technologies AD3, USA). Tone burst stimuli (5-ms duration, 0.5-ms rise-fall time) were generated, and each average response was based on 1000 repeated stimuli at a rate of 11 stimuli/s applied at frequencies of 8, 16, 24 and 32 KHz. Neuronal activity was amplified (100,000U) and filtered (0.3-3.0 kHz). The hearing threshold at each frequency was determined based on a clear wave when reducing the stimulus intensity from the suprathreshold level. Beginning at a sound pressure level (SPL) of 100 dB, decreasing steps in 10-dB SPL increments were applied, after which 5-dB increments were applied until a clear wave response occurred. The mean threshold value was determined after repeating the ABR test at least two times.
Immunohistochemistry
The paraffin-embedded slices were sectioned (4 um thick) and mounted on poly-lysine-coated glass slides. The sections were washed 3× with 0.01 M PBS (5 min each) and blocked using 1% BSA for 30 min at room temperature. The sections were incubated overnight in a primary antibody (calmodulin 1:300; calbindin 1:200; Abcam Company). The sections were rinsed 3× with 0.01 M PBS (5 min each) and incubated for 2 h in biotinylated goat anti-rabbit antibody (1:200). The sections were incubated for another 2 h in ABC solutions after identical rinsing steps. After washing with PBS as indicated above, the sections were stained using diaminobenzidine (DAB) and counterstained using hematoxylin. The slides were coverslipped using neural gum mounting medium. The data were quantified by measuring the mean optical density.
Statistical analysis
All data were expressed as means ± SD and were analyzed using SPSS software, version 17.0 (SPSS, USA). One-way analysis of variance (ANOVA) with the LSD post hoc test was used to analyze the ABR test, RT-PCR and immunohistochemistry data sets. Differences between the groups were considered significant for P≤0.05.
Results
Expression of three T-type channel subunits by real-time quantitative RT-PCR
The expression level of each receptor was calculated using the formula 2-ΔΔCT (Figure 1). Compared to the other two subunits, the expression level of α1H in the SGNs was the greatest at the age of 6-8 W (P<0.05). The expression levels of α1G and α1I were lower and there was no significant difference between them (P>0.05). The expression levels of three receptors decreased with age. The expression levels of α1G and α1H significantly decreased at the age of 42-44 W compared to the age of 6-8 W (P<0.05).
Figure 1.
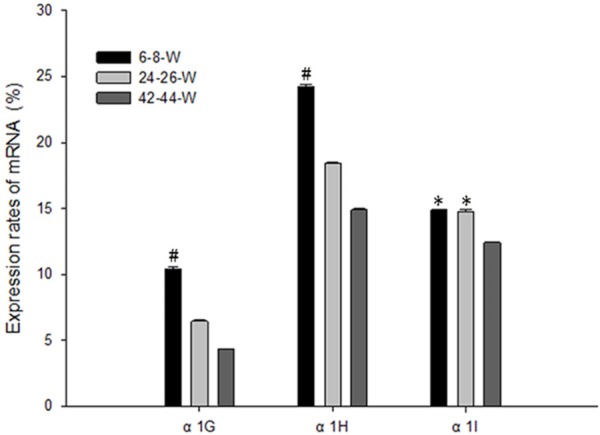
The quantified expression of three T-type channel subunits via real-time quantitative RT-PCR analysis revealed that the expression level of α1H was the highest, whereas that of α1G and that of α1I were the lowest at the age of 6-8 W. The expression levels of three receptors especially for α1G and α1H significantly decreased with age. #P<0.05, *P>0.05.
ABR test
The hearing loss was profound at 42-44 weeks of age of C57BL/6J mice. Compared to the saline-treated group, the hearing threshold at 24 kHz significantly decreased in the zonisamide-treated group (P<0.05). The hearing threshold at 32 kHz decreased in the zonisamide- and felodipine-treated groups compared to the saline-treated group, but these differences were not significant (Figure 2).
Figure 2.
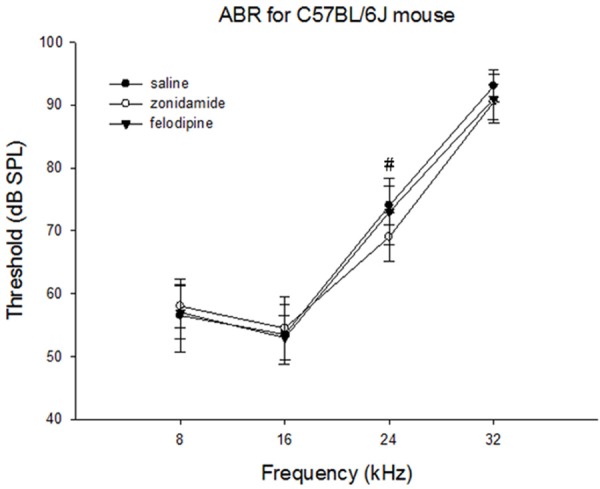
The hearing threshold of C57BL/6J mice at 42-44 weeks of age after treatment. The hearing threshold at 24 kHz significantly decreased in the zonisamide-treated group compared to threshold of the felodipine-treated group and the saline-treated group. #P<0.05.
Expression of calmodulin and calbindin based on immunohistochemistry
In the SGNs of 42-46-W C57BL/6J mice (Table 1), the expression level of calmodulin was significantly lower in the zonisamide-treated group (Figure 3A) than in the saline-treated group (Figure 3C) (P<0.05). However, the expression level of calmodulin was not significantly different between the saline-treated group and the felodipine-treated group (Figure 3B) (P>0.05). The expression level of calbindin was not significantly different in the zonisamide-treated group (Figure 4A) or the felodipine-treated group (Figure 4B) than in the saline-treated group (Figure 4C) (P>0.05).
Table 1.
The expression levels of calmodulin and calbindin in the SGNs of 42-44-W C57BL/6J mice after treatment (mean optical density ± S.E.M)
| Zonisamide | Felodipine | Saline | |
|---|---|---|---|
| Calmodulin | 0.4877 ± 0.0262# | 0.5744 ± 0.0254 | 0.5851 ± 0.0196# |
| Calbindin | 0.4813 ± 0.0168* | 0.4812 ± 0.0220* | 0.4792 ± 0.0281* |
<0.05;
P>0.05.
Figure 3.
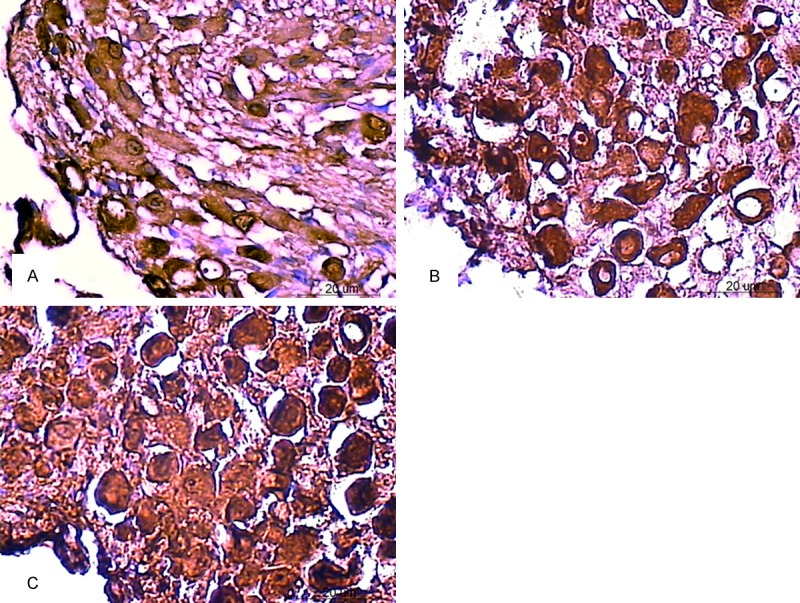
Expression of calmodulin in the SGNs of C57BL/6J mice at 42-44 weeks of age after treatment. The immunoreactive product appears as brown particles in the SGNs. The expression of calmodulin was significantly decreased in the zonisamide-treated group (A) compared to that of the saline-treated group (C). The expression level was not significantly different between the felodipine-treated group (B) and the saline-treated group (Scale bar=20 um).
Figure 4.
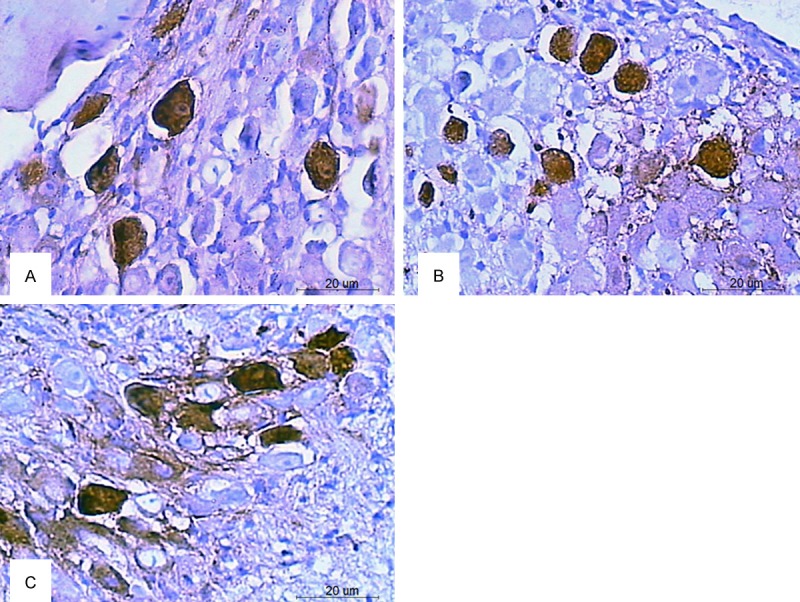
Expression of calbindin in the SGNs of C57BL/6J mice at 42-44 weeks of age after treatment. The brown particles are the immunoreactive product in the SGNs. The expression of calbindin was not significantly decreased in both the zonisamide-treated group (A) and the felodipine-treated group (B) compared to that of the saline-treated group (C) (Scale bar=20 um).
Discussion
Research on the etiology of presbycusis has generated much significant progress, but the ideal method for the prevention and treatment of presbycusis has yet to be discovered. Some studies on the relationship between calcium channels and deafness have been reported recently. A missing gene, CACNA1D, closely associated with congenital deafness and heart disease was reported [13]. A variety of calcium channel blockers have been broadly used to treat diseases of the cardiovascular system and other organ systems. There is evidence that these drugs can reduce the cytotoxicity of calcium ions in ischemic heart disease [14]. There is controversy, especially with respect to L-type calcium channels, over whether calcium channel blockers exert a protective effect on the inner ear. The L-type calcium channel blocker nifedipine was observed to decrease noise damage to the guinea pig cochlea [15]. In contrast, nimodipine, another L-type calcium channel blocker, did not protect the cochlea from hearing loss and even worsened the damage to the organ of Corti when administered in combination with pentoxifylline [16]. However, the T-type calcium channel blocker flunarizine prevented cisplatin-induced cochlear hair cell death in an in vitro study [9].
We chose 42-44-W C57BL/6J mice experiencing profound hearing loss as our animal model mimic old people to determine effect of T-type calcium channel blockers. Age-related progressive retrograde degeneration of SGNs had been demonstrated in C57BL/6J mice [17]. Therefore, we observed the effect of T-type calcium channel blockers on the SGNs of 42-44-W C57BL/6J mice. First, we confirmed that three T-type calcium channel subtypes were distributed in the SGNs of C57BL/6J mice. Among these three subunits, α1H was highly expressed and the other two subunits were weakly expressed based on RT-PCR. The expression levels of three receptors especially for α1G and α1H significantly decreased with age. We chose the T-type calcium channel blocker zonisamide and the L-type blocker felodipine, saline as control group. Zonisamide is an antiepileptic drug that also reduces the symptoms of Parkinson’s disease [18]. The possible mechanism of action of this drug is related to its inhibition of T-type calcium channels in neurons [19,20]. Felodipine is a preferred drug to reduce high blood pressure by inhibiting L-type calcium channel [21]. The amelioration of hearing is associated with the peripheral or central auditory system or both. SGNs may represent one neuron type that benefits from this treatment. The finding that the zonisamide-treated group exhibited better hearing than the felodipine-treated group may be due the effect of zonisamide on T-type calcium channel.
Calcium homeostasis is maintained in hair cells and SGNs by regulating proteins such as calcium-binding proteins [22,23]. Calcium-binding proteins play important roles in intracellular Ca2+ signaling, modulating neuronal excitability and plasticity [24]. To determine the changes in SGNs after the administration of T-type calcium channel blockers, we measured the expression levels of two calcium-binding proteins, calbindin and calmodulin. Calbindin is a calcium-binding protein belonging to the troponin C superfamily. Calmodulin is known to modulate the calcium-dependent inactivation of calcium channels [25]. Calmodulin and calbindin are both EF hand-containing calcium-binding proteins that are widely expressed in neurons and act as Ca2+ buffers to regulate calcium signal transduction [26,27]. Increased calcium-binding protein immunoreactivity in the cochlea nucleus of old mice has been observed to reflect an endogenous protective strategy to counteract calcium overload [28]. Interestingly, our results revealed that the expression level of calmodulin decreased after administration of the T-type blocker zonisamide. The results indicate that T-type blocker can alleviate calcium overload in the SGNs of 42-44-W C57BL/6J mice. Our results demonstrate that the more pronounced effects of T-type channel blockers may be due to the larger dose of the drugs provided by administration via gavage rather than via drinking water for four consecutive weeks. One benefit of this route of administration is that the dosage of the drugs could be accurately controlled. However, the side effects must be considered if a larger dose is administered to humans.
Nevertheless, these drugs cannot be immediately used clinically for presbycusis because the results of this study were obtained using a limited number of mice. Many factors may affect these results, such as the sex of the animals, the type of calcium channel blockers used, use of these drugs alone or in combination, and the route of administration. Future studies will be focused on the manner in which to select and administer these calcium channel blockers and the reduction of their side effects.
Acknowledgements
This study was supported by grants from the Science and Technology Bureau of Suzhou (SYS201228 and SYS201449).
Disclosure of conflict of interest
None.
References
- 1.Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- 2.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 3.Farooqui AA, Ong WY, Horrocks LA. Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A2. Neurochem Res. 2004;29:1961–1977. doi: 10.1007/s11064-004-6871-3. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. J Bioenerg Biomembr. 2004;36:353–356. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- 5.Giacomello M, De Mario A, Primerano S, Brini M, Carafoli E. Hair cells, plasma membrane Ca2+ ATPase and deafness. Int J Biochem Cell Biol. 2012;44:679–683. doi: 10.1016/j.biocel.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Karlstad J, Sun Y, Singh BB. Ca(2+) signaling: an outlook on the characterization of Ca(2+) channels and their importance in cellular functions. Adv Exp Med Biol. 2012;740:143–57. doi: 10.1007/978-94-007-2888-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fern R. Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J Neurosci. 1998;18:7232–43. doi: 10.1523/JNEUROSCI.18-18-07232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikonenko I, Bancila M, Bloc A, Muller D, Bijlenga P. Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. Mol Pharmacol. 2005;68:84–89. doi: 10.1124/mol.104.010066. [DOI] [PubMed] [Google Scholar]
- 9.So HS, Park C, Kim HJ, Lee JH, Park SY, Lee JH, Lee ZW, Kim HM, Kalinec F, Lim DJ, Park R. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hear Res. 2005;204:127–139. doi: 10.1016/j.heares.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao X, Wang Q, Ohlemiller KK, Piccirillo J, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KR, Erway LC, Cook SA. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 12.Yu YF, Zhai F, Dai CF, Hu JJ. The relationship between age-related hearing loss and synaptic changes in the hippocampus of C57BL/6J mice. Exp Gerontol. 2011;46:716–722. doi: 10.1016/j.exger.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nürnberg P, Striessnig J, Bolz HJ. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 14.Ghyasi R, Sepehri G, Mohammadi M, Badalzadeh R, Ghyasi A. Effect of mebudipine on oxidative stress and lipid peroxidation in myocardial ischemic-reperfusion injury in male rat. J Res Med Sci. 2012;17:1150–1155. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Niu YG, Li WX, Yuan YY, Han WJ, Yu N, Yang SM, Li XQ. Interaction of a calcium channel blocker with noise in cochlear function in guinea pig. Acta Otolaryngol. 2012;132:1140–1144. doi: 10.3109/00016489.2012.690534. [DOI] [PubMed] [Google Scholar]
- 16.Kansu L, Ozkarakas H, Efendi H, Okar I. Protective effects of pentoxifylline and nimodipine on acoustic trauma in Guinea pig cochlea. Otol Neurotol. 2011;32:919–925. doi: 10.1097/MAO.0b013e3182267e06. [DOI] [PubMed] [Google Scholar]
- 17.White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141:12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- 18.Murata M, Hasegawa K, Kanazawa I. Japan Zonisamide on PD Study Group, Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology. 2007;68:45–50. doi: 10.1212/01.wnl.0000250236.75053.16. [DOI] [PubMed] [Google Scholar]
- 19.Schauf CL. Zonisamide enhances slow sodium inactivation in Myxicola. Brain Res. 1987;413:185–188. doi: 10.1016/0006-8993(87)90168-5. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Kawakami K, Nishimura S, Watanabe Y, Yagi K, Seino M, Miyamoto K. Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 1992;12:21–27. doi: 10.1016/0920-1211(92)90087-a. [DOI] [PubMed] [Google Scholar]
- 21.Paterna S, Di Pasquale P, Parrinello G, Tuttolomondo A, Follone G, Cardinale A, Ortoleva A, Giambanco F, Abruzzese G, Colomba D, Bologna P, Fernández P, Giubilato A, Valdes L, Albano V, Licata G. Comparison of the effects of felodipine and cilazapril on exercise performance in patients with mild to moderate hypertension. A crossover study. Drugs Exp Clin Res. 2000;26:125–131. [PubMed] [Google Scholar]
- 22.Hansen MR, Bok J, Devaiah AK, Zha XM, Green SH. Ca2+/calmodulin-dependent protein kinases II and IV both promote survival but differ in their effects on axon growth in spiral ganglion neurons. J Neurosci Res. 2003;72:169–184. doi: 10.1002/jnr.10551. [DOI] [PubMed] [Google Scholar]
- 23.Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci. 2005;25:7867–7875. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christel CJ, Schaer R, Wang S, Henzi T, Kreiner L, Grabs D, Schwaller B, Lee A. Calretinin regulates Ca2+-dependent inactivation and facilitation of Ca(v)2.1 Ca2+ channels through a direct interaction with the α12.1subunit. J Biol Chem. 2012;287:39766–39775. doi: 10.1074/jbc.M112.406363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovalevskaya NV, van de Waterbeemd M, Bokhovchuk FM, Bate N, Bindels RJ, Hoenderop JG, Vuister GW. Structural analysis of calmodulin binding to ion channels demonstrates the role of its plasticity in regulation. Pflugers Arch. 2013;465:1507–1519. doi: 10.1007/s00424-013-1278-0. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki H, Nakayama S, Kretsinger RH. Classifi cation and evolution of EF-hand proteins. Biometals. 1998;11:277–295. doi: 10.1023/a:1009282307967. [DOI] [PubMed] [Google Scholar]
- 27.Nelson MR, Thulin E, Fagan PA, Forsen S, Chazin WJ. The EF-hand domain: a globally cooperative structural unit. Protein Sci. 2002;11:198–205. doi: 10.1110/ps.33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idrizbegovic E, Salman H, Niu X, Canlon B. Presbyacusis and calcium-binding protein immunoreactivity in the cochlear nucleus of BALB/c mice. Hear Res. 2006;216-217:198–206. doi: 10.1016/j.heares.2006.01.009. [DOI] [PubMed] [Google Scholar]


