Abstract
Objective: The purpose of this experimental study was to evaluate the efficacy of carvacrol (CVR) and pomegranate (PMG) against methotrexate (MTX)-induced intestinal damage using histopathological and immunohistochemical techniques. Methods: Thirty-two male Sprague-Dawley rats, weighing 195-250 g, were divided into four groups: control, MTX treatment alone, MTX plus CVR and MTX plus PMG. A single dose of CVR (73 mg/kg) was administered intraperitoneally to group III on the first day of the experiment, PMG (225 mg/kg/day) was administered orogastrically (with a gavage needle) once daily for 7 days and a single dose of MTX (20 mg/kg) was administered intraperitoneally on the second day of the experiment. Intestinal tissues were obtained on 8th day, and examined for villus damage, crypt damage, and inflammation. Ki-67 and Caspase 3 staining was used for immunohistochemical evaluation. Results: MTX treatment induced villus shortening and fusion, epithelial atrophy, crypt loss, inflammatory infiltrate in the lamina propria, and goblet cell depletion. The CVR and PMG decreased the severity of intestinal damage caused by MTX treatment. In the MTX-received group, significant inflammatory cell infiltration was observed in the lamina propria. Compared to the MTX-received group, the PMG and CVR groups showed less villus and crypt damage and less inflammation in the lamina propria. Fewer Ki-67 positive cells were observed in the crypts of the MTX-received groups compared to the control group. There were more Ki-67 positive cells in the CVR and PMG groups compared to MTX group. The MTX-received group exhibited more caspase-3 positive cells than the control group, and the number of caspase-3 positive cells were decreased in the CVR and PMG treated groups. Conclusion: This study is the first to show that PMG and CVR decrease MTX-related damage and apoptotic activity in intestinal tissue.
Keywords: Carvacrol, intestinal damage, methotrexate, pomegranate
Introduction
Methotrexate (MTX), a folic acid antagonist, is commonly used in the treatment of certain neoplasms like leukaemia and lymphoma and some autoimmune diseases such as rheumatoid arthritis, lupus and psoriasis for more than 60 years [1,2]. MTX, as a dihydrofolic acid analogue, inhibits the dihydrofolate reductase (DHFR) enzyme which catalyses the conversion of dihydrofolate to the active tetrahydrofolate in the tetrahydrofolate synthesis. MTX, therefore, inhibits the DNA, RNA, thymidylate, and protein synthesis and eventually causes apoptosis [3]. MTX exerts its cytotoxic effects not only against cancer cells but also normal cells. MTX-related toxicity has been reported in normal tissues, particularly tissues with a high proliferative rate like hematopoietic system, bone marrow and gastrointestinal system (GIS) mucosa [4,5].
Gastrointestinal side effects, like intestinal mucosal damage and enterocolitis are the major MTX-related toxic reactions. Damage in the mucosa of the small intestine results in malabsorption and diarrhea [6,7]. Recent studies support the idea that apoptosis is causative in MTX-related GIS cell loss. However there are multiple mechanisms defined for gastrointestinal toxicity, reactive oxygen species (ROS) are mainly responsible for MTX-related small intestinal damage [8]. MTX inhibits NADPH dehydrogenase, an important cytosolic antioxidant that functions against the destructive effect of ROS. MTX leaves vulnerable the enterocytes against ROS via decreasing glutathione levels [9]. On the other hand, MTX-induced activation of nuclear factor kappa B was reported to cause intestinal mucosal damage and pro-inflammatory cytokine and chemokine production in intestinal epithelial cells in vivo and in vitro [10].
Different anti-inflammatory, antioxidant, anti-proliferative and immunomodulatory drugs have been used to decrease the toxic effects of MTX [11,12]. However, none have provided complete protection against MTX toxicity. Thus, there is a wide field for new agents that can be used to prevent MTX-related damage on normal tissues.
Pomegranate (PMG) is an herbal supplement having anti-inflammatory, anti-proliferative, anti-oxidant, anti-carcinogenic and immunomodulatory effects [13-15]. Carvacrol (CVR), another herbal supplement, has been shown to have a curative effect on lung, liver, testicle and breast cancer, in addition to anti-oxidant and anti-hepatotoxic effects [16-18].
According to English medical literature search we did not find any study on the effect of CVR and PMG on MTX-related toxicity in the small intestine. The purpose of this experimental study was to evaluate the efficacy of CVR and PMG against MTX-induced intestinal damage using histopathological and immunohistochemical techniques.
Materials and methods
Thirty-two Sprague-Dawley adult male rats weighing 195-250 g were used in this study. The animals were kept in the animal laboratory of our university in a 12-h light/12-h dark cycle in suitable cages, and water and food were provided freely. All the rats in each group were given normal pellet food and water during the experiment period. The local ethics board provided consent for the animal experiments performed in this study.
Experimental protocol
The animals (n = 32) were randomly divided into four groups of eight rats each: control, MTX, CVR and PMG groups.
Group 1 (control): The rats in this group were not given any drugs and were fed with a normal diet.
Group 2 (MTX): On the second day, the rats were administered 20 mg/kg of MTX (MTX, Onco-Tain, Faulding Pharmaceutics Plc., U.K.) intra-peritoneally. They were fed with a normal diet for 7 days.
Group 3 (CVR): A 73 mg/kg dose of oregano oil containing 83% CVR was injected intra-peritoneally on the first day. On Day 2, group III rats received a single dose of 20 mg/kg MTX intraperitoneally. The plant substance tested in this study, CVR (2-methyl-5-(1-methylethyl)-phenol), was isolated from steam-distilled essential oil of Origanum onites L. collected from Western Anatolia, as described by Canbek et al [19].
Group 4 (PMG): Pomegranate extract (Pomella®, Verdure Sciences, Noblesville, IN, USA) at a dose of 225 mg/kg/day was administered orogastrically (with a gavage needle) once daily for 7 days. On Day 2, a single dose of 20 mg/kg MTX was administered by intraperitoneal injection.
Surgical procedures
The total duration of the experiment was 8 days. None of the animals died during the experiment. After completion of the experimental and control treatments, all the animals were anesthetized on the eight day with an intramuscular injection of ketamine HCl (50 mg/kg) and a peritoneal injection of xylazine (Rompun®; Bayer AG, Leverkusen, Germany), 10 mg/kg) and euthanized by decapitation.
Histopathological assessment
After excision, the intestines of the euthanized animals were fixed in 10% formalin solution for 48 h. After routine histological tissue preparation, all the specimens were infiltrated with wax, sectioned at 4-5 μm thickness and stained with Hematoxylin and Eosin (H&E) and periodic acid-Schiff (PAS) according to standard methods. For histopathological evaluation, all the specimens were examined and photographed using light microscopy (Olympus BX53, Tokyo, Japan). H&E-stained tissue sections were examined for villus damage, crypt damage and inflammation. The length of the intestinal villi was also measured.
Villus damage and crypt damage was scored as follows: no damage = 0, mild damage = 1 moderate damage = 2 and severe damage = 3. Inflammation observed in the lamina propria was scored as follows [20]: no inflammation = 0, mild inflammation = 1 moderate inflammation = 2 and severe inflammation = 3. The heights of 10 intestinal villi (× 10 magnification) in each slice were measured on H&E slides on a light microscope equipped with a high-resolution digital camera (Olympus DP21, Tokyo, Japan).
Goblet cell loss was evaluated in the PAS-stained cross-sections [20]. Goblet cell numbers were scored as follows: 0-49 cells = 3, 50-99 cells = 2; 100-149 cells = 1 and ≥ 150 cells = 0.
Immunohistochemistry
Ki-67 (Pre-diluted, ready-to-use) (PRM 325 AA, rabbit monoclonal, Biocare, Concord, USA), demonstrating proliferation, and caspase-3 Ab-4 (dilution 1:100) (RB-1197-P1, rabbit polyclonal, Thermo, Fremont, USA) antibodies, demonstrating apoptosis, were used in immunohistochemical staining. Four micrometer cross-sections were taken on the positively charged slide from each paraffin block. The tissue sections were stained using a standard immunohistochemical technique with a Ventana BenchMark Ultra Automated İmmunostainer (BenchMark Ultra; Ventana Medical Systems, Tucson, AZ, USA) using heat-induced epitope retrieval and a standard diaminobenzidine detection kit (Ventana). Immunopositive cells with Ki-67 and caspase-3 in crypts were counted in 10 areas at × 1000 enlargement.
Statistical analysis
For statistical analyses, the SPSS 18.0 (SPSS Inc., Ca, IL, USA) software package program was used. The mean (± standard deviation) values of the data were calculated. The compatibility of the variables with a normal distribution pattern was investigated with Kolmogorov-Smirnov test. Since the data was not complying with the normality of distribution the Mann-Whitney U test was used in comparison. Categorical values were compared with a chi-square test. P values of < 0.05 were established as the threshold of statistical significance. All the values were presented as the median (range).
Results
Histopathological analysis
Intact epithelial layer, long crypts, normal cellularity with lamina propria and goblet cells containing sharp-edged villi were observed in the histopathological cross-sections of the control group (Figures 1A, 2A).
Figure 1.
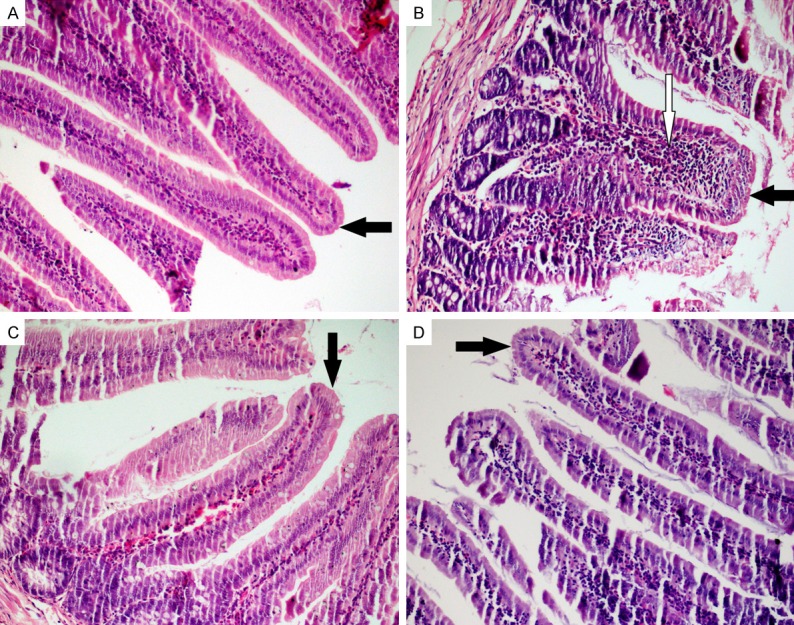
Staining with hematoxilin eosin (HE). A. Control; sharp-edged and long villi, normal cellularity with lamina propria; B. MTX; shortening and fusion of the villi, crypt loss and significant inflammatory cell infiltration in the lamina propria; C and D. PMG and CRV; improvement in the villus damage and less inflammation in the lamina propria (HE, × 200). Black arrows = Villus; White arrow = inflammatory cell.
Figure 2.
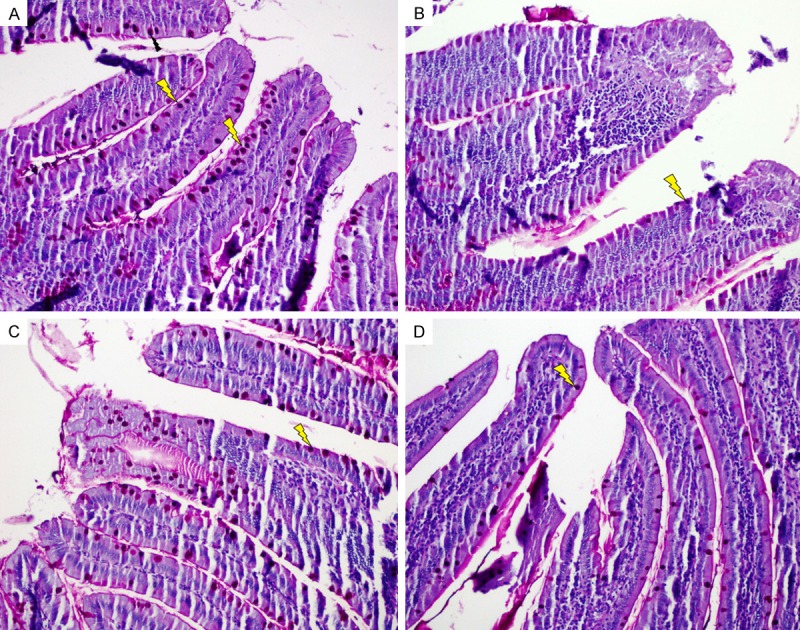
Staining with periodic acid schiff (PAS). A. Control; goblet cells in the control group; B. MTX; Significant goblet cells depletion; C and D. PMG and CRV; Increased goblet cells (PAS, × 200). Lightning bolt = goblet cell.
The microscopic evaluation of the intestinal tissues of the MTX-received group revealed severe shortening and fusion in villi, with a variable degree of epithelial atrophy (Figure 1B). The numbers of goblet cells were lower (P < 0.01) in the MTX-received group compared with those of the other groups, and crypt loss was also observed (Figure 2B). In the MTX-received group, significant inflammatory cell infiltration was observed in the lamina propria. The histopathological changes in the MTX-received group are detailed in Table 2.
Table 2.
Total histopathological scores of intestinal tissue
| Villus damage | Crypt damage | Cellular infiltration | Goblet cell depletion | |
|---|---|---|---|---|
| Control (I) | 0 | 0 | 0.25±0.46 | 0 |
| MTX (II) | 2.38±0.52 | 2.13±0.64 | 2.50±0.53 | 2.63±0.52 |
| CVR (III) | 1.5±0.53 | 1.38±0.52 | 1.5±0.53 | 1.75±0.71 |
| PMG (IV) | 2.0±0.53 | 1.63±0.74 | 2.13±0.64 | 2.13±0.64 |
| p value | ||||
| I-II | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| I-III | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| I-IV | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| II-III | 0.02 | 0.05 | 0.01 | 0.02 |
| II-IV | 0.03 | 0.02 | 0.03 | 0.01 |
| III-IV | 0.161 | 0.574 | 0.105 | 0.328 |
CVR; carvacrol, PMG; pomegranate, MTX; methotrexate.
Compared to the MTX-received group, the PMG and CVR groups showed less villus and crypt damage and less inflammation in the lamina propria (Figure 1C, 1D). The numbers of goblet cells were also higher in the PMG and CVR groups compared with those in the MTX-received group (Figure 2C, 2D).
Morphometric measurements revealed significantly shorter villi (P < 0.001) in the MTX-received group than control group. A decrease in the shortening of the villus length was observed in both CVR and PMG groups. The mean length of the villi in the CVR group was greater than that in the PMG group but not statistically significant (Table 1).
Table 1.
Caspase-3, Ki-67 and villus height values in intestinal tissue
| Villus height | Caspase-3 | Ki-67 | |
|---|---|---|---|
| Control (I) | 650.70±30.80 | 12.38±2.67 | 343.50±44.60 |
| MTX (II) | 339.44±50.51 | 36.13±7.04 | 246.50±43.34 |
| CVR (III) | 554.76±31.99 | 21.75±2.38 | 342.38±22.47 |
| PMG (IV) | 433.79±72.27 | 27.50±3.12 | 317.13±16.72 |
| p value | |||
| I-II | < 0.001 | < 0.001 | 0.001 |
| I-III | < 0.001 | < 0.001 | 0.959 |
| I-IV | < 0.001 | < 0.001 | 0.195 |
| II-III | < 0.001 | < 0.001 | < 0.001 |
| II-IV | 0.003 | 0.007 | < 0.001 |
| III-IV | 0.002 | 0.002 | 0.028 |
CVR; carvacrol, PMG; pomegranate, MTX; methotrexate.
Immunohistochemistry
Fewer Ki-67 positive cells were observed in the crypts of the MTX-received groups compared to the control group (Figure 3A, 3B). There were more Ki-67-positive cells in the CVR and PMG groups compared to MTX group (< 0.001, < 0.001, respectively) (Figure 3C, 3D).
Figure 3.
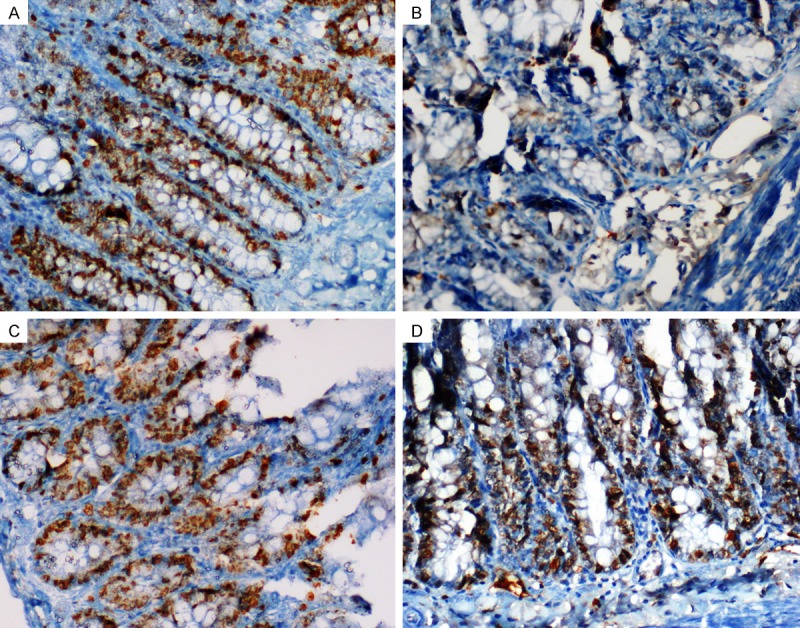
Staining with Ki-67. A. Control; A great number of Ki-67 positive cells in the crypts; B. MTX; Fewer Ki-67 positive cells; C and D. PMG and CRV; An increased number of Ki-67 positive cells (× 400).
The MTX-received group exhibited more caspase-3 positive cells than the control group, and the number of caspase-3 positive cells was decreased in the CVR and PMG treated groups (Table 1, Figure 4A-D).
Figure 4.
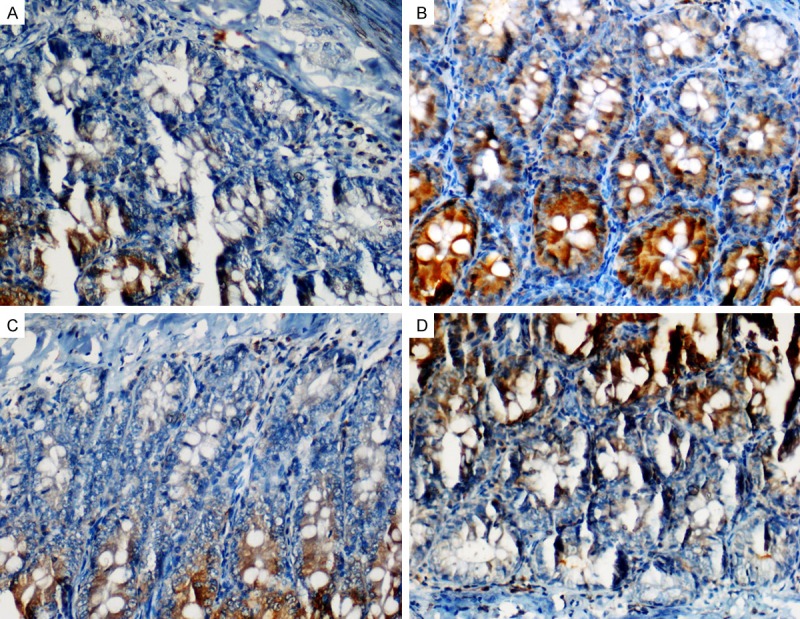
Staining with caspase 3. A. Control: Caspase 3 positive cells in the control group; B. MTX: More caspase-3 positive cells in the MTX group; C and D. PMG and CRV; Decreased caspase-3 positive cells in the CVR and PMG treated groups (× 400).
Discussion
Small intestine toxicity is one of the most common side effect of MTX treatment. The treatment duration and the dose of MTX, type of disease, genetic, molecular and apoptotic factors have a role in MTX-induced small intestine toxicity. In the present study, we demonstrated significant decrease in MTX-related toxicity, inflammation and apoptosis in the rats treated either with CVR or PMG. Our study is the first to demonstrate a protective effect of CVR and PMG on MTX-related toxicity in the small intestine.
Polyglutamation of folylpolyglutamate synthase is necessary for MTX to exert its cytotoxic effect. Polyglutamation takes place in both normal tissues and malignant cells in the body. The accumulation of these polyglutamates in cells inhibits enzymes involved in folate metabolism [21,22]. MTX exerts a cytotoxic effect against not only cancer cells but also other cells in the body, especially cells with rapid proliferation, such as those in the gastrointestinal system mucosa [11,23].
A research has shown that oxidative stress, especially neutrophil infiltration, plays a role in MTX-related small intestine damage [24]. By decreasing glutathione levels, MTX impairs the ability of the antioxidant defense system to protect against ROS, such as superoxide anions, hydroxyl radicals, hydrogen peroxide and hydrochloric radicals [21]. Intestinal mucositis a serious side effect of MTX treatment. The dose of MTX should be decreased in case of mucositis symptom and findings. In our study, the administration of CVR and PMG, which are anti-oxidant, anti-inflammatory and anti-carcinogenic agents, showed amelioration of the toxic effects in intestine tissue. Histopathologically, we observed that MTX caused villus shortening and fusion, epithelial atrophy, crypt loss, inflammatory infiltrate in the lamina propria, goblet cell depletion and loss of mucosal integrity in the GIS. We found significant recovery in these findings in CRV and PMG groups.
MTX mainly exerts its toxic effects in the intestinal tissue due to a redox imbalance, which results in apoptosis. In previous studies, apoptosis was increased significantly in crypt cells and developed in small intestines in MTX-treated rats. In addition, caspase-3 activity was increased significantly following MTX-related intestinal damage [12,25]. In the present study, we observed a significant increase in small intestinal cells stained with caspase-3, a significant indicator of apoptosis. We found fewer such cells in the groups treated with PMG and CVR. Earlier studies showed apoptosis in small intestine tissue with p53 staining and the TUNEL technique in rats given MTX, as well as death in intestinal crypt tissues with p53 staining [11]. In our study, the PMG and CVR treatment decreased the tissue damage observed in histopathological and immunohistochemical examinations. Previous studies demonstrated that PMG and CVR treatment resolved MTX-related damage and oxidative stress in the lung and kidney [26,27].
The most common immunohistochemical stain used for evaluation of cell proliferation is the Ki-67 antibody [28]. Leitão et al. showed that MTX treatment significantly decreased the numbers of Ki-67-positive cells [29]. Although the numbers of Ki-67-positive cells significantly decreased in the MTX-only group in the present study, this decrease was not observed in the PMG- and CVR-treated groups.
In conclusion, small intestine toxicity is a serious complication of MTX treatment. We demonstrated that PMG and CVR treatment ameliorated the MTX induced intestinal toxicity. As the previous studies reported the oxidant effect of MTX, we suggest that the anti-oxidant nature of PMG and CVR may have protective effect on apoptosis in intestinal tissue. Further studies are warranted to understand the protective effects of PMG and CVR in MTX induced intestinal toxicity.
Acknowledgements
This study was supported by Scientific Research Fund of Dicle University. The authors have no financial interest in any of the products mentioned in the manuscript. We are grateful to Dicle University DUBAP for their sponsorship about English editing of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Braun J, Rau R. An update on methotrexate. Curr Opin Rheumatol. 2009;21:216–223. doi: 10.1097/BOR.0b013e328329c79d. [DOI] [PubMed] [Google Scholar]
- 2.Schilsky RL. Methotrexate: An effective agent for treating cancer and building careers. The polyglutamate era. Stem Cells. 1996;14:29–32. doi: 10.1002/stem.140029. [DOI] [PubMed] [Google Scholar]
- 3.Genestier L, Paillot R, Quemeneur L, Izeradjene K, Revillard JP. Mechanisms of action of methotrexate. Immunopharmacology. 2000;47:247–257. doi: 10.1016/s0162-3109(00)00189-2. [DOI] [PubMed] [Google Scholar]
- 4.Cronstein BN. Molecular therapeutics. Methotrexate and its mechanism of action. Arthritis Rheum. 1996;39:1951–1960. doi: 10.1002/art.1780391203. [DOI] [PubMed] [Google Scholar]
- 5.Grosflam J, Weinblatt ME. Methotrexate: mechanism of action, pharmacokinetics, clinical indications, and toxicity. Curr Opin Rheumatol. 1991;3:363–368. [PubMed] [Google Scholar]
- 6.Nagakubo J, Tomimatsu T, Kitajima M, Takayama H, Aimi N, Horie T. Characteristics of transport of fluoresceinated methotrexate in rat small intestine. Life Sci. 2001;69:739–747. doi: 10.1016/s0024-3205(01)01162-6. [DOI] [PubMed] [Google Scholar]
- 7.Goodman TA, Polisson RP. Methotrexate: adverse reactions and major toxicities. Rheum Dis Clin North Am. 1994;20:513–528. [PubMed] [Google Scholar]
- 8.Gao F, Horie T. A synthetic analog of prostaglandin E1 prevents the production of reactive oxygen species in the intestinal mucosa of methotrexate treated rats. Life Sci. 2002;71:1091–1099. doi: 10.1016/s0024-3205(02)01795-2. [DOI] [PubMed] [Google Scholar]
- 9.Cody V, Luft JR, Pangborn W. Understanding the role of Leu22 variants in methotrexate resistance: comparison of wild-type and Leu22Arg variant mouse and human dihydrofolate reductase ternary crystal complexes with methotrexate and NADPH. Acta Crystallogr D Biol Crystallogr. 2005;61:147–155. doi: 10.1107/S0907444904030422. [DOI] [PubMed] [Google Scholar]
- 10.van’t Land B, Blijlevens NM, Marteijn J, Timal S, Donnelly JP, de Witte TJ, M’Rabet L. Role of curcumin and the inhibition of NF-kappaB in the onset of chemotherapy-induced mucosal barrier injury. Leukemia. 2004;18:276–284. doi: 10.1038/sj.leu.2403233. [DOI] [PubMed] [Google Scholar]
- 11.Dadhania VP, Tripathi DN, Vikram A, Ramarao P, Jena GB. Intervention of alpha-lipoic acid ameliorates methotrexate-induced oxidative stress and genotoxicity: A study in rat intestine. Chem Biol Interact. 2010;183:85–97. doi: 10.1016/j.cbi.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I. Dietary L-arginine supplementation reduces Methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol. 2012;12:41. doi: 10.1186/1471-230X-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gümüs M, Tekin R, Firat U, Onder A, Kapan M, Böyük A, Aldemir M, Kilinç C. The effects of pomegranate on bacterial translocation in rats with obstructive jaundice. Eur Rev Med Pharmacol Sci. 2013;17:1488–1494. [PubMed] [Google Scholar]
- 14.Prashanth D, Asha MK, Amit A. Antibacterial activity of Punica granatum. Fitoterapia. 2001;72:171–173. doi: 10.1016/s0367-326x(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 15.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Mizutani A, Kira S, Mori M, Iwasaka H, Noguchi T. Effect of Ulinastation, a human urinary trypsin inhibitor, on the oleic acid-induced acute lung injury in rats via the inhibition of activated leukocytes. Injury. 2005;36:387–394. doi: 10.1016/j.injury.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Bozkurt M, Bodakci MN, Turkcu G, Kuyumcu M, Akkurt M, Sula B, Em S, Oktayoglu P, Batmaz I, Yüksel H. Protective Effects of Carvacrol Against Methotrexate-induced Liver Toxicity in Rats. Acta Chir Belg. 2014;114:404–409. [PubMed] [Google Scholar]
- 18.Daggulli M, Dede O, Utangac MM, Bodakci MN, Hatipoglu NK, Penbegul N, Sancaktutar AA, Bozkurt Y, Türkçü G, Yüksel H. Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. Int J Clin Exp Med. 2014;7:5511–5516. [PMC free article] [PubMed] [Google Scholar]
- 19.Canbek M, Uyanoglu M, Bayramoglu G, Senturk H, Erkasap N, Koken T, Uslu S, Demirustu C, Aral E, Husnu Can Baser K. Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomedicine. 2008;15:447–452. doi: 10.1016/j.phymed.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Acipayam C, Bayram I, Daglioglu K, Doran F, Yilmaz S, Sezgin G, Totan Ateş B, Ozkan A, Tanyeli A. The protective effect of hesperidin on methotrexate-induced intestinal epithelial damage in rats: an experimental study. Med Princ Pract. 2014;23:45–52. doi: 10.1159/000355900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. Nacetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274–8. [PubMed] [Google Scholar]
- 22.Chabner BA, Allegra A, Curt C. Polyglutamation of methot-rexate. Is methotrexate a prodrug? J Clin Invest. 1985;76:907–912. doi: 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Tian L, Zhang M, Sun Q, Zhang X, Li X, Cao X, Liu Q, Li X, Hao L. Protective effect of amifostine on high-dose methotrexate-induced small intestinal mucositis in mice. Dig Dis Sci. 2013;58:3134–3143. doi: 10.1007/s10620-013-2826-3. [DOI] [PubMed] [Google Scholar]
- 24.Miyazono Y, Gao F, Horie T. Oxidative stress contributes to methotrexate induced small intestinal toxicity in rats. Scand J Gastroenterol. 2004;39:1119–1127. doi: 10.1080/00365520410003605. [DOI] [PubMed] [Google Scholar]
- 25.Ciralik H, Bulbuloglu E, Cetinkaya A, Kurutas EB, Celik M, Polat A. Effects of N-acetylcysteine on methotrexate-induced small intestinal damage in rats. Mt Sinai J Med. 2006;73:1086–1092. [PubMed] [Google Scholar]
- 26.Selimoğlu Şen H, Şen V, Bozkurt M, Türkçü G, Güzel A, Sezgi C, Abakay Ö, Kaplan I. Carvacrol and pomegranate extract in treating methotrexate-induced lung oxidative injury in rats. Med Sci Monit. 2014;20:1983–1990. doi: 10.12659/MSM.890972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt M, Em S, Oktayoglu P, Turkcu G, Yuksel H, Sarıyıldız MA, Caglayan M, Batmaz I, Nas K, Bozkurt Y, Kuyumcu M. Carvacrol prevents methotrexate-induced renal oxidative injury and renal damage in rats. Clin Invest Med. 2014;37:E19–25. doi: 10.25011/cim.v37i1.20865. [DOI] [PubMed] [Google Scholar]
- 28.Lebe B, Pabuççuoğlu U, Ozer E. The significance of Ki-67 proliferative index and cyclin D1 expression of dysplastic nevi in the biologic spectrum of melanocytic lesions. Appl Immunohistochem Mol Morphol. 2007;15:160–164. doi: 10.1097/01.pai.0000209868.58699.64. [DOI] [PubMed] [Google Scholar]
- 29.Leitão RF, Brito GA, Oriá RB, Braga-Neto MB, Bellaguarda EA, Silva JV, Gomes AS, Lima-Júnior RC, Siqueira FJ, Freire RS, Vale ML, Ribeiro RA. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 2011;11:90. doi: 10.1186/1471-230X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]


