Abstract
Chitosan-derived biomaterials have been reported to adhere when in contact with blood by encouraging platelets to adhere, activate and aggregate at the sites of vascular injury, thus enhanced wound healing capacity. This study investigated platelet morphology changes and the expression level of transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor-AB (PDGF-AB) in the adherence of two different types of chitosans in von Willebrand disease (vWD): N,O-carboxymethylchitosan (NO-CMC) and oligo-chitosan (O-C). Fourteen vWD voluntary subjects were recruited, and they provided written informed consent. Scanning electron microscopy and enzyme-linked immunosorbent assay test procedures were employed to achieve the objective of the study. The results suggest that the O-C group showed dramatic changes in the platelet’s behaviors. Platelets extended filopodia and generated lamellipodia, leading to the formation of grape-like shaped aggregation. The platelet aggregation occurred depending on the severity of vWD. O-C was bound to platelets on approximately 90% of the surface membrane in vWD type 1; there was 70% and 50% coverage in vWD type II and III, respectively. The O-C chitosan group showed an elevated expression level of TGF-β1 and PDGF-AB. This finding suggests that O-C stimulates these mediators from the activated platelets to the early stage of restoring the damaged cells and tissues. This study demonstrated that the greater expression level of O-C assists in mediating the cytokine complex networks of TGF-β1 and PDGF-AB and induces platelet activities towards wound healing in vWD. With a better understanding of chitosan’s mechanisms of action, researchers are able to accurately develop novel therapies to prevent hemorrhage.
Keywords: Wound healing, platelet morphology, growth factors, von Willebrand disease, chitosan
Introduction
Wound healing is a complicated process of replacing missing cells and tissue structures to restore skin integrity. Wound healing phases are characterized as non-linear due to the progression of both back and forward steps in the restoration process. Wound healing depends on the intrinsic and extrinsic levels of wound injury [1]. Although the wound healing phases comprise 3 different phases (inflammatory, proliferation and maturation), the degree of healing is mainly regulated by cytokines and growth factors. Cytokines are a unique family of growth factors that are released from lymphocytes known as lymphokines. Cytokines are produced by both hematopoietic and non-hematopoietic cells and are potentially used for cellular communications. Additionally, growth factors are proteins that are able to bind to the receptor on the surface of the cells to activate cellular proliferation and differentiation. Two different crucial growth factors, namely transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor-AB (PDGF-AB), have been identified to promote wound healing [2]. TGF-β molecules are highly pleiotropic cytokines that regulate immune responses, cell growth, proliferation and epithelial-mesenchymal transition [2,3]. The main role of TGF-β1 is to assist the wound healing process [4]. PDGF is composed of a heterodimer, which is composed of 2 disulfide-linked polypeptide chains assigned as A and B [5-7]. PDGF can regulate growth factors and cell division. This recombinant PDGF-AB plays a crucial role in the angiogenesis process, which is required in wound healing [8-10]. Von Willebrand disease (vWD) is a genetic disorder that alters the hemostasis process, leading to excessive bleeding after an injury. This disease is caused by the insufficiency or defect in a blood clotting protein known as von Willebrand factor (vWF), which intermediates the initial adherence of platelets by tethering and stabilizing factor VIII (FVIII). Hence, a deficiency of vWF can lead to abnormal hemorrhage by reducing the concentration level of FVIII [11]. The complication of hemorrhage in vWD can lead to delayed wound healing. Although many therapies have already been implemented to control excessive bleeding in persons who have vWD, the use of topical hemostatic dressings is integral to promote hemostasis, as it controls the bleeding and seals the wound. Among the various hemostatic agents used in medical and surgical interventions, chitosan-formulated hemostatic dressings have been shown to be most adherent when in contact with blood by encouraging platelets to adhere, activate and aggregate at the site of vascular injury [12,13]. Chitosan is a naturally obtained biomaterial that is derived from the deacetylation form of chitin and is composed of β (1→4)-linked 2-acetamido-2-deoxy-β-D-glucose (N-acetylglu-cosamine). Chitosan-based hemostatic agents are promising marine polysaccharides that can potentially assist in triggering hemostasis and wound healing by improving cell adhesion, proliferation and differentiation in vitro [12-16]. Chitosan has been shown to elevate the release of TGF-β1 and PDGF-AB [16,17]. Additionally, chitosan formulated hemostatic dressings have been widely studied in biomedical research and been shown to accelerate coagulation and growth factor release in wound healing. This study was constructed to evaluate the potential of two different types of chitosans in elevating growth factor release by supporting platelet adherence in vWD in vitro. Although extensive research has been undertaken to elucidate the importance of chitosan biomaterials in various fields, to the best of our understanding, no research has been conducted to assist wound healing in vWD patients with vascular injury or hemorrhage. Therefore, the current approach was believed to elucidate the influence of chitosan in expediting the wound healing process in vWD patients by examining platelet adhesion and cytokine mediator (TGF-β1 and PDGF-AB) abilities.
Materials and methods
Materials
N,O-carboxymethylchitosan (NO-CMC) and oligo-chitosan (O-C) were produced by Standard and Industrial Research Institute of Malaysia (SIRIM Berhad), with a degree of deacetylation (DDA) of 75-95%. Chitosan sponges with variable chitosan formulations [7% NO-CMC with 0.45 mL collagen, 8% NO-CMC, O-C and a powdered type of chitosan termed O-C 53] were used. Lyostypt was used as the positive control.
Subject selection
Fourteen vWD patients aged 18 to 50 years who had not consumed any drugs in the 2 weeks prior to recruitment participated in the study. Informed written consent was obtained prior to blood collection. None of the women were taking oral contraceptives when blood samples were obtained. Ethical clearance was granted by the Medical Research & Ethics Committee at the Malaysian National Medical Research Registry (Ref Num.: NMRR-13-873-17276). All laboratory experiments were performed in duplicate. The participants were interviewed, and the consent forms were completed by physicians. The participants were required to sign both bilingual consent forms which were written in Malay and English, and they were fully informed about the procedures and the risks that were involved in this research. The researchers also ensured that the participants were not at risk of harm as a result of their participation.
Blood collection
Twelve mL of blood was withdrawn from antecubital veins and collected in vials containing ethylenediaminetetraacetic acid (EDTA) and citrated blood tubes. Blood samples were obtained from the vWD patients (n=14). The blood samples were collected in BD Vacutainer [K2 EDTA 3.6 mg (REF 367842)] tubes. Subject selection was contingent on a hematocrit level between 38% and 45% and a normal platelet count between 150 × 103/μL and 350 × 103/μL [12,13,16].
Platelet adhesion study
Each chitosan biomaterial measuring 5 mm × 5 mm was placed in a 12-well tissue culture plate. Platelets were isolated by differential centrifugation with 150 times gravity (× g) for 15 minutes (min) and 900 × g for 5 min at room temperature. Five hundred mL of isolated platelets was combined with each chitosan sample and incubated for 30 min in 12-well tissue culture plates. Each well was then washed with penicillin-infused phosphate-buffered saline (PBS) for 1 hour (hr), fixed in 100 µL of glutaraldehyde for 1 hr, and then washed with distilled water. Various concentrations of ethanol (30%, 70% and 100%) were added to dehydrate the chitosan biomaterials. Finally, all the samples were dried in an incubator (58°C) overnight. Chitosan biomaterials were sputter-coated with gold using a gold sputter coater (Leica SCD 005, Germany) with a vacuum millibar of 5 × 10-2 [Current: 20 milliampere; Timer: 150 seconds] after the biomaterials were completely dried. The gold sputtered chitosan biomaterials were examined with scanning electron microscope (SEM) (FEI-QUANTA FEG 450, Netherlands), in which their surface and cross-section platelet morphology were examined [12,17-20].
Expression of TGF-β1 & PDGF-AB in vWD patients
The levels of TGF-β1 and PDGF-AB in platelet-rich plasma (PRP) upon adhesion to chitosans were measured using enzyme-linked immunosorbent assay (ELISA) kits. These growth factor tests were performed using 2 different ELISA kits with distinct types of assay procedures. Blood samples were collected as mentioned above. Blood samples were centrifuged at 1000 × g for 15 min. Supernatants were harvested after centrifugation. Chitosan samples, each weighing 5 mg, were dissolved or pre-moistened in 50 µL of (PBS; pH 7.4) and subjected to incubation at 37°C for 60 min. Five hundred µL of PRP was then mixed with each prepared chitosan sample for 30 min [12,13,16,17,21,22]. An ELISA kit was used to detect TGF-β1 (Cat. No. CSB-E04725h) and PDGF-AB (Cat. No. CSB-E04701h) (Cusabio Biotech Co., LTD, China) expression levels in vWD patients after the adherence of chitosan-derived biomaterials according to the manufacturer’s instructions.
Assay procedures
One-hundred µL of prepared standard and samples were loaded on each well, and the plate was covered using adhesive strips. The sample-loaded plate was incubated for 2 hr at 37°C. One-hundred µL of Biotin-Ab (1 ×) were loaded after the standards and samples were completely removed from each well. The plate was incubated for 1 hr at 37°C. Followed by the Biotin-Ab (1 ×) addition, each well was aspirated and washed with washing buffer (200 µL) 2 times for a total of 3 washes using a multichannel pipette every 2 min. One-hundred µL of Biotin-Av (1 ×) were added to each well, and the plate was incubated again for 1 hr at 37°C.Aspiration or washing procedures were repeated at least 5 times as described previously. After the final washing was completed, 90 µL of TMB substrate was added to each well, and the plate was protected from light exposure and incubated for 15 to 30 min. As a final solution to the plate, 50 µL of stop solution was added to the entire well, and the plate was gently tapped to ensure thorough mixing. Eventually, all the reactions were stopped, and the absorbance was determined at 450 nanometers (nm) utilizing an ELISA reader (Tecan Infinite 200 PRO NanoQuant, Switzerland). A standard curve was generated, and the concentration of each sample was determined in ng/mL. Protein expression was calculated based on the volume of supernatant obtained after clot retraction.
Quantification procedures
The Stock solutions were used to produce a 2-fold dilution series as follows for TGF-β1 (50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0 ng/mL) and PDGF-AB (2000, 1000, 500, 250, 125, 62.5, 31.25, 0 pg/mL). The undiluted standard served as the high concentration of standard, and the sample diluent served as the zero standard. Eventually, all the reactions were stopped, and the absorbance was determined at 450 nanometers (nm) utilizing an ELISA reader (Tecan Infinite 200 PRO NanoQuant, Switzerland). A standard curve was generated, and the concentration of each sample was determined in ng/mL. The protein expression was calculated based on the volume of supernatant obtained after clot retraction. The standard curve was generated by plotting the absorbance for each standard on the y-axis against its concentration on the x-axis, and a 4-parametric logistic (4-PL) curve-fit was plotted at all the data points [23]. No significant cross-reactivity or interference was observed among all the measurement levels. Experimental outcomes are shown as the mean ± standard error of means (S.E.M). Statistical significance was defined as P ≤ 0.05, and these values were calculated using Statistical Package for the Social Sciences (SPSS) software, version 20.0.
Results
Platelet adhesion
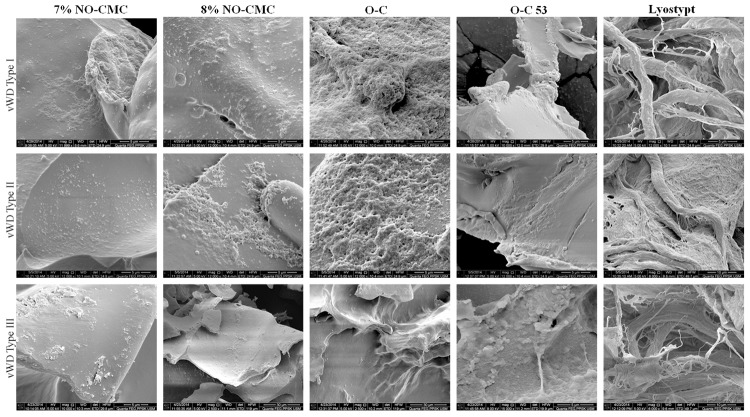
vWD patients were categorized based on the type of severity (types I, II and III). The chitosan-adhered blood samples were analyzed using SEM. SEM was used to analyze the surface morphology of platelet behavior because it utilizes a high energy level of electrons to generate signals to read the surface membrane of biomaterials. Figure 1 shows the platelet shape changes at various magnifications (2500 ×, 6000 ×, 8000 ×, 10000 ×, 12000 × and 15000 ×) with diameter ranges of 5 µM, 10 µM and 30 µM. All tested biomaterials were able to alter the platelet morphology depending on the scaffold characteristics and chemical compositions.
Figure 1.
Platelet morphology after the adherences of NO-CMC, O-C and lyostypt according to the severity of vWD. Observations at various magnifications (2500 ×, 6000 ×, 8000 ×, 10000 ×, 12000 × and 15000 ×) with diameter ranges of 5 µM, 10 µM and 30 µM.
The results suggest that the O-C chitosan group showed dramatic changes in platelet behavior. Platelets extended filopodia and generated lamellipodia, leading to the formation of aggregation in grape-like shapes. The platelet aggregation depended on the severity of the vWD. O-C was bound to platelets on approximately 90% of the surface membrane in vWD type 1 patients; there was 70% and 50% coverage in vWD type II and III patients, respectively. The platelets were observed to be more spherical and swollen when they were in contact with O-C. Additionally, 7% and 8% NO-CMC showed mild platelet groupings by covering approximately 30-40% of the chitosan surface membrane. Lyostypt, which was composed of extremely flexible networks, permitted platelets to form a fibrin web. Because the rolling of platelets with the support of vWF was found more in vWD type I patients, the thread-like layers became thicker compared to those of vWD type II and III patients. Nevertheless, platelet morphological changes under the influence of chitosan biomaterials, particularly their physiological and mechanical relevance, remain unknown.
Expression of TGF-β1
TGF-β1 was continuously expressed at moderate level in the adherence to various forms of chitosans. The reference range for the TGF-β1 expression is 31.2 pg/mL-2000 pg/mL. O-C recorded the highest release with 49.4 ± 8.29 pg/mL. The 8% NO-CMC showed only slight changes in the mean expression value of TGF-β1 with 48.1 ± 7.15 pg/mL. The tested groups were compared with blood alone to elucidate the significant values. All the examined biomaterials indicated promising increases similar to the blood alone. The expression levels noted in vWD patients were elevated as well: O-C 53 (12.3%), 7% NO-CMC (11.3%) and lyostypt (3.3%). No significant differences were noted in the TGF-β1 expression in vWD patients (Figure 2).
Figure 2.
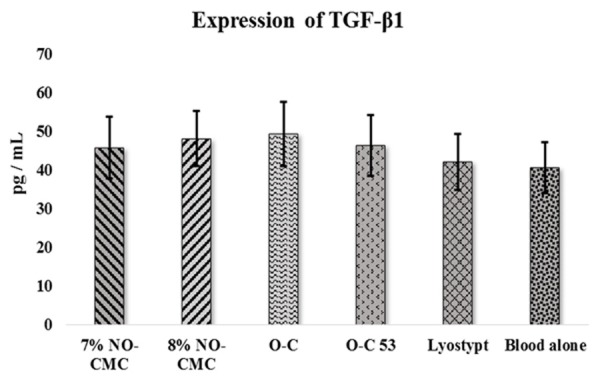
Mean expression of TGF-β1 in vWD patients after the adherence of chitosan biomaterial. The error bars represent the S.E.M.
Expression of PDGF-AB
According to the manufacturer’s manual, the PDGF-AB quantification detection range is 31.2 pg/mL-2000 pg/mL. O-C and lyostypt showed an elevated release level of PDGF-AB by 29.8% and 23.8%, respectively, in vWD patients. No significant differences were noted within the tested group of biomaterials compared to the blood alone (Figure 3). In contrast, 8% NO-CMC recorded a decreased level of PDGF-AB release with 1435.17 ± 237.41 pg/mL, which is a 7.6% decrease that is similar to the blood alone group. Except for the 8% NO-CMC, all the other remaining chitosan biomaterials demonstrated increased levels of PDGF-AB. Followed by O-C and lyostypt, the 7% NO-CMC and O-C 53 groups showed increased release of PDGF-AB by 15.2% and 11.0%, respectively (Figure 3).
Figure 3.
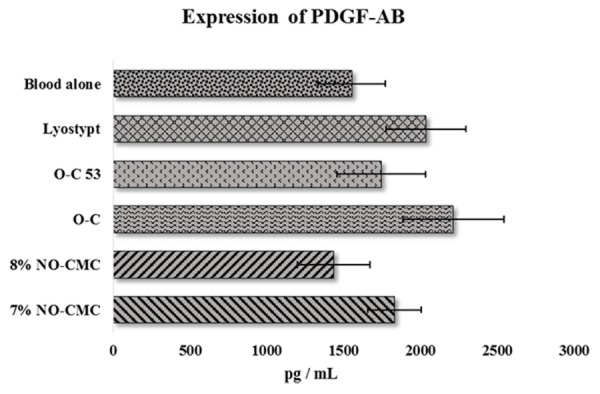
Mean expression of PDGF-AB in vWD patients after the adherence of chitosan biomaterial. Error bars represent the S.E.M.
Discussion
vWD is an inherited bleeding disorder that affects blood coagulation ability. The National Blood Center (PDN, Malaysia) reported that vWD is an uncommon hemostatic disorder in Malaysia with a rate of 0.002% among 30 million individuals in Malaysia (unpublished source). This clinically heterogeneous hemorrhagic disorder arises from a deficiency resulting from defective vWF, which interrupts the platelet signals in blood coagulation. vWF is a blood glycoprotein that serves as sticky glue or an adhesive protein, which can be bound to other proteins to form a platelet plug [24-26]. Although chitosan plays a significant role in recruiting more platelets at the damaged area, we also found that the adhesive protein vWF plays a crucial role in assisting platelets to clump together. The reason for focusing on vWD is because it is a disease resulting in abnormalities in blood coagulation ability in which the platelets require a prolonged period to achieve hemostasis. vWD is always associated with delayed wound healing.
Wound healing is the final process that follows hemostasis after tissue injury in all individuals. Wound healing is becoming a target of research, and the development of new biomaterials is gaining substantial interest. Chitosan biomaterial is an attractive candidate in promoting wound healing because it recruits more platelets at the sites of vascular injury. C. Rouget and G. Bizzozero were the first successful scientists who investigated and described chitosans and platelets in 1859 and 1881 [27-31]. This unique feature offered by chitosan will lead to fabricating improved hemostatic agents. Because growth factors act as important mediators in the wound healing process, the expression level of 2 different important growth factors (TGF-β1 and PDGF-AB) were evaluated after the adherence of chitosan biomaterials in this study by examining vWD patients’ blood samples in vitro. The expression levels of TGF-β1 and PDGF-AB were analyzed due to their crucial function as biochemical intermediators that are involved in wound healing [16]. It was previously reported that chitosan-derived biomaterials were able to assist platelet adherence and aggregation [12,13].
Generally, vWD is divided into 3 types. vWD type I is the most common and mildest compared to vWD type II and III, which comprise < 70% cases in Malaysia. A total of 572 vWD cases were registered in Malaysia from 1979-2013 (unpublished source). In this study, among the 14 vWD patients, 11 subjects had vWD type 1, one person had type II, and remaining patients had vWD type III. We discovered more platelets rolling in vWD type I after the chitosan adherence compared those in the other types of vWD. This finding may be because the platelet-binding capacity of vWF was higher compared to that in the other types of vWD. The biophysical mechanism of vWF in this type demonstrated a modestly decreased vWF level, which is always associated with mild bleeding after injury [26,32].
The vWF levels vary depending on the 3 vWD types. Type I (common) shows reduced levels of vWF, which is always associated with mild bleeding; Type II (uncommon with variable pattern) presents with abnormal structure and function of vWF; Type III is the rarest type in which there is a limited amount or total absence of vWF [26]. Based on the results of the platelet morphology with the various formulations of chitosan derivatives, the platelets showed remarkable changes in forming aggregations. In the platelet clumps, O-C covered 60-80% of the chitosan membrane in vWD type I and II. Because the tethering and rolling of the vWF is very limited in type III, the platelet adhesion capacity was found to be very weak to form aggregation. Overall, platelet morphology and platelet aggregation demonstrated modifications of platelet shape after chitosan adherence. Platelets clumped into grape-like and swollen shapes, released granules and aggregated into pseudopodia shapes depending on the severity of the vWD. O-C is fully able to invite and encourage platelets to form platelet plugs to assist the primary hemostasis process. Generally, the platelet plug formation is only a temporary solution to cease a hemorrhage. Wound healing is another specific biological process that involves growth factors and tissue regeneration [33].
Chitosan was reported to enhance the tensile strength of wounds. Chitosan-derivatives accelerate the process of wound healing by stimulating crucial inflammatory cells, such as macrophages, fibroblasts, osteoblasts and polymorphonuclear leukocytes [33,34]. In this study, O-C was found to elevate the expression level of TGF-β1. This result suggests that O-C is able to stimulate TGF-β1 from the activated platelets and trigger the early stage of restoring the damaged cells and tissues.
In a recent in vivo study, Dexter et al. discovered an elevated expression of TGF-β1 at day 3 after application of a chitosan dressing. The expression level of TGF-β1 decreased at day 7, indicating that chitosan treatment will not lead to any scar formation. The authors recommended that chitosan hemostatic dressing might be beneficial in arresting scar formation [35]. The release of TGF-β1 from a tested chitosan gel biomaterial was shown to trigger oral mucosa healing [36].
This study’s finding were consistent with those of Okamoto et al. in which the authors also concluded that chitosan-derived biomaterials increased the expression level of TGF-β1 and PDGF-AB from platelets. Their study highlighted that the release reaction actually was contingent on the degree of membrane injury in the platelets. They stated that the thickness, molecular weight (MW) and DDA of the chitosan membrane plays an important role in damaging the platelets. Accordingly, they can recruit more mediators to assist platelet activities and wound healing processes [17]. A previous report revealed that solid-based chitosan biomaterials were able to absorb platelets to expedite the hemostasis process [20]. PDGF-AB plays a significant role in blood vessel formation by enhancing cell growth and division. We can state clearly that O-C bound platelets can initiate the release of PDGF-AB. Shen et al. also suggested that chitosan-derived biomaterials might be a suitable substitute for thrombin generation associated with the PRP preparation because TGF-β1 and PDGF-AB are released from the activated platelets after chitosan application [37].
In our earlier study, we reported that O-C induced the following platelet activation markers: P-selectin and Glycoprotein IIb/IIIa [16,38]. These proteins are activation markers that eventually follow the series of hemostasis processes that trigger wound healing mediators such as TGF-β1 and PDGF-AB. Consequently, both mediators’ outcomes raise many questions in regard to chitosan’s mechanism of action following its adhesion to blood cells. It is still not understood how and why chitosan biomaterials possibly affect the release of these studied factors, other than the potential influence of chitosan properties such as DDA, MW, biodegradability, temperature, pH level, viscosity and absorbability. According to the results, the NO-CMC chitosan group assists in the platelet shape changes and growth factor release but is not as promising as the O-C group of chitosans. Lyostypt was used as the positive control hemostatic agent to compare the potential differences between the tested biomaterials. Lyostypt is a commercially available hemostatic dressing because it is rich in a membrane that potentially provides an ideal framework for platelet adherence.
We also conducted a scaffold characterization study to examine the morphological and characteristic features of the O-C and NO-CMC groups, respectively. We have reported that the functional groups represented in O-C closely resemble the standard features of chitin. In the NO-CMC group of chitosan-derivatives, we noted an absence of various functional groups and chemical structures [39]. We believe that chitosan-based hemostatic agents could be helpful in hemostatic disorders, particularly vWD.
The most important limitation of this study was the small sample size. Because vWD is a rare disease in Malaysia, we only managed to recruit 14 subjects. In accordance with the disease, most of the patients had vWD type I, and only a few patients had vWD types II and III. Additionally, we also would like to emphasize that although a considerable amount of literature has been published on chitosan as a hemostatic agent, its mechanisms of action remain undetermined. Further research should be conducted to examine the exact MW, DDA, surface thickness, temperature, pH and tensile strength, which could elucidate the properties and mechanisms of action of NO-CMC and O-C. These factors could be the determinants of the enhanced hemostatic activity found in this study.
Conclusion
Chitosan is a versatile, naturally obtained polysaccharide that has numerous applications in the biomedical field due to its unique characteristics. Therefore, this study showed that the high expression level of O-C assists in mediating cytokine complex networks, particularly those of TGF-β1 and PDGF-AB. Additionally, we demonstrated chitosan’s ability to induce platelet activities towards wound healing in vWD. These results support the use of chitosan derivatives as a potential hemostatic agent for surgical and injury-induced wound healing in vWD patients. It is expected that in the future, with a better understanding of chitosan as a hemostatic agent, researchers will be able to better treat vWD and eventually develop novel therapies to prevent hemorrhage.
Acknowledgements
This project was funded by a USM Research Grant, numbered 1001/PPSP/813068. We would like to thank Miss Faridah MD Afandi and Madam Jamaliah Daud from PDN for helping us recruit vWD patients for this study. Additionally, we thank all the vWD patients who donated their blood. We would also like to express our gratitude to Director General of Health YBhg Datuk Dr. Noor Hisham bin Abdullah from the MOH for approving this paper to be published.
Disclosure of conflict of interest
None.
References
- 1.Hutchinson J. The wound programme. Centre for Medical Education: Dundee; 1992. [Google Scholar]
- 2.Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle) 2013;2:215–224. doi: 10.1089/wound.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendt MK, Allington TM, Schiemann WP. Mechanisms of epithelial-mesenchymal transition by TGF-β. Future Oncol. 2009;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derynck R, Jarrett J, Chen E, Eaton D, Bell J, Assoian R, Roberts A, Sporn M, Goeddel D. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985;316:701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- 5.Antoniades HN, Scher CD, Stiles CD. Purification of human platelet-derived Growth factor. Proc Natl Acad Sci U S A. 1979;76:1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldin CH, Westermark B, Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979;76:3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raines EW, Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982;257:5154–5160. [PubMed] [Google Scholar]
- 8.Betsholtz C, Johnsson A, Heldin CH, Westermark B, Lind P, Urdea MS, Eddy R, Shows TB, Philpott K, Mellor AI, knott TJ, Scott J. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986;320:695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- 9.Rao CD, Igarashi H, Chiu IM, Robbins CK, Aaronson SA. Structure and sequence of the human c-sis/platelet-derived growth factor 2 (sis/PDGF-2) ranscriptional unit. Proc Natl Acad Sci U S A. 1986;83:2392–2396. doi: 10.1073/pnas.83.8.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart CE, Bailey M, Curtis DA, Osborn S, Raines E, Ross R, Forstrom JW. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochem. 1990;29:166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- 11.Nichols WL, Hultin MB, James AH, Manco-johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 12.Periayah MH, Halim AS, Hussein AR, Mat Saad AZ, Abdul Rashid AH, Noorsal K. In vitro capacity of different grades of chitosan derivatives to induce platelet adhesion and aggregation. Int J Biol Macromol. 2013;52:244–249. doi: 10.1016/j.ijbiomac.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Periayah MH, Halim AS, Yaacob NS, Mat Saad AZ, Hussein AR, Rashid AHA, Ujang Z. In vitro comparative coagulation studies of novel biodegradable N, O-Carboxymethylchitosan (NO-CMC) and Oligo-Chitosan (O-C) Int J of Pharma Sci Res. 2014;5:4689–4698. [Google Scholar]
- 14.Lim CK, Halim AS. In Vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int J Mol Sci. 2009;10:1300–1313. doi: 10.3390/ijms10031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim CK, Yaacob NS, Zainol I, Halim AS. In vitro biocompatibility of chitosan porous skin regenerating templates (PSRTs) using primary human skin keratinocytes. Toxicol In Vitro. 2010;24:721–727. doi: 10.1016/j.tiv.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Periayah MH, Halim AS, Yaacob NS, Mat Saad AZ, Hussein AR, Abdul Rashid AH. Expression of P-selectin, TXA2, TGF-β1 and PDGF-AB in the presence of bioadhesive chitosan derivatives. Online Int Interdiscip Res J. 2014;4:5–14. [Google Scholar]
- 17.Okamoto Y, Yano R, Miyatake K, Tomohiro I, Shigemasa Y, Minami S. Effects of chitin and chitosan on blood coagulation. Carbohydr Polym. 2003;53:337–342. [Google Scholar]
- 18.Salas A. Separation of platelets from whole blood. Available from: http://springerlab.tch.harvard.edu/springer/uploads/Protocols/Separationof Platelets from Whole Blood. pdf. 2000. Accessed date 14 October 2014.
- 19.Maurer SE, Pfeiler G, Maurer N, Lindner H, Glatter O, Devine DV. Room temperature activates human blood platelets. Lab Invest. 2001;81:581–592. doi: 10.1038/labinvest.3780267. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Tian F, Wang Z, Wang Q, Zeng Y, Chen SQ. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res B Appl Biomater. 2007;84:131–137. doi: 10.1002/jbm.b.30853. [DOI] [PubMed] [Google Scholar]
- 21.Wagner WR, Pachence JM, Ristich J, Johnson PC. Comparative in vitro analysis of topical hemostatic agents. J Surg Res. 1996;66:100–108. doi: 10.1006/jsre.1996.0379. [DOI] [PubMed] [Google Scholar]
- 22.Zhou M, Yang JH, Ye X, Zheng AR, Li G, Yang PF, Zhu Y, Cai L. Blood platelet’s behavior on nanostructured superhydrophobic surface. J Nano Res. 2008;2:129–136. [Google Scholar]
- 23.Garbaraviciene J, Diehl S, Varwig D, Bylaite M, Ackermann H, Ludwig RJ, Boehncke WH. Platelet P-selectin reflects a state of cutaneous inflammation: Possible application to monitor treatment efficacy in psoriasis. Exp Derma. 2010;19:736–741. doi: 10.1111/j.1600-0625.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th edition. Saunders Elsevier; 2009. [Google Scholar]
- 25.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 26.Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB Working Party on von Willebrand Disease Classification. Update on the pathophysiology and classification of von Willebrand disease: A report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 27.Coller BS. Bizzozero and the discovery of the blood platelets. Lancet. 1984;1:804. doi: 10.1016/s0140-6736(84)91330-8. [DOI] [PubMed] [Google Scholar]
- 28.Muzzarelli RAA. Chitin. Oxford: Pergamon Press; 1977. [Google Scholar]
- 29.Muzzarelli RAA, Boudrant J, Meyer D, Manno N, DeMarchis M, Paoletti MG. A tribute to Henri Braconnot precursor of the carbohydrate polymers science on the chitin bicentennial. Carbohyd Polym. 2012;87:995–1012. [Google Scholar]
- 30.Paweletz N. From Galen to Golgi: Birth of the life sciences in Italy. Nat Rev Mol Cell Bio. 2001;2:475–480. doi: 10.1038/35073102. [DOI] [PubMed] [Google Scholar]
- 31.Ribatti D, Crivellato E. Giulio Bizzozero and the discovery of platelets. Leukemia Res. 2007;31:1339–1341. doi: 10.1016/j.leukres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Sadler JE. Von Willebrand disease type 1: A diagnosis in search of a disease. Blood. 2003;6:101. doi: 10.1182/blood-2002-09-2892. [DOI] [PubMed] [Google Scholar]
- 33.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9:857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degim Z, Celebi N, Sayan H, Babul A, Erdogan D, Take G. An investigation on skin wound healing in mice with a taurine chitosan gel formulation. Amino Acids. 2002;22:187–198. doi: 10.1007/s007260200007. [DOI] [PubMed] [Google Scholar]
- 35.Baxter RM, Dai T, Kimball J, Wang E, Hamblin MR, Wiesmann WP, McCarthy SJ, Baker SM. Chitosan dressing promotes healing in third degree burns in mice: Gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J Biomed Mater Res A. 2013;101:340–348. doi: 10.1002/jbm.a.34328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senel S, Kremer MJ, Kas S, Wertz PW, Hincal AA, Squier CA. Enhancing effect of chitosan on peptide drug delivery across buccal mucosa. Biomaterials. 2000;21:2067–2071. doi: 10.1016/s0142-9612(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 37.Shen EC, Chou TC, Gau CH, Tu HP, Chen YT, Fu E. Releasing growth factors from activated human platelets after chitosan stimulation: a possible biomaterial for platelet-rich plasma preparation. Clin Oral Impl Res. 2006;17:572–578. doi: 10.1111/j.1600-0501.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- 38.Periayah MH, Halim AS, Yaacob NS, Mat Saad AZ, Hussein AR, Rashid AHA, Ujang Z. Glycoprotein IIb/IIIa and P2Y12 induction by oligochitosan accelerates platelet aggregation. BioMed Res Int. 2014;2014:653149. doi: 10.1155/2014/653149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Periayah MH, Halim AS, Gomathysankar S, Sukari AAA, Saad AZM, Rashid AHA, Ujang Z, Muslim NZM. N,O-Carboxymethylchitosan (NO-CMC) and Oligo-Chitosan (O-C): Scaffold characterization. Int J of Basic Appl Sci. 2014;3:532–540. [Google Scholar]