Abstract
Vascular endothelial cell growth factor (VEGF) combined with bone morphogenetic protein (BMP) was used to repair avascular necrosis of the femoral head, which can maintain the osteogenic phenotype of seed cells, and effectively secrete VEGF and BMP-2, and effectively promote blood vessel regeneration and contribute to formation and revascularization of tissue engineered bone tissues. To observe the therapeutic effect on the treatment of avascular necrosis of the femoral head by using bone marrow mesenchymal stem cells (BMSCs) modified by VEGF-165 and BMP-2 in vitro. The models were avascular necrosis of femoral head of rabbits on right leg. There groups were single core decompression group, core decompression + BMSCs group, core decompression + VEGF-165/BMP-2 transfect BMSCs group. Necrotic bone was cleared out under arthroscope. Arthroscopic observation demonstrated that necrotic bone was cleared out in each group, and fresh blood flowed out. Histomorphology determination showed that blood vessel number and new bone area in the repair region were significantly greater at various time points following transplantation in the core decompression + VEGF-165/BMP-2 transfect BMSCs group compared with single core decompression group and core decompression + BMSCs group (P < 0.05). These suggested that VEGF-165/BMP-2 gene transfection strengthened osteogenic effects of BMSCs, elevated number and quality of new bones and accelerated the repair of osteonecrosis of the femoral head.
Keywords: Osteonecrosis of the femoral head, stem cells, transfection, vascular endothelial growth factor, bone morphogenetic protein-2
Introduction
The femoral head necrosis is also known as the avascular necrosis (AN) of femoral head, caused by different disease causes that damaged the blood supply of femoral head, thus resulting in the necrosis of femoral head bone and cartilage cells, as well as the collapse of articular surface, it’s one of the common clinical diseases. In its early stages, the early detection and treatment could relieve the patients’ symptoms, delay or avoid the occurrence of femoral head collapse, thus postponing or avoiding the replacement of artificial total hip joint [1,2].
Although the efficacy of marrow core decompression therapy in the treatment of early femoral head necrosis was still controversial, most scholars hold a positive attitude. The recent studies had found that the numbers of hematopoietic stem cells and mesenchymal stem cells of bone marrow in the AN patients were decreased, as well as their activities were reduced. It was also believed that after the marrow core decompression, the incomplete bone repair was the reason of femoral head collapse, and related to the number reduction of bone marrow mesenchymal stem cells [3-5].
The bone marrow mesenchymal stem cells were also called the bone marrow stem cells (BMSCs) and bone marrow multipotential stem cells, as a class of fiber colony-forming cells that obtained the attention from 1970s, BMSCs exhibited the potentials of multi-directionally differentiating into the bones, cartilages and epithelium, and they were easy for the sampling and isolation, with weak immunogenicity, so they had been favored in the studies of femoral head necrosis. Though most clinical studies reported that the simple application of BMSCs in the treatment of femoral head necrosis was not satisfactory, the treatment time was long, while the expected results were not reachable.
Many cytokines had been found to be able to promote the BMSCs to differentiate into the osteocytes, chondrocytes and other directions [6-8]. The related cytokines included: bone morphogenie protein (BMP), basic fibroblast growth factor (bFGF), osteogenic growth peptide and vascular endothelial growth factor (VEGF), etc.
This study aimed to investigate the effects of vascular endothelial growth factor 165/bone morphogenetic protein-2 (VEGF-165/BMP-2) in the in vitro modification of bone marrow mesenchymal stem cells, which were then transplanted for the treatment of rabbit avascular necrosis.
Methods
Establishment of animal model
36 healthy New Zealand white rabbits, 6 months old, purchased from Dalian University experimental animal center, body mass between 2.0-2.5 kg. With lottery method, the rabbits were randomly divided into 3 groups: single core decompression group, core decompression + BMSCs group, core decompression + VEGF-165/BMP-2 transfect BMSCs group, (the ratio of BMP-2 and VEGF-165 was 3:1) [9], 12 rats in each group. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Dalian University (Permit Number: 20060828001).
Using liquid nitrogen freezing method, all animals were induced into the right avascular necrosis of the femoral head, after radiological detection as the successful models, which can be used for the next step experiment [10].
Acquisition, culture, transfection and identification of BMSCs
At the time of modeling the core decompression group + BMSCs, core decompression +BMP-2/VEGF-165 transfect BMSCs group rabbits were performed obtain and culture of the BMSCs. Two groups of rabbits were underwent bone marrow puncture and extraction of bone marrow in the bilateral lateral condyle of femur and proximal tibia, 4 mL on each side. The mononuclear cell layer was isolated with Ficoll-Hypaque solution, adding in 10 mL of culture medium for culture, main components of medium including 1640 (GIBCO, Grand Island, NY, USA), 10% volume fraction of fetal bovine serum (Biochrom AG, Berlin, Germany), penicillin and streptomycin (Amresco, Solon, Ohio, USA) each 100 U/mL, pH value was 7.2, changing the medium 2 times a week. The adherent cells were identified by microscopy.
When the cells grew to 80% confluence, the cells were subcultured. When the second generation cells grew to 80% confluence, the cells were extracted, and the cell concentration was adjusted to achieve 1 × 1012 L-1. The core decompression + BMSCs group animals were performed marrow core decompression and arthroscopic BMSCs replantation. For the core decompression and BMP-2/VEGF-165 (Vector Gene Technology Company LTD, Beijing, China) transfect BMSCs group, when the BMSCs cultured to the first generation, the cell concentration was adjusted to achieve 1 × 109 L-1, after the cells reached 90% cell confluence, which were performed virus transfection for overnight, the virus being rAAV-2-hVEGF-165 and rAAV-2-BMP-2 (Vector Gene Technology Company LTD, Beijing, China). At the infection rate (MOI) of 100, 1 week after transfection, which were performed core decompression and arthroscopic BMSCs replantation. The single core decompression group was only added equal volume of 1640 medium. VEGF-165 and BMP-2 protein expression was detected by Western blot assay.
Operation method
3 groups were selected 3 mm core decompression channel and 2.7 mm arthroscopy for removal of the necrotic bone, to observe the changes of bone trabecular, and to clear out the dead bone, and 1.0-1.5 mL BMSCs was transplanted into the necrotic area of the femoral head, closing the incision layer by layer. Rabbits of each group were sacrificed at 4, 8 weeks after transplantation.
Histology examination
When the cells grew to 80% confluence, the cells were subcultured. When the second generation cells grew to 80% confluence, the cells were extracted, and the cell concentration was adjusted to achieve 1 × 1012 L-1. The core decompression + BMSCs group animals were performed marrow core decompression and arthroscopic BMSCs replantation. For the core decompression + BMP-2/VEGF-165 transfect BMSCs group, when the BMSCs cultured to the first generation, the cell concentration was adjusted to achieve 1 × 109 L-1. After the cells reached 90% cell confluence, which were performed virus transfection for overnight, the virus being rAAV-2-hVEGF-165 and rAAV-2-BMP-2, at the infection rate (MOI) of 100, 1 week after transfection, which were performed core decompression and arthroscopic BMSCs replantation. The single core decompression group was only added equal volume of 1640 medium. VEGF-165 and BMP-2 protein expression was detected by Western blot assay.
The main observation index
The blood area ratio and new bone ratio in the restoration area.
Statistical analysis
All the data were performed analysis of variation, P < 0.05 as statistic significant difference.
Results
The number of experimental animal
36 rabbits were involved in the result analysis.
Isolation, culture and identification of the BMSCs
It was seen adherent growth in 24 h under microscopic observation, and adherent cells were fusiform or irregular long polygon. BMSCs subcultured after growth to 80% confluence (Figure 1).
Figure 1.
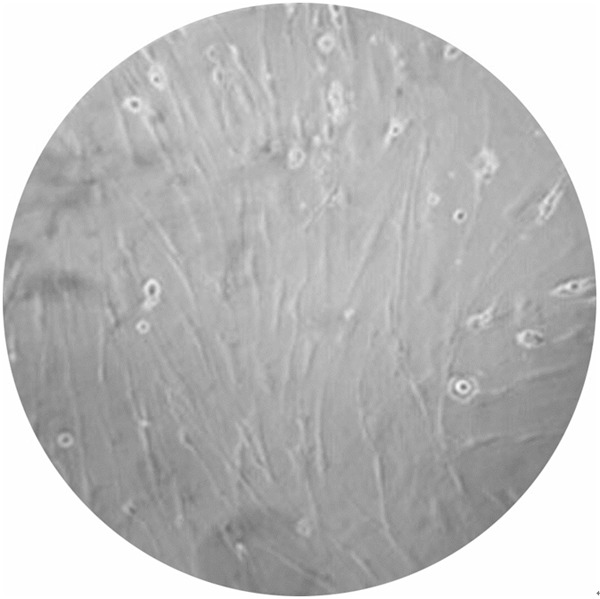
The second generation of bone marrow mesenchymal stem cells on 10 d after transfection, cells showing pie-shaped, polygonal growth, and no contact inhibition (× 40).
West blot assay showed that the BMP-2 protein expression was strong positive in core decompression + VEGF-165/BMP-2 transfect BMSCs group, and only weak positive in single core decompression group and core decompression + BMSCs group, (Figure 2A), indicating that there was BMP-2 antigen expression, 48 h after recombinant adenovirus transfection of BMSCs. VEGF-165 protein was strong positive in core decompression + VEGF-165/BMP-2 transfect BMSCs group, and only weak positive or negative in single core decompression group and core decompression + BMSCs group (Figure 2B), indicating that there was VEGF-165 antigen expression, 48 h after recombinant adenovirus transfect of BMSCs, and there was large amount of VEGF-165 antigen expression after 10 days (Figure 2A, 2B).
Figure 2.
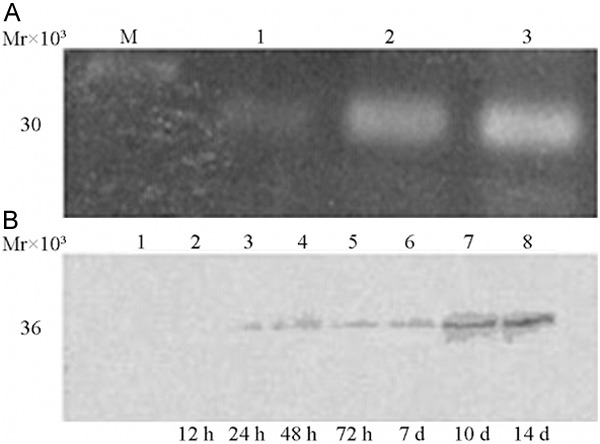
Western blot assay results of bone marrow mesenchymal stem cells following bone morphogenetic protein-2 (BMP-2) (A) and Vascular endothelial cell growth factor-165 transfection (B). (A) 1: Control group; 2, 3: rAAV2-BMP2; Mr30 000 size: BMP-2. (B) 1. Control group; 2-8: VEGF-165 transfection group; 36 KD: VEGF165; control group and 12 h transfection group: negative; 24 h-7 d group: weakly positive; 10 d, 14 d group: positive.
Arthroscopic observation
It was shown under the arthroscopy that most microvessels had embolism or were difficult to find out; bone trabecular irregularly arranged in yellow and black color; parts of bone trabular ruptured or were difficult to distinguish. After removal of the necrotic bone, it was visible of the surrounding normal bone trabecular, showing regular arranged in reddish and white, and among which the microvessels in the surface of the wound had errhysis and no block, as shown in Figure 3A-C.
Figure 3.
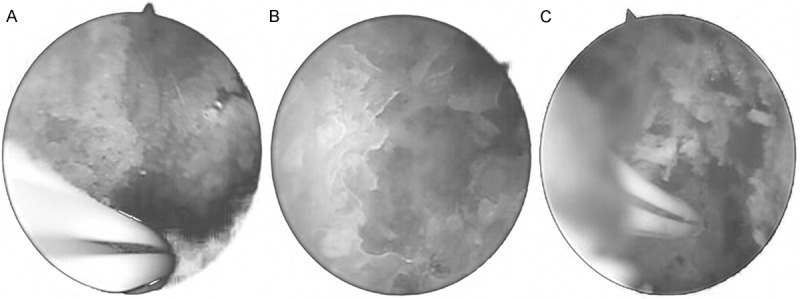
A. Arthroscopic dark color of femoral head, blood supply was reduced, trabecular bone was arranged in irregular yellow and black color, showing obvious sequestrum formation. B. Arthroscopic bone necrosis of the femoral head was removed; removal of necrotic bone and bone trabecular can be seen around a normal bleeding. C. Normal bone tunnel, the femoral head subchondral bone and trabecular bone were detected after arthroscopic removal of bone necrosis.
Histological observation
Single core decompression group: 2 weeks after transplant of BMCs, it was visible of the surrounding bone trabecula mild necrosis and collapse. 4 weeks after transplant, it was visible of subchondral osteoclast reaction, and some necrotic bone. 8 weeks after transplant, it was visible of the newly born capillaries and newly born bone. 12 weeks after transplant, it was visible of irregular contour bone trabecula and not rich in newly bone capillaries. Core decompression + BMSCs group: 2 weeks after transplant of BMCs, it was visible of the surrounding bone trabecula bleeding accompany with mild necrosis and collapse. 4 weeks after transplant, it was visible of bone trabecula collapse. Core decompression + VEGF-165/BMP-2 transfect BMSCs group: it was visible of many newly born capillaries surrounding the bone trabecula and normal bone trabecula, as shown in Figure 4.
Figure 4.
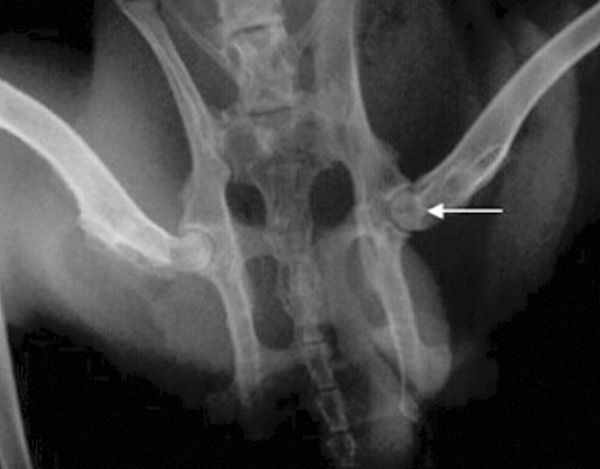
Core decompression and rAAV2-hBMP-2 and rAAV-2-hVEGF-165 autologous bone marrow mesenchymal stem cells transfected plants at 8 wk after X-ray film; the femoral head surface was smooth; cystic lesions disappeared, with a large number of new bone.
Femoral head blood vessel count
During histological observation, number of femoral head blood was counted in femoral head necrosis area, and new bone area ratio was measured in restoration area. As shown in Table 1, the vessel numbers and new bone areas in the marrow core decompression + BMP-2/VEGF-165-transfected BMSCs group were significantly higher than the other 2 groups at each time point after the transplantation (P < 0.05).
Table 1.
Femoral head blood vessel count in femoral head necrosis area and new bone area ratio in restoration area in each group (X̅ ± s, n = 12)
| Group | Femoral head count (number) | Femoral head area ratio (%) | ||
|---|---|---|---|---|
|
| ||||
| Postoperation 4 wk | Postoperation 8 wk | Postoperation 4 wk | Postoperation 8 wk | |
| A | 6.97 ± 1.44 | 7.81 ± 1.95 | 4.02 ± 0.87 | 7.12 ± 1.14 |
| B | 2.31 ± 0.93 | 2.82 ± 1.21 | 4.91 ± 1.97 | 4.83 ± 0.98 |
| C | 1.98 ± 1.68 | 2.85 ± 0.62 | 7.99 ± 2.15 | 7.13 ± 1.17 |
| F | 21.32 | 16.87 | 82.32 | 68.32 |
| P | < 0.01 | < 0.05 | < 0.01 | < 0.05 |
A: Single core decompression group; B: Core decompression + BMSCs group; C: Core decompression + VEGF-165/BMP-2 transfect BMSCs group.
Discussion
The main pathologic process of avascular necrosis of femoral bone is the necrosis of the activity component of the bone. The hip joint-preserving surgery to prevent the femoral head from collapse is currently the hot point and difficult point in the avascular necrosis of femoral bone research area, in which vascularized autologous bone grafts and core decompression are currently widely used methods [11-17]. From early 2006, our team used the lateral circumflex femoral vascularized greater trochanter and (or) iliac bone graft in treatment of osteonecrosis of the femoral head in 1005 cases, after follow-up for 1.5-15 years, the clinical success rate was 89.4%, and the imaging success rate was up to 75.4% [18,19].
However, after long term follow-up, the core decompression can’t thoroughly solve the restoration problem of the femoral head, and if the vicious spiral of increased pressure within the bone would continue, it could lead to reduced femoral head biological strength, and eventually lead to the collapse of the femoral head.
Recent studies showed that BMSCs had abundant source, exuberant differentiation ability, and could secrete a variety of osteogenic activity factor, therefore owing its unique advantage in the repair of avascular necrosis of the femoral head. Fialkov et al. [20] think that BMSCs only has significance on the early bone formation and has no obvious advantages in long-term effect. Suh et al. [21] think that BMSCs has very significant effect in induction of bone formation in alcoholic avascular necrosis of femoral bone. Cui et al. [22] used BMSCs in vitro amplification for autologous replantation in the treatment of rat bone defect model, and had achieved good results. The results in this study between single core decompression group and core decompression + BMSCs group were consistent with above findings. Furthermore, animal experiments have confirmed that systemic and local injection of autologous in vitro BMSCs rarely cause rejection reaction [23].
Latest research showed that many cytokines could promote the cell differentiation from BMSCs to osteocytes and chondrocytes, including VEGF and BMP-2. Currently, VEGF tissue engineering applications mainly focus on: ① using VEGF secreted by vascular endothelial cells to promote engineering bone vascularization [24]; ② using the slow released VEGF in two or three weeks to promote engineering bone vascularization [25]; ③ using the VEGF expressed and secreted by the body to promote engineering bone vascularization [26]. As to BMP-2, only when it reaches a certain concentration in local tissues, can it play the function of induction of osteogenesis. Here in this study, 4 weeks after operation, the core decompression + BMP-2/VEGF-165 transfect BMSCs group had obvious osteogenic reaction and the formation of the new bone, indicating that BMSCs transfected by the human VEGF-165 gene has obvious biological effects. 8 weeks after operation, the bone defect of the femoral head area was repaired, the bone quality had been improved and the physiology of bone repair had been shortened. This results were consistent with the previous study conducted by Dr. Hang et al. [27], who demonstrated that VEGF (165) transgenic autologous BMSCs enhanced bone reconstruction and blood vessel regeneration in the ONFH (osteonecrosis of the femoral head) model. Compared with non-transgenic BMSCs, this approach could provide advanced benefits in the treatment of ONFH.
The arthroscopic core decompression for treatment of avascular necrosis of the femoral head is mainly applicable to the early lesions (ARCO I C to ARCO IIC) [28] and the cases that the articular surface of the femoral head is smooth, and the hip joint biomechanics relationship has no obvious abnormal, and can provide the seed cells for reconstruction of the femoral head. This experiment underwent arthroscopic core decompression combined with autologous BMSCs transplanted into femoral head, which improved the intraosseous high pressure and the pathological state of bone microcirculation obstacles. At the same time, provided seed cells for reconstruction of femoral head, to promote the repair of avascular necrosis of the femoral head. The blood vessel number femoral head had increased, which indicated that human BMP-2 gene transfection of BMSCs had obvious biological effect. There are advantages when applying arthroscopy in the treatment of avascular necrosis of the femoral head. Firstly, it can achieve minimally invasion by magnified 20 times to; secondly, under direct view of arthroscopy, the necrosis bone was completely removed, and it was visible of the fresh blood bleeding, so that the blood supply was saved; thirdly, the seed cells can play the function of the vector; fourthly, the arthroscopy channel that was the core decompression channel, can be sealed by the bone wax to prevent from the loss of BMSCs.
This experiment had provided a new direction for treatment of avascular necrosis of femoral head, but its treatment is a very complex process, and there are still many theoretical and practical problems for further resolve, such as ① the optimum BMSCs dosage and concentration; ② the optimum time and frequency; ③ the induction of bone-formation; ④ the detection of in vivo osteogenic capability of BMSCs.
This article innovation: this article used the avascular necrosis of the femoral head, stem cells, gene transfection as keyword to search in CNKI, PubMed 1980/2010 article, and which decided the experiment has the advancement. The innovative application of two kinds of gene transfection of stem cell transplantation in the treatment of avascular necrosis of the femoral head suggested that the two genes transfect bone marrow mesenchymal stem cells can promote vascularization, and osteogenesis, which provides new technical means for the treatment of avascular necrosis of the femoral head.
Acknowledgements
This study was supported by National Natural Science Foundation of China (30471752), research on one marrow stromal cells in vitro transduction and modification and arthroscopic autologous replantation for the treatment of avascular necrosis of femoral head.
Disclosure of conflict of interest
None.
References
- 1.Yasunaga Y, Terayama H, Yamasaki T, Ishikawa M, Ochi M. [Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow mononuclear cells] . Clin Calcium. 2007;17:910–915. [PubMed] [Google Scholar]
- 2.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87:106–112. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 3.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81:349–355. doi: 10.1302/0301-620x.81b2.8818. [DOI] [PubMed] [Google Scholar]
- 4.Bennett M. Hyperbaric oxygen therapy improved both pain scores and range of motion in patients with early idiopathic femoral head necrosis (Ficat stage II) Diving Hyperb Med. 2011;41:105. [PubMed] [Google Scholar]
- 5.Balla B, Pintér C, Kósa JP, Podani J, Takács I, Nagy Z, Speer G, Horváth B, Korányi L, Lakatos P. Gene expression changes in femoral head necrosis of human bone tissue. Dis Markers. 2011;31:25–32. doi: 10.3233/DMA-2011-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez J, Osses N, Brandan E. Changes in secreted and cell associated proteoglycan synthesis during conversion of myoblasts to osteoblasts in response to bone morphogenetic protein-2: role of decorin in cell response to BMP-2. J Cell Physiol. 2006;206:58–67. doi: 10.1002/jcp.20428. [DOI] [PubMed] [Google Scholar]
- 7.Beck LS, Amento EP, Xu Y, Deguzman L, Lee WP, Nguyen T, Gillett NA. TGF-beta 1 induces bone closure of skull defects: temporal dynamics of bone formation in defects exposed to rhTGF-beta 1. J Bone Miner Res. 1993;8:753–761. doi: 10.1002/jbmr.5650080614. [DOI] [PubMed] [Google Scholar]
- 8.Canalis E, Centrella M, McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest. 1988;81:1572–1577. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farhadi J, Jaquiery C, Barbero A, Jakob M, Schaeren S, Pierer G, Heberer M, Martin I. Differentiation-dependent up-regulation of BMP-2, TGF-beta1, and VEGF expression by FGF-2 in human bone marrow stromal cells. Plast Reconstr Surg. 2005;116:1379–1386. doi: 10.1097/01.prs.0000182355.67397.5a. [DOI] [PubMed] [Google Scholar]
- 10.Rackwitz L, Eden L, Reppenhagen S, Reichert JC, Jakob F, Walles H, Pullig O, Tuan RS, Rudert M, Nöth U. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther. 2012;3:7. doi: 10.1186/scrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amanatullah DF, Strauss EJ, Di Cesare PE. Current management options for osteonecrosis of the femoral head: part II, operative management. Am J Orthop (Belle Mead NJ) 2011;40:E216–E225. [PubMed] [Google Scholar]
- 12.Rajagopal M, Balch Samora J, Ellis TJ. Efficacy of core decompression as treatment for osteonecrosis of the hip: a systematic review. Hip Int. 2012;22:489–493. doi: 10.5301/HIP.2012.9748. [DOI] [PubMed] [Google Scholar]
- 13.Korompilias AV, Beris AE, Lykissas MG, Kostas-Agnantis IP, Soucacos PN. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2011;31:223–228. doi: 10.1002/micr.20837. [DOI] [PubMed] [Google Scholar]
- 14.Mont MA, Marulanda GA, Seyler TM, Plate JF, Delanois RE. Core decompression and nonvascularized bone grafting for the treatment of early stage osteonecrosis of the femoral head. Instr Course Lect. 2007;56:213–220. [PubMed] [Google Scholar]
- 15.Etemadifar M, Kooskzari M, Khalilollah N, Ali MK, Mahsa B. The results of core decompression treatment in patients with avascular necrosis of femoral head in patients at Isfahan City educational hospitals in 2010-2011. Adv Biomed Res. 2014;3:93. doi: 10.4103/2277-9175.129363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, Bian C, Huang X, Shi A, Wang C, Wang K. Core decompression in combination with nano-hydroxyapatite/polyamide 66 rod for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2014;134:103–112. doi: 10.1007/s00402-013-1885-4. [DOI] [PubMed] [Google Scholar]
- 17.Tran TN, Warwas S, Haversath M, Classen T, Hohn HP, Jäger M, Kowalczyk W, Landgraeber S. Experimental and computational studies on the femoral fracture risk for advanced core decompression. Clin Biomech (Bristol, Avon) 2014;29:412–417. doi: 10.1016/j.clinbiomech.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhao DW, Sun Q, Wang BJ, Cui DP. Free iliac crest grafts with periosteum for treatment of old acetabular defects. Chin J Traumatol. 2006;9:338–340. [PubMed] [Google Scholar]
- 19.Yu XB, Zhao DW, Zhong SZ, Liu BY, Wang BJ, Liu YP, Zhang Y, Cui DP, Fu DP, Xie H. Prospective and comparative analysis of internal fixation of femoral neck fractures with or without vascularized iliac graft in young adults. Orthopedics. 2013;36:e132–e138. doi: 10.3928/01477447-20130122-12. [DOI] [PubMed] [Google Scholar]
- 20.Fialkov JA, Holy CE, Shoichet MS, Davies JE. In vivo bone engineering in a rabbit femur. J Craniofac Surg. 2003;14:324–332. doi: 10.1097/00001665-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Suh KT, Kim SW, Roh HL, Youn MS, Jung JS. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005:220–225. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 22.Cui Q, Xiao Z, Li X, Saleh KJ, Balian G. Use of genetically engineered bone-marrow stem cells to treat femoral defects: an experimental study. J Bone Joint Surg Am. 2006;88:167–172. doi: 10.2106/JBJS.F.00891. [DOI] [PubMed] [Google Scholar]
- 23.Johnstone B, Yoo JU. Autologous mesenchymal progenitor cells in articular cartilage repair. Clin Orthop Relat Res. 1999;367(Suppl):S156–S162. doi: 10.1097/00003086-199910001-00017. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FW, Schoen FJ, Mayer JE Jr, Bischoff J. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–H487. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 25.Murphy WL, Simmons CA, Kaigler D, Mooney DJ. Bone regeneration via a mineral substrate and induced angiogenesis. J Dent Res. 2004;83:204–210. doi: 10.1177/154405910408300304. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Shansky J, Del Tatto M, Ferland P, Wang X, Vandenburgh H. Recombinant vascular endothelial growth factor secreted from tissue-engineered bioartificial muscles promotes localized angiogenesis. Circulation. 2001;104:594–599. doi: 10.1161/hc3101.092215. [DOI] [PubMed] [Google Scholar]
- 27.Hang D, Wang Q, Guo C, Chen Z, Yan Z. Treatment of osteonecrosis of the femoral head with VEGF165 transgenic bone marrow mesenchymal stem cells in mongrel dogs. Cells Tissues Organs. 2012;195:495–506. doi: 10.1159/000329502. [DOI] [PubMed] [Google Scholar]
- 28.Ellenrieder M, Tischer T, Kreuz PC, Fröhlich S, Fritsche A, Mittelmeier W. [Arthroscopically assisted therapy of avascular necrosis of the femoral head] . Oper Orthop Traumatol. 2013;25:85–94. doi: 10.1007/s00064-011-0072-4. [DOI] [PubMed] [Google Scholar]


