Abstract
Background: To evaluate the association of MMP11 and P14ARF expression in laryngeal squamous cell carcinoma (LSCC) with clinical pathological characteristics and survival. Methods: The mRNA and protein levels for both genes were determined in 65 LSCC patients. A log-rank test and Cox models were used to compare survival among different groups. Results: The mRNA expressions of MMP11 and P14ARF were significantly different between LSCC and their corresponding adjacent tissues (All P < 0.001). The expressions of MMP11 and P14ARF were correlated with several clinical characteristics (All P < 0.05). Patients with low MMP11 and high P14ARF expression had significantly better survival compared with those with high MMP11 and low P14ARF expression, respectively (All P < 0.05). The patients with surgery only had significantly better survival than those with chemoradiotherapy (log rank: P = 0.016), particularly in patients with low MMP11 and high P14ARF expression (log rank: P = 0.006). Furthermore, multivariable analysis showed that patients with low MMP11 and high P14ARF expression alone had a significantly reduced risk of death compared with those with high MMP11 and low P14ARF expression. The reduced risk for overall death was pronounced for patients with low and high expression of both genes (HR, 0.2; 95% CI, 0.1-0.5) compared with any other co-expression status of both genes, particularly for patients with surgery only (HR, 0.1; 95% CI, 0.0-0.9). Conclusion: These results suggest that altered expression of MMP11 and P14ARF in tumors may individually, or in combination, predict poor prognosis of LSCC, particularly for patients with surgery only.
Keywords: P14ARF, MMP11, LSCC, survival, biomarker
Introduction
Head and neck squamous cell carcinomas (HNSCC) is the sixth most common type of cancer worldwide, with poor clinical outcomes [1] and laryngeal squamous cell carcinoma (LSCC) ranks second among the HNSCC [2]. It accounts for nearly half of all HNSCC cases in China [3]. The progression and metastasis of LSCC involve multiple factors and molecular processes, with a notably expression imbalance of numerous cellular molecules.
The matrix metalloproteinases (MMPs) is a family of extracellular or membrane-bound Zn2+-dependent proteases that are capable of digesting various proteinaceous components of the extracellular matrix (ECM) which serves as a medium for cell-cell interactions and can directly signal cells through cell surface ECM receptors [4]. MMP11, also known as Stromelysin-3 (ST3), is one of members in MMPs family. It was first identified by its over-expression in primary breast cancer and isolated as a breast carcinoma-associated gene [5]. Extensive studies have shown that high expression of MMP11 were associated with tumor invasion, metastasis, tumor progression, and prognosis [6].
P14ARF, located on human chromosome 9p21, is a tumor suppressor gene encoded by theunusual INK4a/P14ARF locus, which is frequentlyinactivated in tumors [7]. Itis a multi-functional gene that is involved in many physic processes. P14ARF can induce both G1 and G2 arrest due to its stabilizing effects on the p53 transcription factor, and is involved in cellular senescence and apoptosis [8]. Moreover, P14ARF has also significant association with autophagy and angiogenesis [9,10].
Although several studies have reported that these two genes may play important roles in development, progression and prognosis in human cancers [5-10], such potential roles and their relationship between MMP11 and P14ARF in LSCC is not unknown. This study aimed to compare the expression ofMMP11 and P14ARF in LSCC and their matched adjacent normal laryngeal tissues, explore their relationships with clinical pathological characteristics, and evaluate the potential association between MMP11 and P14ARF expression and survival of LSCC, which may thus lead to better understanding of the functional contribution of MMP11 and P14ARF to progress and prognosis of LSCC.
Materials and methods
Patients and biological samples
Sixty-five patients were included in this study and all biopsies were obtained with patients’ consent. We included consecutive patients diagnosed with LSCC between 2009 and 2010. Immediately after surgical excision, a tumor sample was obtainedfrom the tumor area, while its corresponding peripheral normal laryngeal tissues were obtained from theassociated non-cancerous tissue within 5 cm of the tumor,without affecting the assessment of tumor margins. The biopsies were divided into two parts: one snap-frozen in liquid nitrogen immediately after surgery, and stored at -80°C; the other formalin fixed and paraffin embedded. Histopathological assessment was performed on the paraffin blocks. In addition, patients who were categorized as “ever drinkers” are related to those who had drunk at least one alcoholic beverage per week for at least 1 year during their lifetime, and patients who were categorized as “never drinkers” are related to those who had never had such a manner of drinking. Patients who were categorized as “ever smokers” are related to those who had smoked at least 100 cigarettes in their lifetime, and patients who had smoked fewer than 100 cigarettes in their lifetime were categorized as “never smokers”. This study was approved by the ethics committee of Beijing Tongren Hospital of the Capital Medical University, and all of the patients had signed the informed consent forms.
Quantitative analysis
Total RNA was extracted for QPCR. The primers and internal reference primers were synthesized by Beijing Tiangen Biomedical Development Co., Ltd. (Beijing, China) and the Ct value comparison method was used to detect the gene expression level, with β-actin as an internal reference9. The relative expression levels of MMP11 and P14ARF were calculated with the Ct value referring to the number of cycles when the fluorescence signal reached the set threshold in each reaction tube. The primers used in this study are shown in Table 1.
Table 1.
Primers used in this study
| Primers | Sequences |
|---|---|
| β-actin | F: 5-CACCCTTTCTTGACAAAACCT-3’ |
| R: 5’-AGTGGGGTGGCTTTTAGGA-3’ | |
| MMP11 | F: 5-TGAGTGCCCGCAACCG 3’ |
| R: 5’-GGCGTCACATCGCTCCATA-3’ | |
| P14ARF | F: 5-TGGAGGCGGCGAGAACA-3’ |
| R: 5’-TCAGTAGCATCAGCACGAGGG-3’ |
Immunohistochemistry
MMP11 and P14ARF antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), while streptavidin-peroxidase, 4-dimethylaminoazobenzene, and the streptavidin-peroxidase immunohistochemical staining kit were purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). The LSCC tissues and its corresponding adjacent normal laryngeal tissues were preserved. A positive biopsy in the kit was used for the staining of the positive control, and the antibody was replaced by phosphate-buffered saline for the staining of the negative control. The immunohistochemical staining is shown in Figure 1. Yellow to brownish-yellow granules in cells were considered to indicate positive cells. Positive cells were counted in a total of 100 cells under high-power microscopy, and scored according to the positive expression rate (0 points for < 10%; 1 point for 11-20%; 3 points for 21-50%; 4 points for > 50%) and staining intensity (0 points for no staining; 2 points for weak; 3 points for strong). The sum of the points for staining intensity and positive expression rate was used as the expression score. A score ≥ 3 was considered to indicate a positive case while a score of 0-2 was classified as a negative case.
Figure 1.
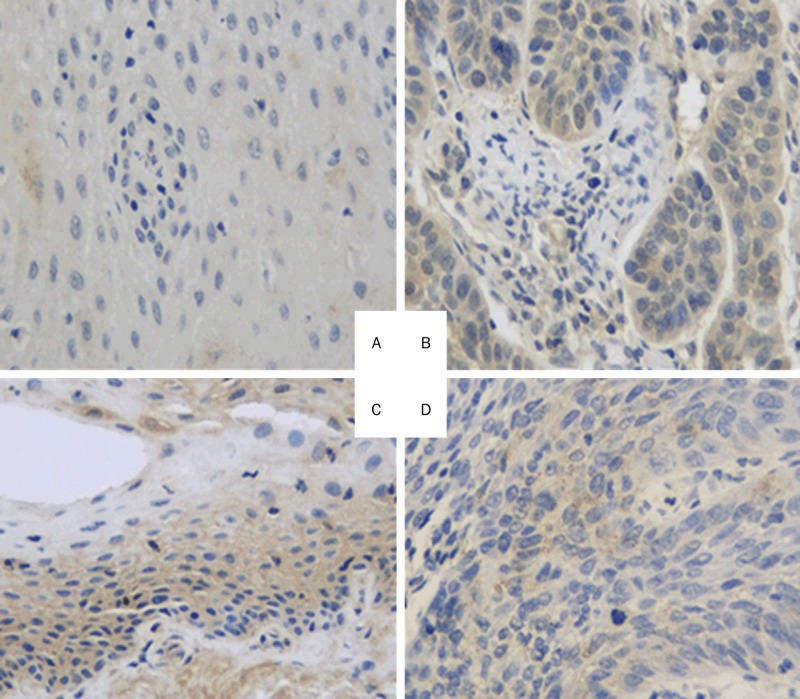
Immunostaining of MMP11 and P14ARF in laryngeal carcinoma tissues. A. MMP11 weakly expressed in the adjacent tissue; B. Laryngeal carcinoma tissues with high MMP11 expression in the cytoplasm; C. P14ARF positively expressed in the adjacent tissue; D. P14ARF weekly expressed in the laryngeal carcinoma tissue.
Statistical analysis
The primary endpoint in this study is overall death. Overall survival (OS) was defined as the time from first appointment to death from any cause or date of last follow-up. Participants who were alive at the end of the study period or lost to follow-up were considered censored. Medical record review for follow-up status of all patients was performed under direct supervision of staff head and neck surgeon. Primary tumor subsite, clinical stage, treatment, and vital status were reviewed from medical records as assessed between the initial and final patient contact recorded. Data were analyzed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL). Measurement data were compared using a paired t-test, while the correlation of MMP11 and P14ARF expression was analyzed using Spearman’s rank correlation analysis. Survival analysis was performed by using Kaplan-Meier analysis, and the significance was analyzed with the log-rank test. Univariate cox proportional hazards regressions were applied to estimate the individual hazard ratio (HR) for the overall survival. The difference was considered significant when the P value was less than 0.05.
Results
mRNA levels of MMP11 and P14ARF
QPCR was performed to detect the potential changes in the mRNA levels of MMP11 and P14ARF expression in LSCC and its corresponding adjacent normal laryngeal tissues. The QPCR results showed significant gene expression level differences between the cancerous and adjacent tissues (P < 0.05). The expression of MMP11 in the cancerous tissue was significantly increased (P < 0.05) while the P14ARF mRNA expression in the cancerous tissue was notably decreased compared with that in the adjacent tissue (P < 0.05), as shown in Table 2. These results indicate that the mRNA levels of MMP11 in LSCC are increased while the mRNA levels of P14ARF are decreased compared with those in the adjacent tissues.
Table 2.
mRNA expression of MMP11 and P14ARF in LSCC and adjacent tissues
| Tissue types | n | MMP11 | P | P14ARF | P |
|---|---|---|---|---|---|
| Adjacent tissues | 65 | 0.961 ± 0.294 | 1.050 ± 0.202 | ||
| Tumor tissues | 65 | 2.346 ± 1.995 | < 0.001 | 0.794 ± 0.248 | < 0.001 |
Protein expression of MMP11 and P14ARF
Immunohistochemical analyses of MMP11 and P14ARF expression in LSCC and their corresponding adjacent normal laryngeal tissues were performed to detect the expression of MMP11 and P14ARF proteins (Table 3). Both MMP11 and P14ARF proteins were expressed in the cytoplasm. The positive expression rates and scores of MMP11 were significantly increased in the cancerous tissue compared with those in the adjacent tissue (P < 0.05). For P14ARF, however, its protein expression levels were significantly decreased in LSCC compared with those in the adjacent tissues (P < 0.05). These results indicate that the expression levels of MMP11 in LSCC are increased while the levels of P14ARF are decreased compared with those in adjacent tissues.
Table 3.
MMP11 and P14ARF protein expression level in LSCC and adjacent tissues
| Tissue type | n | MMP11 (+) | P | P14ARF (+) | P |
|---|---|---|---|---|---|
|
|
|
||||
| N % | N % | ||||
| Adjacent tissues | 65 | 14 (21.5) | 53 (81.5) | ||
| Tumor tissues | 65 | 50 (76.9) | < 0.001 | 28 (43.1) | < 0.001 |
Correlation between the mRNA and protein expression of MMP11 and P14ARF and the clinical pathological characteristics
The mRNA expression levels were analyzed to explore the potential relationship between the protein expression and mRNA levels of MMP11 and P14ARF and their relationships with clinical pathological characteristics (Table 4). It was revealed that MMP11 and P14ARF were negatively correlated (correlation coefficient, -0.376, P = 0.002). In the 65 cases of LSCC tissues, MMP11 was correlated with the tumor stage, lymph node metastasis; and the patients with later tumor stage and lymph node metastasis had shown a much stronger MMP11 expression compared with those with early tumor stage and negative lymph node metastasis (P < 0.05). In contrast, P14ARF expression was also associated with the tumor stage; and the patients in the later stage had shown a much lower expression level (P < 0.05). Both MMP11 and P14ARF had a significant correlation with tobacco smoking. These results suggest that the elevated expression of MMP11 may be correlated with the malignancy of tumors and that P14ARF expression may be negatively correlated with the degree of malignancy.
Table 4.
Correlations between the protein/mRNA expressions of MMP11 and P14ARF with clinical pathological characteristics in 65 LSCC cases
| Factors | Cases (%) | MMP11 | P14ARF | ||
|---|---|---|---|---|---|
|
| |||||
| Protein (+) | High mRNA | Protein (+) | High mRNA | ||
|
| |||||
| N, % | N, % | N, % | N, % | ||
| Age (years) | |||||
| ≥ 60 | 28 (43.1) | 22 (78.6) | 28 (100)a | 10 (35.7) | 8 (28.6) |
| < 60 | 37 (56.9) | 28 (75.7) | 30 (81.1) | 18 (48.6) | 5 (13.5) |
| Gender | |||||
| Male | 58 (89.2) | 45 (77.6) | 52 (89.7) | 25 (43.1) | 12 (20.7) |
| Female | 7 (10.8) | 5 (71.4) | 6 (85.7) | 3 (42.9) | 1 (14.3) |
| Tumor stage | |||||
| I-II | 20 (30.8) | 11 (55.0) | 14 (70.0) | 15 (75.0) | 10 (50.0) |
| III-IV | 45 (69.2) | 39 (86.7)a | 44 (97.8)a | 13 (28.9)a | 3 (6.7)a |
| LNM | |||||
| Absent | 26 (38.2) | 16 (61.5) | 20 (76.9) | 8 (30.8) | 6 (23.1) |
| Present | 39 (61.8) | 34 (87.8)a | 38 (97.4)a | 20 (51.3) | 7 (17.9) |
| Differentiation | |||||
| High | 23 (35.4) | 16 (69.6) | 20 (86.9) | 13 (56.5) | 6 (26.1) |
| M./low | 42 (64.6) | 34 (81.0) | 38 (90.5) | 15 (36.7) | 7 (16.7) |
| Treatment | |||||
| Surgery only | 45 (63.1) | 32 (71.1)a | 43 (95.5)a | 24 (53.3)a | 12 (26.7)a |
| C/X/CX/CXS | 20 (36.9) | 18 (90.0) | 15 (75.0) | 4 (20.0) | 1 (5.0) |
| Smoking | |||||
| Ever | 51 (78.5) | 44 (88.0) | 49 (96.1) | 16 (31.4) | 5 (9.8) |
| Never | 14 (21.5) | 6 (42.9)a | 9 (64.3)a | 12 (85.7)a | 8 (57.1)a |
| Alcohol | |||||
| Ever | 55 (84.6) | 42 (76.4) | 50 (90.9) | 23 (41.8) | 10 (18.2) |
| Never | 10 (15.4) | 8 (80.0) | 8 (80.0) | 5 (50.0) | 3 (30.0) |
P < 0.05: significant for either MMP and P14ARF protein or mRNA expressions between then comparison groups for the factors.
LNM: lymph node metastasis; M: moderate; C: chemotherapy; X: radiation; S: surgery.
Association of MMP11 and P14ARF expression with survival in LSCC
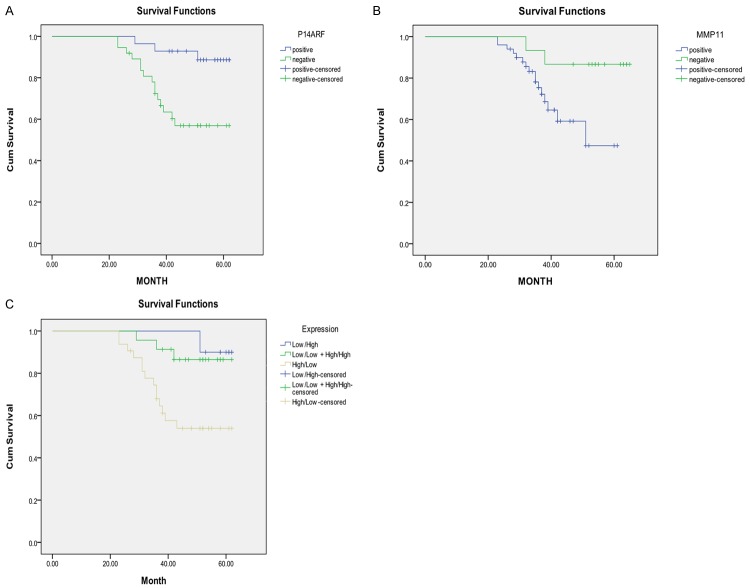
Figure 2 shows the univariate Kaplan-Meier analyses of survival with respect to the death from all causes. Among the 65 patients, 45 had surgery only and 20 received chemotherapy, radiation or their combination (Table 4). At a median follow-up time of 67 months (range, 4-67 months), 15 deaths occurred from any causes. There were 10 deaths from 45 patients with surgery only and 5 deaths from 20 patients with chemoradiotherapy or in combination. The patients with low MPP11 and high P14ARF expression had a significantly better overall survival than patients with high MPP11 and low P14ARF expression (Log-rank: P = 0.035 for MMP11 and P = 0.005 for P14ARF, Figure 2A, 2B); and patients with low and high expression of both MMP11 and P14ARF had a significantly better survival than patients with high and low expression of either of genes or any other co-expression status of both genes (P = 0.009, Figure 2C). Furthermore, the patients with surgery only had significantly better survival than those with chemoradiotherapy or their combination with surgery (log rank, P = 0.016) (Figure 3A). Among the patients with surgery only, the patients with low MMP11 and high P14ARF expressions had significantly better survival than those with high and low expression of either of genes or any other co-expression status of both genes (P = 0.006, Figure 3B). The multivariable Cox proportional hazards regression analysis regarding the association between expression of both genes and risk of overall death are shown in Table 5. Estimates of association were adjusted for potential confounders including age, gender, tumor differentiation, TNM stage, postoperative treatment, smoking and alcohol use, and Lymph node metastasis. Compared with patients having high and low expression of either of genes, the patients with low and high expression had significantly reduced risk of overall death (HR, 0.5; 95% CI, 0.2-0.8 for MMP11 and HR, 0.4; 95% CI, 0.2-0.7 for P14ARF). The reduced risk for overall death was pronounced for patients with low and high expression of both genes (HR, 0.2; 95% CI, 0.1-0.5) compared with any other co-expression status of both genes (Table 5), particularly for patients with surgery only (HR, 0.1; 95% CI, 0.0-0.9) (data not shown).
Figure 2.
Overall survival analysis of LSCC patients by expression of P14ARF, MMP11, and their Combination: (A) P14ARF; (B) MMP11; and (C) combined P14ARF and MMP11.
Figure 3.
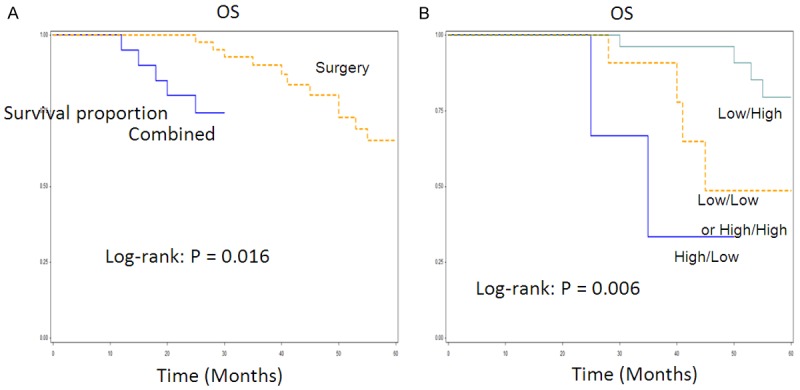
A. Overall survival analysis of LSCC patients by treatments among all 65 patients. B. Overall survival analysis of LSCC patients by expression of P14ARF in combination. With MMP11 among 45 patients with surgery only.
Table 5.
Multivariable survival analysis by expression of MMP11 and P14ARF alone or in combination in 65 LSCC patients
| Gene expression | Events/Total (15/65) | Survival (OS) | Survival (OS) |
|---|---|---|---|
|
|
|
||
| Crude HR, 95% CI | aHR, 95% CI | ||
| MMP11 | |||
| High | 13/50 | 1.0 | 1.0 |
| Low | 2/15 | 0.6 (0.3-0.9) | 0.5 (0.2-0.8) |
| P14ARF | |||
| Low | 12/37 | 1.0 | 1.0 |
| High | 3/28 | 0.4 (0.3-0.8) | 0.4 (0.2-0.7) |
| Combined MMP11 and P14ARF | |||
| High/Low | 5/12 | 1.0 | 1.0 |
| Low/Low + High/High | 6/25 | 0.5 (0.2-0.9) | 0.6 (0.3-1.0) |
| Low/High | 4/28 | 0.3 (0.2-0.7) | 0.2 (0.1-0.5) |
Adjusted for age, sex, smoking, alcohol, overall stage, differentiation, node metastasis, and treatment in Cox’s models.
Discussion
Few studies have comprehensively evaluated the role of MMP11 and P14ARF in LSCC. Our current study evidently shows that both MMP11 and P14ARF individually, or in combination, affect survival and might be served as prognostic biomarkers of LSCC patients, particularly for those with surgery only. There is increasing evidence that genetic differences are involved in the occurrence of cancer and are independent predictors of cancer outcome in many cancer types. Characterizing patients according to their difference could help in clinical management and enhance personalized therapy. In the current study, we explored the contribution of MMP11 and P14ARF expressions in LSCC, which we believe is a step toward personalized prevention and treatment of thyroid cancer. We did find that the combined expressions of both genes were significantly associated with survival and disease stage of patients with LSCC. Such findings could have implications for early detection, diagnosis, and treatment strategies and may help provide clinicians with additional information for personalized treatment, such as surgery only or surgery plus adjuvant treatment (chemotherapy/radiation).
The MMPs and play important roles in angiogenesis, tumor cell invasion and malignant cell proliferation [11-16]. Normal MMP11 expression has been shown to be involved in tissue remodeling during embryogenesis, tissue involution, wound healing and metamorphosis [17,18]. Recent studies have shown that the expression level of MMP11 was elevated in several cancers, including lung, colorectal, and ovariancarcinomas [19-21]. MMP11 expression has also been observed in HNSCC [22,23]. The tumor-suppressor P14ARF is a multi-functional gene which is involved in a lot of physic processes, such as cell cycle and apoptosis [8,24-26]. To date, whether the expression level of P14ARF during tumor progression is increased or decreased is still controversial. Zhang Y et al. demonstrated that P14ARF promoter genetic polymorphisms, which may affect expression of P14ARF, were associated with the susceptibility to second primary malignancy in patients with index squamous cell carcinoma of the head and neck [27]. Some studies suggest that P14ARF is widely down-regulated in several solid tumors, including breast, urinary bladder, pancreatic and esophageal carcinomas, as well as gliomas [28,29]. On the contrary, others indicate that that P14ARF expression level was up-regulated in some tumors [30].
Our study illustrated that MMP11 was over-expressed while the expression level of P14ARF was decreased in LSCC in both mRNA and protein level. MMP11 was correlated with the tumor stage, lymph node metastasis of LSCC patients while P14ARF was associated with tumor stage. For MMP11, its expression was much higher in patients with deeper tumor stage and lymph node metastasis, suggesting that MMP11 was strongly associated with tumor malignance. For P14ARF, the expression was decreased and negatively correlated with tumor stage, which is in agreement with the investigation in HNSCC [31].The possible mechanisms that cause altered expression could be due to its promoter hypermethylation, genomic loss, and epigenetic repression [32,33]. In addition, both MMP11 and P14ARF had a significant relationship with tobacco smoking, patients with ever tobacco smoking resulted in higher MMP11 expression as well as lower P14ARF expression than those who were never smoking, suggesting that tobacco smoking is another influence factor which affect the abnormal expression of MMP11 and P14ARF in LSCC.
Moreover, our data showed that patients with high-level MMP11 expression had significantly lower survival compared with those with low levels of MMP11. Several studies have demonstrated that over-expression of MMP-11 is related to a lower survival among patients with human breast cancer and non-small cell lung cancer [34,35]. The function of MMP-11 in cellular is closely associated with decreasing cancer cells death through necrosis and apoptosis during malignancy [36]. In contrast, patients with low-level of P14ARF had significantly shorter survival compared with those with low expression levels. One study found similar result in hepatocellular carcinoma [37]. These results thus might indicate that both MMP11 and P14ARF might individually or jointly affect the prognosis of LSCC.
Interestingly, in this study we found that there exists a negative correlation between the expression levels of MMP11 and P14ARF. The potential mechanism may due to the fact that P14ARF may inhibit angiogenesis by down-regulating the expression of VEGF [38,39], while the knockdown of MMP11 inhibited the proliferative activities and invasive potential of SGC-7901 GAC cells with decreased expression of VEGF [40]. Therefore, more studies are needed to further investigations to understand the relationship between these two genes.
In conclusion, over-expressed MMP11 and down-regulated P14ARF in LSCC were correlated with malignancy and prognosis of LSCC. Moreover, patients with low MMP11 and high P14ARF expressions had better survival and reduced risk of overall deaths, particularly for LSCC patients with surgery only. In addition, we found a negative correlation between the expression levels of MMP11 and P14ARF. Therefore, our results may provide evidence that both MMP11 and P14ARF may contribute to development, progression and prognosis of LSCC. However, further larger studies are required for validation of our findings and an exploration of the molecular mechanisms underlying the observed associations.
Acknowledgements
The authors thank all clinical staff from the department for their help with subject recruitment for this study and laboratory personnel for laboratory management. This work was sup-ported by the grants from the National Natural Science Foundation of China (grant number: 81302374 and 81241084), Beijing Natural Science Foundation (grant numbers: 7121005 and 5122016), and Beijing Nova Program (grant number: xx2013043), Capital Development Fund for medical research (grant numbers: 2011-2005-06).
Disclosure of conflict of interest
None.
Abbreviations
- LSCC
laryngeal squamous cell carcinoma
- HNSCC
head and neck squamous cell carcinomas
- MMPs
matrix metalloproteinases
- RT-PCR
reverse transcription polymerase chain reaction
- HR
hazard ratio
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, Song F, He M, Li H, Qian B, Zhang W, Wei Q, Hao X. Trends in head and neck cancer incidence in Tianjin, China, between 1981 and 2002. Head Neck. 2009;31:175–182. doi: 10.1002/hed.20946. [DOI] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 5.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- 6.Wei L, Shi YB. Matrix metalloproteinase stromelysin-3 in development and pathogenesis. Histol Histopathol. 2005;20:177–185. doi: 10.14670/HH-20.177. [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 8.Ivanchuk SM, Mondal S, Dirks PB, Rutka JT. The INK4A/ARF locus: role in cell cycle control and apoptosis and implications for glioma growth. J Neurooncol. 2001;51:219–229. doi: 10.1023/a:1010632309113. [DOI] [PubMed] [Google Scholar]
- 9.Pimkina J, Humbey O, Zilfou JT, Jarnik M, Murphy ME. ARF induces autophagy by virtue of interaction with Bcl-xl. J Biol Chem. 2009;284:2803–2810. doi: 10.1074/jbc.M804705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerrouqi A, Pyrzynska B, Febbraio M, Brat DJ, Van Meir EG. P14ARF inhibits human glioblastoma-induced angiogenesis by upregulating the expression of TIMP3. J Clin Invest. 2012;122:1283–1295. doi: 10.1172/JCI38596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cikos S, Bukovská A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol. 2007;8:113. doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasi F, Stoppelli MP. Proteases and cancer invasion: from belief to certainty. AACR meeting on proteases and protease inhibitors in cancer, Nyborg, Denmark, 14-18 June 1998. Biochim Biophys Acta. 1999;1423:R35–44. doi: 10.1016/s0304-419x(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 13.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–1630. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 14.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 15.Mañes S, Mira E, del Mar Barbacid M. Identification of insulin-like growth factor-binding protein-1 as a potential physiological substrate for human stromelysin-3. J Biol Chem. 1997;272:25706–25712. doi: 10.1074/jbc.272.41.25706. [DOI] [PubMed] [Google Scholar]
- 16.Murphy G, Segain JP, O’Shea M. The 28-kDa N-terminal domain of mouse stromelysin-3 has the general properties of a weak metalloproteinase. J Biol Chem. 1993;268:15435–15441. [PubMed] [Google Scholar]
- 17.Wyke JA. Overview-burgeoning promise in metastasis research. Eur J Cancer. 2000;36:1589–1594. doi: 10.1016/s0959-8049(00)00182-9. [DOI] [PubMed] [Google Scholar]
- 18.Rio MC. Stromelysin-3, a particular member of the matrix metalloproteinase family/Proteases and Their Inhibitors in Cancer Metastasis. Springer Netherlands. 2002;6:81–107. [Google Scholar]
- 19.Anderson IC, Sugarbaker DJ, Ganju RK. Stromelysin-3 is overexpressed by stromal elements in primary non-small cell lung cancers and regulated by retinoic acid in pulmonary fibroblasts. Cancer Res. 1995;55:4120–4126. [PubMed] [Google Scholar]
- 20.Porte H, Chastre E, Prevot S. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer. 1995;64:70–75. doi: 10.1002/ijc.2910640114. [DOI] [PubMed] [Google Scholar]
- 21.Mueller J, Brebeck B, Schmalfeldt B, Kuhn W, Graeff H, Höfler H. Stromelysin-3 expression in invasive ovarian carcinomas and tumours of low malignant potential. Virchows Archiv. 2000;437:618–624. doi: 10.1007/s004280000261. [DOI] [PubMed] [Google Scholar]
- 22.Birkedal-Hansen B, Pavelic ZP, Gluckman JL, Stambrook P, Li YQ, Stetler-Stevenson WG. Oral and Maxillofacial Pathology: MMP and TIMP gene expression in head and neck squamous cell carcinomas and adjacent tissues. Oral Dis. 2000;6:376–382. doi: 10.1111/j.1601-0825.2000.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 23.Soni S, Mathur M, Shukla NK, Deo SV, Ralhan R. Stromelysin-3 expression is an early event in human oral tumorigenesis. Int J Cancer. 2003;107:309–16. doi: 10.1002/ijc.11366. [DOI] [PubMed] [Google Scholar]
- 24.Duro D, Bernard O, Della Valle V, Berger R, Larsen CJ. A new type of p16INK4/MTS1 gene transcript expressed in B-cell malignancies. Oncogene. 1995;11:21–29. [PubMed] [Google Scholar]
- 25.Budina-Kolomets A, Hontz RD, Pimkina J, Murphy ME. A conserved domain in exon 2 coding for the human and murine ARF tumor suppressor protein is required for autophagy induction. Autophagy. 2013;9:1553–1565. doi: 10.4161/auto.25831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balaburski GM, Hontz RD, Murphy ME. p53 and ARF: unexpected players in autophagy. Trends Cell Biol. 2010;20:363–9. doi: 10.1016/j.tcb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Sturgis EM, Zafereo ME, Wei Q, Li G. p14ARF genetic polymorphisms and susceptibility to second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Cancer. 2011;117:1227–1235. doi: 10.1002/cncr.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumors. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 29.Forbes S, Clements J, Dawson E, Bamford S, Webb T, Dogan A, Flanagan A, Teague J, Wooster R, Futreal PA, Stratton MR. COSMIC 2005. Br J Cancer. 2006;94:318–322. doi: 10.1038/sj.bjc.6602928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai P, Xiao X, Zou J, Cui L, Bui Nguyen TM, Liu J, Xiao J, Chang B, Wu J, Wang H. Expression of p14ARF, p15INK4b, p16INK4a and skp2 increases during esophageal squamous cell cancer progression. Exp Ther Med. 2012;3:1026–1032. doi: 10.3892/etm.2012.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poi MJ, Knobloch TJ, Sears MT, Warner BM, Uhrig LK, Weghorst CM, Li J. Alterations in RDINK4/ARF-mediated en bloc regulation of the INK4-ARF locus in human squamous cell carcinoma of the head and neck. Mol Carcinog. 2015;54:532–42. doi: 10.1002/mc.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida E, Nakamura M, Ikuta M, Shimada K, Matsuyoshi S, Kirita T, Konishi N. Promoter hypermethylation of p14ARF is a key alteration for progression of oral squamous cell carcinoma. Oral Oncol. 2005;41:614–622. doi: 10.1016/j.oraloncology.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Dreidax D, Gogolin S, Schroeder C, Muth D, Brueckner LM, Hess EM, Zapatka M, Theißen J, Fischer M, Ehemann V, Schwab M, Savelyeva L, Westermann F. Low p14ARF expression in neuroblastoma cells is associated with repressed histone mark status, and enforced expression induces growth arrest and apoptosis. Hum Mol Genet. 2013;22:1735–1745. doi: 10.1093/hmg/ddt020. [DOI] [PubMed] [Google Scholar]
- 34.Cheng CW, Yu JC, Wang HW, Huang CS, Shieh JC, Fu YP, Chang CW, Wu PE, Shen CY. The clinical implications of MMP-11 and CK-20 expression in human breast cancer. Clin Chim Acta. 2010;411:234–41. doi: 10.1016/j.cca.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Tetu B, Trudel D, Wang CS. [Proteases by reactive stromal cells in cancer: an attractive therapeutic target] . Bull Cancer. 2006;93:944–948. [PubMed] [Google Scholar]
- 36.Boulay A, Masson R, Chenard MP, El Fahime M, Cassard L, Bellocq JP, Sautès-Fridman C, Basset P, Rio MC. High cancer cell death in syngeneic tumors developed in host mice deficient for the stromelysin-3 matrix metalloproteinase. Cancer Res. 2001;61:2189–2193. [PubMed] [Google Scholar]
- 37.Aigelsreiter A, Ress AL, Bettermann K, Schauer S, Koller K, Eisner F, Kiesslich T, Stojakovic T, Samonigg H, Kornprat P, Lackner C, Haybaeck J, Pichler M. Low expression of the putative tumour suppressor spinophilin is associated with higher proliferative activity and poor prognosis in patients with hepatocellular carcinoma. Br J Cancer. 2013;108:1830–1837. doi: 10.1038/bjc.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herranz S, Través PG, Luque A, Hortelano S. Role of the tumor suppressor ARF in macrophage polarization. Oncoimmunology. 2012;1:1227–1238. doi: 10.4161/onci.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin AC, Thornton JD, Liu J, Wang X, Zuo J, Jablonski MM, Chaum E, Zindy F, Skapek SX. Pathogenesis of persistent hyperplastic primary vitreous in mice lacking the arf tumor suppressor gene. Invest Ophthalmol Vis Sci. 2004;45:3387–3396. doi: 10.1167/iovs.04-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kou YB, Zhang SY, Zhao BL, Ding R, Liu H, Li S. Knockdown of MMP11 inhibitsproliferation and invasion of gastric cancer cells. Int J Immunopathol Pharmacol. 2013;26:361–370. doi: 10.1177/039463201302600209. [DOI] [PubMed] [Google Scholar]