Abstract
Salvianolic acid B (Sal B), which is purified from Danshen, is a popular herb extract. Sal B has anti-oxidative, anti-inflammatory, anti-hypoxic, anti-arteriosclerotic and anti-apoptotic properties. This substance can also ameliorate brain injury or neurodegenerative diseases. The listed drug Salvianolate, which contains a substantial amount of Sal B, has been used for the treatment of coronary heart disease. Our present work aimed to evaluate the inhibitory effect of salvianolate on seven cytochrome P450 isoforms (CYP450), namely, CYP1A2, CYP2A6, CYP2E1, CYP2C9, CYP2C19, CYP2D6 and CYP3A4, in human liver microsomes (HLMs) and recombinant enzymes through high-performance liquid chromatography (HPLC) assay. Salvianolate have a potent inhibitory effect on CYP3A4 activity with IC50 values of 1.438 (HLMs) and 3.582 (recombinant cDNA-expressed CYP3A4) mg/L, respectively. Salvianolate strongly dose, but not time-dependently decreased CYP3A4 activity in HLMs. The typical Lineweaver-Burk plots showed that Salvianolate inhibited CYP3A4 activity noncompetitively, with a Ki value of 2.27 mg/L in HLMs. Other CYP450 isoforms are not markedly affected by Salvianolate. These findings indicate that salvianolate may be involved in potential drug interactions when co-administrated with CYP3A4 substrates.
Keywords: Salvianolate, CYP3A4, human liver microsomes, noncompetitive inhibition, drug-drug interaction
Introduction
Danshen, the dried root of Salvia miltiorrhiza, is widely used in the treatment of coronary heart disease, hepatitis, cerebrovascular disease, hepatocirrhosis, and chronic renal failure [1-5]. As a highly purified aqueous extract from Danshen, Salvianolate is a listed drug that primarily contains salvianolic acid B (Sal B) (≥85%), rosmarinic acid (≥10.1%), and lithospermic acid (≥1.9%) to protect microvascular reflow against ischaemia/reperfusion injury and acute coronary syndrome [6,7]. It was previously reported that Sal B is the most abundant member of salvianolic acids [8]. Various investigations have shown that Sal B possesses various pharmacologic properties including anti-oxidative, anti-inflammatory, anti-hypoxic, anti-arteriosclerotic, anti-apoptotic properties in vivo and in vitro [9-16].
The CYP450 superfamily is an important enzyme system in humans. This enzyme is responsible for the metabolism of several endogenous compounds and xenobiotics [17]. CYP isozymes can be inhibited by various drugs, such as ketoconazole, ritonavir, and clarithromycin. This process may lead to drug-drug interactions and subsequently exacerbate adverse clinical events [18]. Seven human hepatic CYP isoforms, namely, CYP1A2, CYP3A4, CYP2A6, CYP2C9, CYP2C19, CYP2D6 and CYP2E1, are shown to be responsible for more than 90% drug metabolism [19]. This mechanism may lead to several clinical important drug-drug interactions [20]. The blood concentration of co-administered drugs may be altered significantly and result in adverse reactions or drug withdrawal when concomitant drugs are metabolized by the same enzyme.
Salvianolate has been applied as a therapeutic agent in clinical practices [21]. However, there have are no reports suggesting an adverse drug-drug interaction caused by salvianolate, and no relevant publications of its effects on CYP450s. Therefore, our present work was conducted to investigate the inhibitory effects of salvianolate on the CYP isoforms in HLMs.
Materials and methods
Chemicals
The pooled human liver microsome (HLM) and human recombinant enzyme used in the incubation studies were purchased from BD Gentest Co. (Woburn, MA, USA). Coumarin, 7-hydroxycoumarin, tolbutamide, 4-hydroxytolbutamide, (S)-mephenytoin, 4’-hydroxymephenytoin, metoprolol, α-hydroxymetoprolol, midazolam maleate, 1-hydroxymidazolam, phenacetin, acetaminophen, chlorzoxazone, 6-hydroxychlorzoxazone, propranolol, quinidine hydrochloride, tranylcypromine, fluconazole, ticlopidine, quinidine, ketoconazole, furafylline, and diethyldithiocarbamate were purchased from Sigma Chemicals (St. Louis, MO, USA). Formic acid, ethylic acid, ammonium formate, MgCl2, NADP+, glucose 6-phosphate, glucose 6-phosphate dehydrogenase, and potassium phosphate (monobase and dibase) were chromatographic-grade chemicals purchased from Sigma Chemicals (St. Louis, MO, USA). Acetonitrile and methanol were of analytical grade and purchased from Sigma Chemicals (St. Louis, MO, USA). The deionized water used in the experiments was prepared in the laboratory using a Millipore Milli-Q reverse osmosis system (Bedford, MA, USA). Salvianolate were provided by Shanghai Green Valley Pharmaceutical (Shanghai, China).
Microsomal incubations
The stock solution of salvianolate and subsequent serial dilutions were freshly prepared using deionized water and refrigerated in 4°C until use. Other stock solutions of substrates and inhibitors were made in different ratios of acetonitrile and water. The final percentage of organic solvent in incubation mixtures was less than 1%. Incubations were conducted at 37°C in potassium phosphate buffer (100 mM KH2PO4, 100 mM K2HPO4, 3.3 mM MgCl2, pH 7.4) in the presence of 0.5 mg/mL HLM or human recombinant enzymes. Salvianolate concentrations were set from 0.01 mg/L to 100 mg/L. The incubation mixture was pre-incubated for 10 min prior to the initiation of metabolic reaction with NADPH system (1.3 mM NADP+, 3.3 mM glucose 6-phosphate, 0.4 U/ml glucose 6-phosphate dehydrogenase), with parallel negative and positive controls in triplicate. The reactions were terminated after incubation by adding acetonitrile containing internal standard (IS). Table 1 summarizes each enzyme that corresponds to the agents and incubation conditions for evaluating inhibitory activities of CYP450.
Table 1.
The inhibitory effect of salvianolate on isozyme-specific cytochrome P450 activities in HLMs
| CYP isozyme | Specific probe (μM) | Metabolite | Incubation time (min) | Positive control inhibitor concentration (μM) |
|---|---|---|---|---|
| CYP1A2 | Phenacetin (10) | Acetaminophen | 30 | Furafylline (10) |
| CYP2A6 | Coumarin (5) | 7-hydroxycoumarin | 20 | Tranylcypromine (50) |
| CYP3A4 | Midazolam (5) | 1-hydroxymidazolam | 30 | Ketoconazole (1) |
| CYP2C9 | Tolbutamide (100) | 4-hydroxytolbutamide | 30 | Sulfaphenazole (10) |
| CYP2C19 | S-Mephenytoin (100) | 4’-hydroxymephenytoin | 30 | Ticlopidine (5) |
| CYP2D6 | Metoprolol (7.5) | α-hydroxymetoprolol | 15 | Quinidine (1) |
| CYP2E1 | Chlorzoxazone (40) | 6-Hydroxychlorzoxazone | 20 | Diethyldithiocarbamate (50) |
Inhibitory effects of salvianolate on CYP450
Compound assays were tested in triplicate using individual drug-probe substrates that were specific for each HLM-CYP or recombinant enzyme and HPLC detection. Inhibitor concentrations were set from 0.01 mg/L to 100 mg/L (5 points).
Inhibition mode of salvianolate in HLMs
To investigate the inhibition mode of salvianolate, HLMs were pre-incubated with salvianolate in potassium phosphate buffer (pH 7.4) for 0, 10, or 30 min in the presence of NADPH. Midazolam was then added and incubated for 30 min at 37°C. Midazolam was used as a probe substrate at 5, 10, or 20 μM.
cDNA-expressed CYP3A4 inactivation assay
To confirm the selective inhibition of the CYP3A4 enzyme by salvianolate, 10 pmol of human recombinant cDNA-expressed CYP3A4 was incubated with salvianolate at 0.01 to 100 mg/L, NADPH system and midazolam as a selective CYP3A4 substrate for 30 min at 37°C.
HPLC analysis
HPLC analysis was performed using the SHIMADZU LC-2010CHT with an ultraviolet detector. All chemicals were separated on Hypersil BDS C18 column (4.6×200 mm, 5 μm) (Thermo, USA) with a C18 Pre-column (4.0×3.0 mm) (Phenomenex, USA). The contents of mobile phase, flow rate, wavelength, and IS were summarized in Table 2. A 20 µL aliquot of each sample was subjected to HPLC analysis.
Table 2.
HPLC conditions for the determination of CYP-dependent enzymatic activities
| CYP | Mobile phase (v/v) | IS | Flow rate (mL/min) | LC-UV detection | Retention time (min) (metabolite/IS) |
|---|---|---|---|---|---|
| CYP1A2 | 0.1% Formic acid/ methanol (70/30) | 7-hydroxycoumarin | 0.7 | 297 nm | 5.75/11.29 |
| CYP2A6 | 10 mM ammonium formate /methanol (60/40) | Phenacetin | 0.3 | 230 nm | 2.56/4.97 |
| CYP3A4 | 20 mM ammonium formate /methanol (35/65) | Propranolol | 1 | 225 nm | 8.5/4.0 |
| CYP2C9 | 0.2% ethylic acid/ methanol (45/55) | Phenacetin | 1 | 230 nm | 4.15/5.06 |
| CYP2C19 | 20 mM ammonium formate /methanol (45/55) | Phenacetin | 1 | 204 nm | 3.77/7.47 |
| CYP2D6 | 20 mM ammonium formate (0.1% Formic acid)/methanol (60/40) | Phenacetin | 0.5 | 280 nm | 3.5/10.2 |
| CYP2E1 | Water/Acetonitrile (65/35) | Phenacetin | 0.8 | 282 nm | 4.94/7.3 |
Statistic analysis
The 50% inhibitory concentration (IC50) values were calculated by nonlinear regression using Graphpad Prism 5.0 (San Diego, CA, USA). Lineweaver-Burk plot was obtained from software SigmaPlot (version 10.0, Systat Software, Inc.)
Results
Inhibition of CYP450 activities by salvianolate in HLMs
The inhibitory effects of salvianolate were examined on seven typical reactions catalyzed by seven human CYP enzymes in HLMs. Salvianolate showed a potent inhibition of CYP3A4 activity in HLM with IC50 values of 1.438 mg/L. However, salvianolate caused no significant inhibition of CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, and CYP2E1 activities (IC50 >100 mg/L). Figure 1 illustrates the inhibitory effects of salvianolate on CYP3A4 activity.
Figure 1.
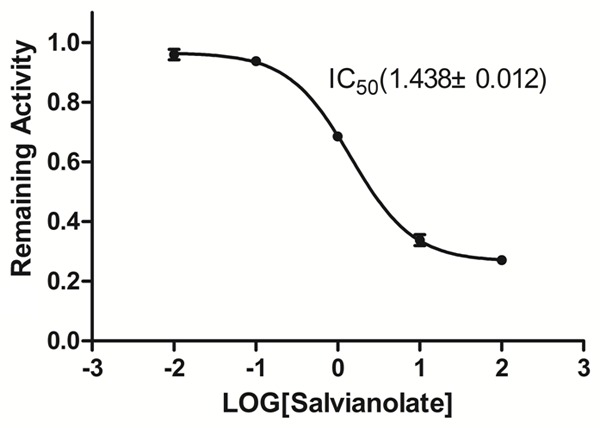
Inhibitory effect of salvianolate (0, 0.01, 0.1, 1, 10, and 100 mg/L) on CYP3A4 enzyme activity in HLM. The data were expressed as mean ± SD.
Mechanism-based inhibition of salvianolate on CYP3A4 enzyme activity
To investigate the mechanism responsible for inhibition of CYP3A4-catalyzed midazolam 1’-hydroxylation by salvianolate, CYP3A4 inhibitory activity was determined with or without pre-incubating microsomal incubation mixtures for 0, 10, or 30 min at 37°C in HLMs. Salvianolate strongly and dose-dependently inhibited CYP3A4-catalyzed midazolam 1’-hydroxylation in HLMs, but not time-dependently (Figure 2). To investigate the mechanism of inhibition of salvianolate (0, 1, or 2 mg/L) on CYP3A4, concentrations of midazolam (5, 10, or 20 µM) were used. From the Lineweaver-Burk plots (Figure 3A) and secondary plots (Figure 3B), both of which linear, they indicated a noncompetitive inhibition type of salvianolate on CYP3A4. Therefore, salvianolate acts as a noncompetitive inhibitor of CYP3A4 with a Ki value of 2.27 mg/L in HLMs.
Figure 2.
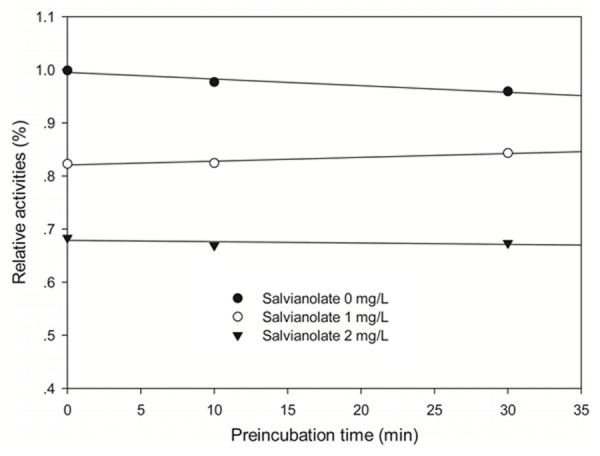
Time-course of CYP3A4-catalyzed midazolam 1’-hydroxylation inactivation by salvianolate in HLMs. HLMs were preincubated with salvianolate at 0 (●), 1 (○), or 2 (▼) mg/L in the presence of NADPH for 0 to 30 min. The results shown are the means of triplicate experiments.
Figure 3.
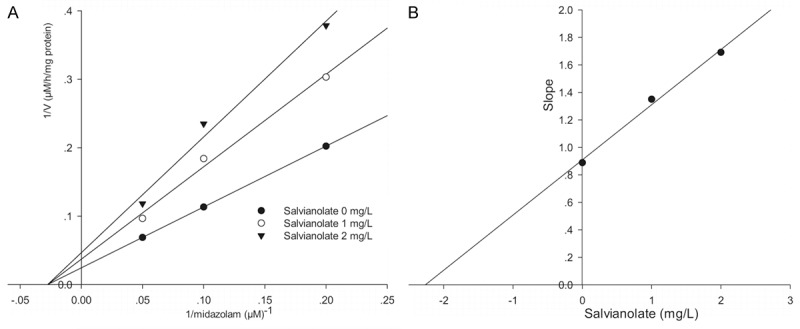
Lineweaver-Burk plot (A) and secondary plot (B) obtained from a kinetic study of CYP3A4-catalyzed midazolam 1’-hydroxylation following a 30 min incubation with salvianolate at 0 (●), 1 (○), or 2 (▼) mg/L. The results shown are the means of triplicate experiments.
Potent inhibition of CYP3A4 by salvianolate
To evaluate the selectivity of the inhibitory effect of salvianolate on CYP3A4, salvianolate was incubated with human recombinant cDNA-expressed CYP3A4. Salvianolate showed a potent inhibition of CYP3A4 activity with IC50 values of 3.582 mg/L (Figure 4).
Figure 4.
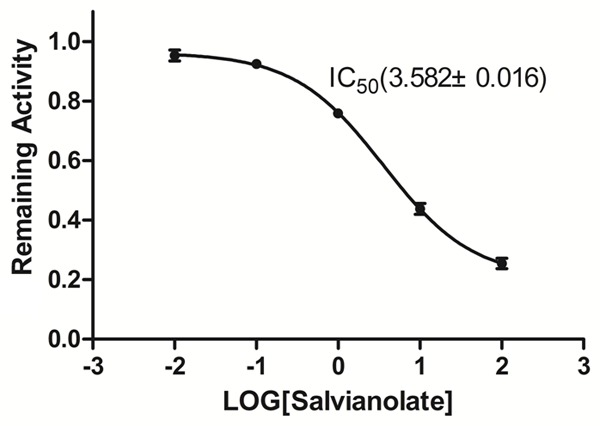
Inhibitory effect of salvianolate on the midazolam 1’-hydroxylation activities of human recombinant cDNA-expressed CYP3A4. The data were expressed as mean ± SD.
Discussion
Our present investigation firstly illustrated the inhibitory effects of salvianolate on seven CYP450 enzymes activities in HLMs, as well as to identify the inhibition in recombinant enzymes. Salvianolate significantly inhibited CYP3A4 enzyme activity in HLMs.
CYP3A4 is responsible for the metabolism of a variety of drugs and endogenous compounds in humans. In general, it contains up to 60% CYP isoforms in liver. Previous reports revealed that over 50% of the marketed drugs were metabolized by CYP3A4 [22,23]. At present, coronary heart disease could be treated by numerous drugs, such as statins [24], antiplatelet drugs [25], anticoagulant drugs [26], ACEI, and β-receptor antagonists [27]. Many of these drugs are related to CYP3A4 metabolism. Salvianolate, a new water-soluble phenolic compound that is one of the most bioactive compounds, has been widely used in treatment of various cardiovascular diseases, especially coronary heart disease [6,7]. Multi-drug therapy for patients with multiple complications was likely to cause interactions due to uncertain alterations in CYP metabolism, which led to serious or even fatal adverse drug reactions (ADRs) [28]. Drug-drug interaction between salvianolate and other drugs mentioned may decrease the safety and increase toxicity. When these drugs are co-administered with salvianolate, the drugs metabolized by CYP3A4 should be taken carefully to avoid severe adverse reactions.
Salvianolate, a series of aqueous extract from Danshen, contained mainly Sal B (≥85%), rosmarinic acid (≥10.1%) and lithospermic acid (≥1.9%) [6,7]. Sal B accounts for the most part of aqueous extract from Danshen. However, Qiu et al. reported that Sal B did not significantly affect the activity of CYP3A4 enzyme in HLM [29]. Hence, other aqueous extracts, such as rosmarinic acid and lithospermic acid, may inhibit the activity of CYP3A4 enzyme, but this requires further investigation to investigate how salvianolate affects activities of CYP450 enzymes.
Moreover, salvianolate showed a potent inhibition of CYP3A4 activity in HLM with IC50 values of 1.438 mg/L. However, salvianolate caused no significant inhibition on CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, and CYP2E1 activities (IC50 >100 µM). As deep into study, salvianolate may be a potent noncompetitive inhibitor of CYP3A4 enzyme activity with Ki value of 2.27 mg/L. The data enriched the CYP3A4 enzyme kinetic research of salvianolate in HLMs.
The in vitro experiment about salvianolate provide medicine co-administration advice to a certain extent. However, animal experiment and clinical trials are still needed to determine whether the salvianolate can favorably influence the treatment of other candidate drugs and the adverse effect that may caused in the condition of co-administration.
Conclusions
In conclusion, this study demonstrates that salvianolate selectively inhibited CYP3A4-catalyzed midazolam 1’-hydroxylation in HLMs and suggested that it might cause drug-drug interactions when co-administrated with CYP3A4 substrates. However, animal experiment and clinical trials are still needed to determine pharmacokinetic effects of salvianolate with respect to CYP3A4 to confirm these results.
Acknowledgements
This work was supported by the Huge Project to Boost Chinese Drug Development (2010ZX09502-003); the Program for Changjiang Scholars and Innovative Research Team in University (IRT0946); and the Hunan Provincial Natural Science Foundation of China (12JJ7006).
Disclosure conflict of interest
None.
References
- 1.Ji X, Tan BK, Zhu YC, Linz W, Zhu YZ. Comparison of cardioprotective effects using ramipril and DanShen for the treatment of acute myocardial infarction in rats. Life Sci. 2003;73:1413–1426. doi: 10.1016/s0024-3205(03)00432-6. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Xu X, Wang J, Yu H, Wang X, Yang H, Xu H, Tang S, Li Y, Yang L, Huang L, Wang Y, Yang S. A system-level investigation into the mechanisms of Chinese Traditional Medicine: Compound Danshen Formula for cardiovascular disease treatment. PLoS One. 2012;7:e43918. doi: 10.1371/journal.pone.0043918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Shen HM, Ong CN. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG(2) cells. Cancer Lett. 2000;153:85–93. doi: 10.1016/s0304-3835(00)00391-8. [DOI] [PubMed] [Google Scholar]
- 4.Wu XJ, Wang YP, Wang W, Sun WK, Xu YM, Xuan LJ. Free radical scavenging and inhibition of lipid peroxidation by magnesium lithospermate B. Acta Pharmacol Sin. 2000;21:855–858. [PubMed] [Google Scholar]
- 5.Zhao SJ, Huang XH, Wang SP. Expression of thermostable alpha-amylase gene in potato. Shi Yan Sheng Wu Xue Bao. 2000;33:157–162. [PubMed] [Google Scholar]
- 6.Han B, Zhang X, Zhang Q, Zhao G, Wei J, Ma S, Zhu W, Wei M. Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch Cardiovasc Dis. 2011;104:313–324. doi: 10.1016/j.acvd.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Yu C, Sun W, Liu G, Jia J, Wang Y. Simultaneous determination of magnesium lithospermate B, rosmarinic acid, and lithospermic acid in beagle dog serum by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2878–2882. doi: 10.1002/rcm.1703. [DOI] [PubMed] [Google Scholar]
- 8.Gu M, Zhang G, Su Z, Ouyang F. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high-speed counter-current chromatography. J Chromatogr A. 2004;1041:239–243. doi: 10.1016/j.chroma.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Guo XW, Chen K, Xu RX, Zheng HZ, Wang J. Inhibition of hypoxia and serum deprivation-induced apoptosis by salvianolic acid in rat mesenchymal stem cells. J Tradit Chin Med. 2012;32:222–228. doi: 10.1016/s0254-6272(13)60015-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen YL, Hu CS, Lin FY, Chen YH, Sheu LM, Ku HH, Shiao MS, Chen JW, Lin SJ. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem. 2006;98:618–631. doi: 10.1002/jcb.20793. [DOI] [PubMed] [Google Scholar]
- 11.Lay IS, Hsieh CC, Chiu JH, Shiao MS, Lui WY, Wu CW. Salvianolic acid B enhances in vitro angiogenesis and improves skin flap survival in Sprague-Dawley rats. J Surg Res. 2003;115:279–285. doi: 10.1016/s0022-4804(03)00226-9. [DOI] [PubMed] [Google Scholar]
- 12.Lin YH, Liu AH, Wu HL, Westenbroek C, Song QL, Yu HM, Ter Horst GJ, Li XJ. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Abeta(25-35)-induced reduction in BPRP in PC12 cells. Biochem Biophys Res Commun. 2006;348:593–599. doi: 10.1016/j.bbrc.2006.07.110. [DOI] [PubMed] [Google Scholar]
- 13.Lin YL, Wu CH, Luo MH, Huang YJ, Wang CN, Shiao MS, Huang YT. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. J Ethnopharmacol. 2006;105:215–222. doi: 10.1016/j.jep.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Shi CS, Huang HC, Wu HL, Kuo CH, Chang BI, Shiao MS, Shi GY. Salvianolic acid B modulates hemostasis properties of human umbilical vein endothelial cells. Thromb Res. 2007;119:769–775. doi: 10.1016/j.thromres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HS, Wang SQ. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol. 2006;41:138–148. doi: 10.1016/j.yjmcc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, Liu Y, Yang X, Isaiah AO, Lin Y, Jiang Y. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One. 2012;7:e35636. doi: 10.1371/journal.pone.0035636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 18.Back DJ, Orme ML. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet. 1990;18:472–484. doi: 10.2165/00003088-199018060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 20.Yan Z, Caldwell GW. Metabolism profiling, and cytochrome P450 inhibition & induction in drug discovery. Curr Top Med Chem. 2001;1:403–425. doi: 10.2174/1568026013395001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Zhang Y, Yang R, Li YJ, Wang M, Miao CL. [Correlation study on effects of salvianolate on inflammatory cytokines of patients with acute coronary syndrome] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:598–601. [PubMed] [Google Scholar]
- 22.Omiecinski CJ, Remmel RP, Hosagrahara VP. Concise review of the cytochrome P450s and their roles in toxicology. Toxicol Sci. 1999;48:151–156. doi: 10.1093/toxsci/48.2.151. [DOI] [PubMed] [Google Scholar]
- 23.Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- 24.Rowan CG, Brunelli SM, Munson J, Flory J, Reese PP, Hennessy S, Lewis J, Mines D, Barrett JS, Bilker W, Strom BL. Clinical importance of the drug interaction between statins and CYP3A4 inhibitors: a retrospective cohort study in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2012;21:494–506. doi: 10.1002/pds.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie IS, Coughtrie MW, MacDonald TM, Wei L. Antiplatelet drug interactions. J Intern Med. 2010;268:516–529. doi: 10.1111/j.1365-2796.2010.02299.x. [DOI] [PubMed] [Google Scholar]
- 26.Becattini C, Vedovati MC, Agnelli G. Old and new oral anticoagulants for venous thromboembolism and atrial fibrillation: a review of the literature. Thromb Res. 2012;129:392–400. doi: 10.1016/j.thromres.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Davis CD, Sinz MW, Rodrigues AD. Cytochrome P450 reaction-phenotyping: an industrial perspective. Expert Opin Drug Metab Toxicol. 2007;3:667–687. doi: 10.1517/17425255.3.5.667. [DOI] [PubMed] [Google Scholar]
- 28.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 29.Qiu F, Zhang R, Sun J, Jiye A, Hao H, Peng Y, Ai H, Wang G. Inhibitory effects of seven components of danshen extract on catalytic activity of cytochrome P450 enzyme in human liver microsomes. Drug Metab Dispos. 2008;36:1308–1314. doi: 10.1124/dmd.108.021030. [DOI] [PubMed] [Google Scholar]


