Abstract
Epithelial-mesenchymal transition (EMT) is a crucial step in tumor progression and has an important role during cancer invasion and metastasis. The proteins of the inhibitor of growth (ING) candidate tumor suppressor family are involved in multiple cellular functions such as cell cycle regulation and apoptosis. ING5 is a member of the family. However, the role of ING5 in breast cancer is still unclear. Thus, the aim of this study is to explore the role of ING5 in breast cancer. In the present study, we showed that ING5 is involved in the pathogenesis of breast cancer. ING5 is down-regulated in breast cancer tissues and cell lines. Overexpression of ING5 significantly inhibited breast cancer cell migration, invasion, and EMT phenotype, moreover, overexpression of ING5 significantly the phosphorylation of PI3K and Aktin in breast cancer cells. In conclusion, our findings show that ING5 can efficiently inhibit the EMT progression in breast cancer cells by suppressing PI3K/Akt signaling pathway. Therefore, ING5 may be a good molecular target for the prevention and treatment of breast cancer.
Keywords: Inhibitor of growth 5 (ING5), breast cancer, epithelial-mesenchymal transition (EMT)
Introduction
Breast cancer is the leading malignancy in women worldwide and the incidence rates have been increasing annually [1]. In the past several decades, the treatment of breast cancer mainly includes the surgical resection of the tumors and high toxicity chemotherapy. Unfortunately, the clinical outcome of patients remains unsatisfactory [2]. This is largely because of a lack of understanding regarding the molecular mechanisms behind breast cancer. Therefore, dissecting the molecular mechanisms that regulate breast cancer progression may facilitate the advancement of clinical treatment.
The inhibitor of growth (ING) gene family includes ING1, ING2, ING3, ING4 and ING5. All ING proteins share a highly conserved carboxy-terminal plant homeodomain (PHD) and are involved in the control of cell growth, senescence, apoptosis, DNA repair and chromatin remodeling [3-5]. One of the ING family genes, ING5, has been demonstrated to play important roles in the progression of tumor. Several studies have reported that ING5 expression is decreased in many tumors, including oral squamous cell carcinoma, head and neck squamous cell carcinoma and gastric carcinoma [6-8]. In addition, it has been reported that ING5 affects adhesion, migration, invasion, epithelial-mesenchymal transition (EMT) and apoptosis of tumor cells [8-10]. However, the role of ING5 in breast cancer is still unclear. Thus, the aim of this study is to explore the role of ING5 in breast cancer. In this study, we have analyzed ING5 expression and function in human breast cancer, identifying apotential tumor suppressor role in breast, mainly involved in inhibition of EMT and cell invasion.
Materials and methods
Tissue specimens
Breast cancer tissues were obtained from patients undergoing surgical treatment at the Department of Oncology, The Second Affiliated Hospital of Medical College Qing Dao University, during the period from 2012 to 2014. The histological types and grades of the primary tumors were determined according to the diagnostic criteria of the WHO Classification of Tumors of the Breast. Healthy tissue samples were from non-pathologic areas distant from tumors in surgical specimens. All fresh samples were immediately frozen after resection and stored at -80°C until use. Informed consent for the scientific use of biological material was obtained from all patients and the work has been approved by the local Ethical Committee.
Cell culture
Human breast cancer cell lines (MDA-MB-231 and MCF-7) and human mammary epithelial cells (HMEC) were purchased from The American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), 2 mmol/l L-glutamine, 100 units/mL penicillin and 100 µg/ml streptomycin (Sigma, St. Louis, MO, USA). All cells were incubated in a 5% CO2 humidified atmosphere at 37°C.
Constructs and establishment of stable cell lines
To generate stable ING5 overexpression cells, breast cancer cells were infected with GV218-EGFP-ING5 lentivirus construct (Genechem). Single-cell clones were isolated by 5 μg/ml puromycin for 48 h followed by 1 μg/ml puromycin treatment. Empty vector-infected cells were used as control.
RNA extraction and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from breast cancer tissues and cells using Trizol reagent (Abcam, Cambridge, UK). The cDNA was synthesized by reverse transcription of total RNA, using the Prime ScriptH RT reagent kit (Takara, Dalian, China) with oligo-dT primers, based on the manufacturer’s instructions. Real-time quantitative PCR reactions were performed on the Bio-Rad iQ5 real-time thermal cyclers using SYBRH Premix Ex TaqTM II kit (Takara, Dalian, China). PCR amplification was performed using the following primers: ING5, 5’-TCCAGAACGCCTACAGCAAG-3’ (sense) and 5’-TGCCCTCCATCTTGTCCTTC-3’ (antisense); and β-actin, 5’-TTAGTTGCGTTACACCCTTTC-3’ (sense) and 5’-ACCTTCACCGTTCCAGTTT-3’ (antisense). The PCR conditions included an initial denaturation step of 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 2 min, and a final elongation step of 72°C for 10 min. For relative quantification, the levels of individual gene mRNA transcripts were first normalized to the control β-actin. Subsequently, the differential expression of these genes was analyzed by the 2-ΔΔCT method and expressed as fold changes.
Western blot
For protein extraction, cells were lysed in lysis buffer containing 1% NP40, 1 mM EDTA, 50 mM Tris-HCl (pH 7.5) and 150 mM NaCl, supplemented with complete protease inhibitors mixture (Roche, Monza, Italy). The equal amount of protein samples was separated by 10% SDS-PAGE and electro transferred onto polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). Immunoblots were blocked with 5% skim milk in TBS/Tween 20 (0.05%, v/v) for 1 hour at room temperature. The membrane was incubated with primary antibody overnight at 4°C. Immunodetection of target proteins [ING5, E-cadherin, N-cadherin, phospho-PI3K, PI3K, phospho-Akt, Aktand β-actin] was performed using mouse monoclonal antibody (1:1,000; Santa Cruz Biotechnology) and anti-β-actin antibody (Sigma, St. Louis, MO, USA), respectively. Blots were then incubated with horseradish peroxidase-conjugated IgG. Protein bands were evaluated by enhanced chemiluminescence (Thermo Fisher Scientific, RockFord, IL, USA).
Transwell migration and invasion assay
For the migration assay, 5 × 104 cells transfected with overexpression-ING5 were suspendedin serum-free medium and plated on chambers (CorningCostar, NY, USA) that were not coated with Matrigel. For the invasion assay, 5 × 104 cells transfected with overexpression-ING5 were seeded into the upper chamber that was precoated with Matrigel (BD Bioscience, CA, USA). For both assays, medium containing 10% FBS was added to the lower chamber as a chemoattractant. After incubating for 24 h at 37°C in the incubator supplemented with 5% CO2, noninvasive cells in the upper chamber were removed by wiping with a cotton swab, and cells adhering to the lower membrane were fixed with 4% paraformaldehyde, stained with crystal violet solution, and then counted in 5 random fields perwell under a light microscope (100 × magnification).
Statistical analysis
All results are reported as means ± SD. Statistical analysis involved using the Student’s t test for comparison of 2 groups or 1-way ANOVA for multiple comparisons. P < 0.05 was considered to be significant.
Results
ING5 is down-regulated in breast cancer tissues and cells
To explore the potential role of ING5 in the tumorigenesis of breast cancer, we detected the ING5 mRNA levels in 12 paired primary breast cancer tissues and the corresponding adjacent normal tissues using RT-qPCR. As shown in Figure 1A, the ING5 mRNA levels in primary breast cancer tissues were obviously lower than those in the adjacent normal breast tissues. Consistent with the expression of ING5 in breast cancer tissues, ING5 mRNA and protein expression were also decreased in MDA-MB-231 and MCF-7 cells (Figure 1B and 1C). These results suggest that ING5 is down-regulated in breast cancer.
Figure 1.
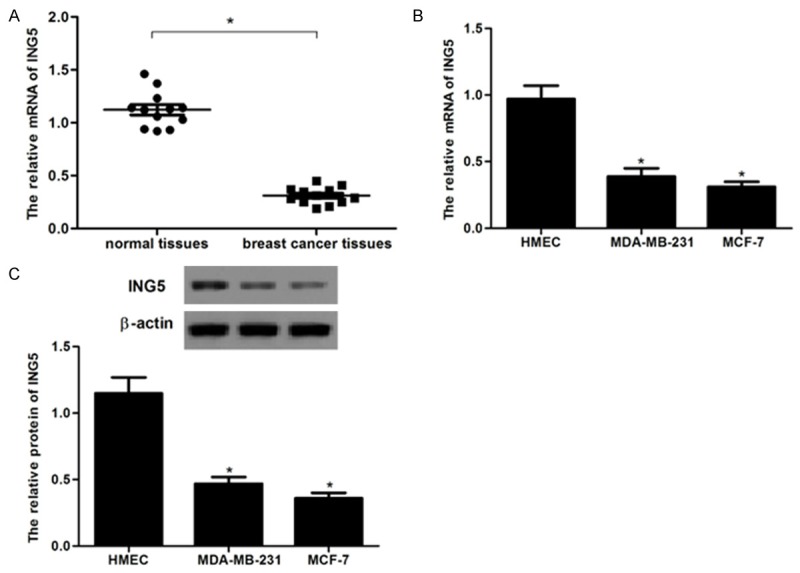
Expression of ING5 in human breast cancer tissue samples and cell lines. A: mRNA expression of ING5 was analyzed by RT-PCR. SOX1 mRNA levels in breast cancer were obviously lower than that in normal breast tissues, *P < 0.05 compared to normal breast tissues; B: Representative mRNA expression of ING5 in breast cancer cell lines; C: Representative Western image of ING5 protein in breast cancer cell lines. All experiments were repeated at least three times. Data are presented as mean ± SD. *P < 0.05 compared to the HMEC group.
ING5 inhibits breast cancer cell migration andinvasion
One characteristic oftumor metastasis is the increased ability of tumor cellsmigration [11]. Thus, we investigated the effect of ING5 on cell migration by transwell migration assay in breast cancer cells. As shown in Figure 2, the expression levels of ING5mRNA and protein were obviously increased in MDA-MB-231 and MCF-7 cells, respectively (Figure 2A and 2B). In addition, ING5 overexpression significantly inhibited migration of MDA-MB-231 cells (Figure 2C). ING5 overexpression could also significantly suppress MDA-MB-231 cells from invading through Matrigel-coated polycarbonate filter in the transwell chamber (Figure 2D). Similar results were observed in MCF-7 cells (Figure 2E and 2F).
Figure 2.
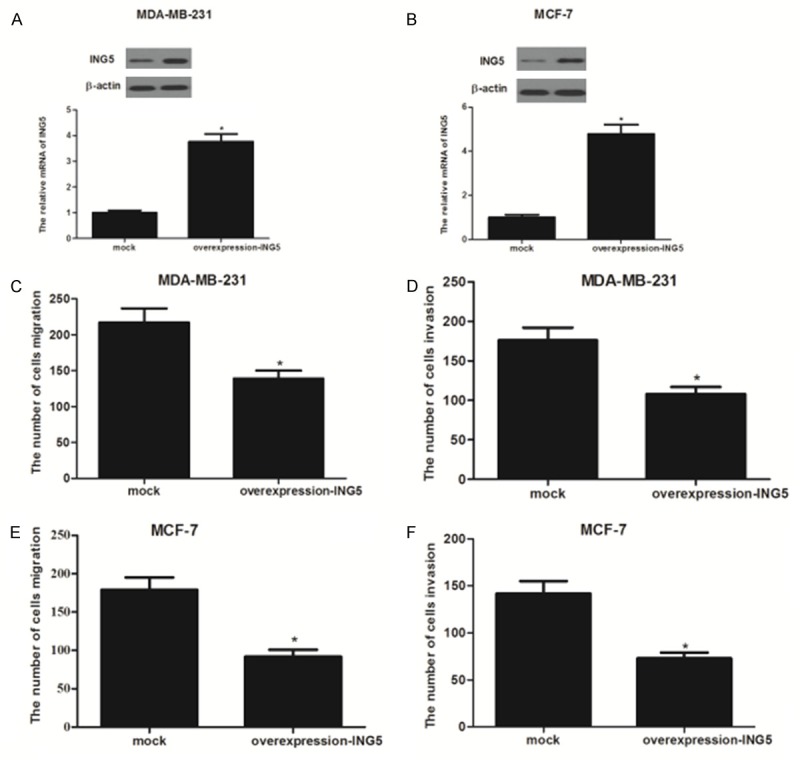
ING5 inhibited the migration and invasion of breast cancer cells. A: The Mrna and protein expression of ING5 in overexpression-ING5-transfected MDA-MB-231 cells; B: The mRNA and protein expression of ING5 in overexpression-ING5-transfected MCF-7 cells; C and D: ING5 inhibited the migration and invasion of MDA-MB-231 cells; E and F: ING5 inhibited the migration and invasion of MCF-7 cells. All experiments were repeated at least three times. Data are presented as mean ± SD. *P < 0.05 compared to the mock group.
ING5 inhibits the EMT process in breast cancer cells
EMT plays an important role in promoting tumor invasion and metastasis [12]. In order to investigate whether ING5 decreased breast cancer cell invasion by inhibiting EMT, we analyzed mRNA level of several EMT markers in MDA-MB-231 and MCF-7 cells transfected with ING5-overexpressing. As shown in Figure 3A, RT-qPCR results showed that ING5 obviously increased mRNA level of E-cadherin, an epithelial marker, and decreased mRNA levels of N-cadherin, a mesenchymal marker, in MDA-MB-231 cells. Similar results were observed in MCF-7 cells (Figure 3B). Moreover, western blot analysis demonstrated that ING5 obviously increased protein level of E-cadherin, an epithelial marker, and decreased protein levels of N-cadherin, a mesenchymal marker, in MDA-MB-231 and MCF-7 cells, respectively (Figure 3C and 3D).
Figure 3.
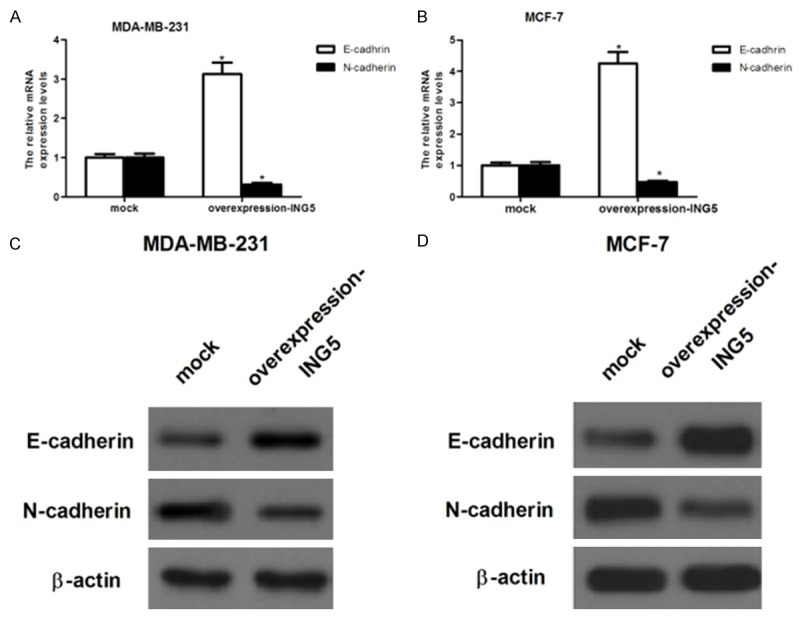
ING5 inhibits the EMT process in breast cancer cells. A and B: Representative images of relative mRNA level of E-cadherin and N-cadherinin overexpression-ING5-transfected MDA-MB-231 and MCF-7 cells, respectively. C and D: Representative images of relative protein level of E-cadherin and N-cadherinin overexpression-ING5-transfected MDA-MB-231 and MCF-7 cells, respectively. All experiments were repeated at least three times. Data are presented as mean ± SD. *P < 0.05 compared to the mock group.
ING5 inhibits PI3K/Akt signal pathway involved in the block of EMT, migration and invasiveness
PI3K/Akt signaling pathway plays an important role in cancer cell growth and invasion [13-15]. To explore the molecular mechanisms by which ING5 contributes to these malignant features, we investigated the effect of ING5 on phosphorylation levels of PI3K and Akt in breast cancer cells. As shown in Figure 4, Western blot showed that ING5 significantly inhibited the phosphorylation of PI3K and Aktin MDA-MB-231 cells.
Figure 4.
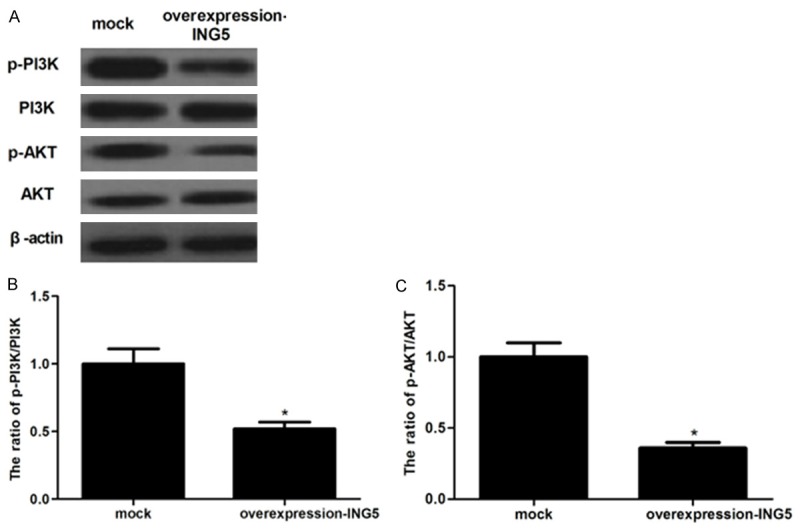
ING5 inhibits PI3K/Akt signaling pathway in breast cancer cells. A: The levels of phosphorylated PI3K, total PI3K, phosphorylated Akt and total Akt in MAD-MB-231 cells transfected with overexpression-ING5 or mock were determined by western blot. B and C: The relative protein expression levels of p-PI3K and p-Akt were quantified using Image-Pro Plus 6.0 software and normalized to β-actin. All experiments were repeated at least three times. Data are presented as mean ± SD. *P < 0.05 compared to the mock group.
Discussion
ING5 belongs to the ING candidate tumor suppressor family. In the present study, we showed that ING5 is involved in the pathogenesis of breast cancer. ING5 is down-regulated in breast cancer tissues and cells. Overexpression of ING5 significantly inhibited breast cancer cell migration, invasion, and EMT phenotype. Moreover, overexpression of ING5 significantly the phosphorylation of PI3K and Aktin in breast cancer cells.
Aberrant expression of ING5 has been found in several tumors. In this study, we observed thatING5 is down-regulated in breast cancer tissues and cells. Our observations are consistent with numerous reports of reduced ING5 expression in many cancers, including lung cancer [16] and hepatocellular carcinoma [17]. Together, these studies indicate that ING5 plays a central role in the pathogenesis of these cancers.
Very recently, several studies demonstrated that other ING family proteins are involved in breast cancer growth, migration and invasion. For example, Xu et al. reported that adenovirus-mediated ING4 (Ad-ING4) treatment could induce in vitro significant growth suppression in both mutant p53 MDA-MB-231 and wild-type p53 MCF-7 breast carcinoma cells despite p53 status, and intratumoral injections of Ad-ING4 in nude mice bearing mutant p53 MDA-MB-231 breast tumors remarkably inhibited the human breast xenografted tumor growth [18]. Thakur et al. reported that ING1 overexpression also blocked cancer cell metastasis in vivo and eliminated tumor-induced mortality in mouse models [19]. Consistent with previous findings, in this study, we observed that ING5 inhibited breast cancer cell migration and invasion. These data suggest that ING5 plays an important role in inhibiting breast cancer cell migration and invasion.
EMT is considered as one of critical steps in breast cancer metastasis and invasion [20-22]. Generally, increased motility and invasion are positively correlated with EMT, which is characterized by repression of epithelial markers and induction of mesenchymal markers, and reduction or a loss of E-cadherin expression is one of the well-established hallmarks of EMT [23]. In this study, we observed that ING5 obviously increased the level of E-cadherin mRNA and protein, and decreased the level of N-cadherin mRNA and proteinin breast cancer cells. Overall, our results suggest that ING5 negatively regulates EMT, consequently markedly affects cell migration and invasion in vitro.
The molecular mechanism of ING5 involvement in the loss of the mesenchymal-like phenotype in breast cancer cells awaits further investigation. Several signaling pathways may contribute to EMT progression [24-27]. The PI3K/Akt pathway is known to play a critical role in human cancer initiation and progression, and is also associated with the induction of EMT [28]. It has been reported that expression of activated Akt in epithelial cells results in loss of cell-cell adhesion, loss of apical-basolateral cell polarization, induction of cell motility, and changes in the expression or the distribution of various epithelial and/or mesenchymal markers [29]. In this study, we found that ING5 significantly inhibited the phosphorylation of PI3K and Aktin breast cancer cells. These results support the notion that ING5 inhibits cell invasion and EMT through suppressing PI3K/Akt signaling pathway.
In conclusion, our findings show that ING5 can efficiently inhibit the EMT progression in breast cancer cells by suppressing PI3K/Akt signaling pathway. Therefore, ING5 may be a good molecular target for the prevention and treatment of breast cancer.
Disclosure of conflict of interest
None.
References
- 1.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maughan KL, Lutterbie MA, Ham PS. Treatment of breast cancer. Am Fam Physician. 2010;81:1339–1346. [PubMed] [Google Scholar]
- 3.Soliman MA, Riabowol K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 2007;32:509–519. doi: 10.1016/j.tibs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Campos E, Chin M, Kuo W, Li G. Biological functions of the ING family tumor suppressors. Cell Mol Life Sci. 2004;61:2597–2613. doi: 10.1007/s00018-004-4199-4. [DOI] [PubMed] [Google Scholar]
- 5.Russell M, Berardi P, Gong W, Riabowol K. Grow-ING, Age-ING and Die-ING: ING proteins link cancer, senescence and apoptosis. Exp Cell Res. 2006;312:951–961. doi: 10.1016/j.yexcr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Cengiz B, Gunduz E, Gunduz M, Beder LB, Tamamura R, Bagci C, Yamanaka N, Shimizu K, Nagatsuka H. Tumor-specific mutation and downregulation of ING5 detected in oral squamous cell carcinoma. Int J Cancer. 2010;127:2088–2094. doi: 10.1002/ijc.25224. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Nishida T, Noguchi A, Zheng Y, Takahashi H, Yang X, Masuda S, Takano Y. Decreased nuclear expression and increased cytoplasmic expression of ING5 may be linked to tumorigenesis and progression in human head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2010;136:1573–1583. doi: 10.1007/s00432-010-0815-x. [DOI] [PubMed] [Google Scholar]
- 8.Xing YN, Yang X, Xu XY, Zheng Y, Xu HM, Takano Y, Zheng HC. The altered expression of ING5 protein is involved in gastric carcinogenesis and subsequent progression. Human Pathol. 2011;42:25–35. doi: 10.1016/j.humpath.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Zheng HC, Xia P, Xu XY, Takahashi H, Takano Y. The nuclear to cytoplasmic shift of ING5 protein during colorectal carcinogenesis with their distinct links to pathologic behaviors of carcinomas. Human Pathol. 2011;42:424–433. doi: 10.1016/j.humpath.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Unoki M, Kumamoto K, Takenoshita S, Harris CC. Reviewing the current classification of inhibitor of growth family proteins. Cancer Sci. 2009;100:1173–1179. doi: 10.1111/j.1349-7006.2009.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1368. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 15.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Zhang X, Meng J, Zhao Y, Liu X, Liu Y, Wang Y, Li Y, Sun Y, Mei Q, Zhang T, Wang Z. ING5 inhibits cancer aggressiveness via preventing EMT and is a potential prognostic biomarker for lung cancer. Oncotarget. 2015;6:16239–52. doi: 10.18632/oncotarget.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gou WF, Shen DF, Yang XF, Zhao S, Liu YP, Sun HZ, Su RJ, Luo JS, Zheng HC. ING5 suppresses proliferation, apoptosis, migration and invasion, and induces autophagy and differentiation of gastric cancer cells: a good marker for carcinogenesis and subsequent progression. Oncotarget. 2015;6:19552–79. doi: 10.18632/oncotarget.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Xie Y, Sheng W, Miao J, Xiang J, Yang J. Tumor-suppressive effect of adenovirus-mediated inhibitor of growth 4 gene transfer in breast carcinoma cells in vitro and in vivo. Cancer Biother Radiopharm. 2010;25:427–437. doi: 10.1089/cbr.2010.0778. [DOI] [PubMed] [Google Scholar]
- 19.Thakur S, Singla AK, Chen J, Tran U, Yang Y, Salazar C, Magliocco A, Klimowicz A, Jirik FR, Riabowol K. Reduced ING1 levels in breast cancer promotes metastasis. Oncotarget. 2014;5:4244. doi: 10.18632/oncotarget.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittekind C, Neid M. Cancer invasion and metastasis. Oncology. 2005;69:14–16. doi: 10.1159/000086626. [DOI] [PubMed] [Google Scholar]
- 21.Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 22.Blick T, Widodo E, Hugo H, Waltham M, Lenburg M, Neve R, Thompson E. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 25.Vincan E, Barker N. The upstream components of the Wnt signalling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin Exp Metastasis. 2008;25:657–663. doi: 10.1007/s10585-008-9156-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11:745. doi: 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 28.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial–mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, Van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]


