Abstract
Vitamin E δ-tocotrienol has been reported to possess anticancer activity both in vitro and in vivo. However, the underlying molecular mechanisms of δ-tocotrienol induced apoptosis in triple-negative breast cancer are not fully understood. Here, we reported that microRNA-429 (miR-429) is up-regulated in two TNBC cell lines (MDA-MB-231 and MDA-MB-468), treated with δ-tocotrienol. Inhibition of miR-429 may partially rescue the apoptosis induced by δ-tocotrienol in MDA-MB-231 cells. We also showed that the forced expression of miR-429 was sufficient to lead to apoptosis in MDA-MB-231 cells. Furthermore, we identified X-linked inhibitor of apoptosis protein (XIAP) as one of miR-429’s target genes. These results suggest that the activation of miR-429 by δ-tocotrienol may be an effective approach for the prevention and treatment of triple-negative breast cancer.
Keywords: δ-Tocotrienol, triple-negative breast cancer (TNBC), microRNA-429 (miR-429), X-linked inhibitor of apoptosis protein (XIAP), apoptosis
Introduction
Breast cancer is one of the most prevalent cancers, with over 1.6 million cases diagnosed worldwide in 2010 [1]. The large number of etiological factors and the complexity of breast cancer pose challenges for prevention and treatment. Triple-negative breast cancer (TNBC) is histologically defined as an invasive carcinoma of the breast that lacks staining for estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor-2 (HER2). TNBC is associated with high proliferative rates, early recurrence, and poor survival rates [2]. Much effort has been spent on the study of the biological behavior of TNBC cells to develop effective treatment strategies.
Vitamin E tocotrienols, including α, β, γ and δ-tocotrienol, are bioactive components of cereal foods such as oats, barley and palm. Γ and δ-tocotrienol have demonstrated anticancer and chemo-preventive activities in various cancer systems, including breast [3], lung [4], colon [5], and hepatocellular cancers [6]. Recently, we have shown that δ-tocotrienol (Figure 1A) is one of the most bioactive tocotrienols against pancreatic cancer, both in vitro and in vivo [7]. However, the molecular mechanism of action of δ-tocotrienol in triple-negative cancer has not been fully elucidated.
Figure 1.
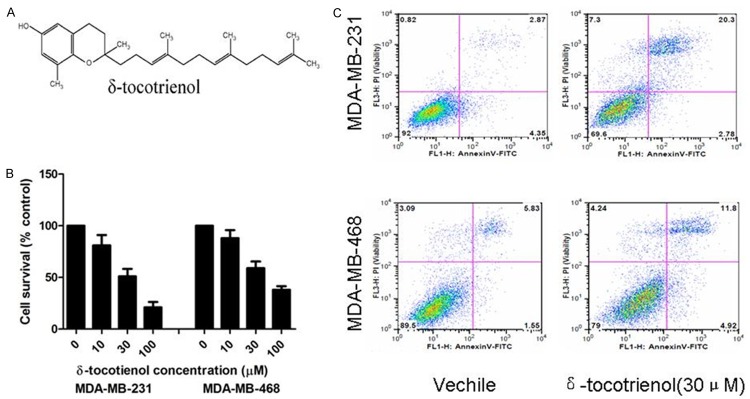
δ-tocotrienol inhibits cell proliferation and induces apoptosis in TNBC cells. A. Structure of δ-tocotrienol. B. The MTT assay was used to measure cell proliferation activity in MDA-MB-231 and MDA-MB-468 cells treated with δ-tocotrienol or vehicle. *P<0.05. C. Annexin V/PI assay was used to measure cell apoptosis in MDA-MB-231 and MDA-MB-468 cells treated with δ-tocotrienol or vehicle. *P<0.05.
MicroRNAs (miRNAs) are small, non-coding RNAs of 19-25 nucleotides in length that are endogenously expressed in mammalian cells. miRNAs post-transcriptionally regulate gene expression by pairing with complementary nucleotide sequences in the 3’-UTRs of specific target mRNAs [8,9]. miRNAs are involved in biological and pathological processes, including cell differentiation, proliferation, apoptosis, and metabolism [10-12].
miR-429, a member of the miR-200 family of microRNAs, was reported to inhibit the expression of transcriptional repressors ZEB1/δEF1 and SIP1/ZEB2 and regulate epithelial-mesenchymal transition [13]. It is significantly down-regulated in several cancers, including renal cell carcinoma [14] and gastric cancer [15]. Emerging evidence has shown that over-expression of miR-429 can inhibit proliferation and induce apoptosis in human osteosarcoma cancer cell lines [16]. However, the function of miR-429 in triple-negative breast cancer remains unclear.
In this paper, we report that miR-429 was up-regulated in TNBC cells treated with δ-tocotrienol. Inhibition of miR-429 may partially rescue the apoptosis induced by δ-tocotrienol in MDA-MB-231 cells. We also showed that the forced expression of miR-429 was sufficient to lead to apoptosis in MDA-MB-231 cells. Furthermore, we identified X-linked inhibitor of apoptosis protein (XIAP) as one of miR-429’s target genes.
Materials and methods
Reagents and antibodies
δ-Tocotrienols were kindly provided as a gift from Dr Malafa (H Lee Moffitt Cancer Center, FL, USA). XIAP and β-actin antibodies were purchased from Santa Cruz Biotechnology (San Diego, CA, USA). miR-429 precursor, miR-429 inhibitor and control miRNA were purchased from Applied Biosystems (Carlsbad, CA, USA). All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified.
Cell culture and treatment
Human triple-negative breast cancer cell lines MDA-MB-231 and MDA-MB-468 were purchased from the American Type Culture Collection (Manassas, VA, USA). MDA-MB-231 and MDA-MB-468 cells were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were collected using 0.05% trypsin EDTA following the specified incubation period.
Precursor miRNA or miRNA inhibitor transfection
Cells were seeded in 6-well plates at a concentration of 2×104 and cultured in medium without antibiotics for approximately 24 h before transfection. Cells were transiently transfected with miR-429 precursor or inhibitor and negative control miRNA at a final concentration of 50 nM (precursor) or 100 nM (inhibitor) using Lipofectamine 2000 (Invitrogen, Carslbad, CA, USA) according to the manufacturer’s protocol.
Cell viability assay
Cell viability was determined at 24 hours by a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltertrazolium bromide (MTT) assay. Cells (5×103 cells per well) were grown overnight in 96-well plates. Fresh medium (200 μL) with different doses of δ-tocotrienol or ethanol was added and incubated at 37°C at 5% CO2 for 24 hours. After 24 hours of incubation, MDA-MB-231 and MDA-MB-468 cells were incubated for an additional 4 hours with 20 μL MTT (5 mg/mL). The supernatant was then removed, and 150 μL DMSO was added. The absorbance at 490 nm was measured with a microplate reader. Experiments were performed in triplicate.
Real-time PCR assay
Total RNA was extracted from cultured cells usingTRIzol reagent (Invitrogen, Carslbad, CA, USA). cDNA was obtained by reverse transcription of total RNA using a TaqManReverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). The expression level of mature miR-429 was measured using a TaqMan miRNA assay (Applied Biosystems, Carlsbad, CA, USA) according to the provided protocol and using U6 small nuclear RNA as an internal control.
Western blot analysis
The cells in each well, including dead cells floating in the medium, were harvested and lysed in RIPA buffer. The protein concentrations of the lysates were determined using a bicinchoninic acid protein assay kit (Pierce Biotech, Rockford, IL, USA). An aliquot of the lysate containing 50 μg proteins was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. The membranes were blocked with blocking buffer (TBST containing 5% non-fat milk) for 1 h at room temperature and then incubated overnight at 4°C with the following specific primary antibodies: XIAP and β-actin. Subsequent incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies was performed for 2 h at room temperature. Signals were detected using enhanced chemiluminescence reagents (Thermo, Carlsbad, CA, USA).
Luciferase reporter assay
To evaluate the function of miR-429, the 3’-UTR of XIAP with a miR-429 targeting sequence was cloned into the pMIR-REPORT luciferase reporter vector (Ambion, Carlsbad, CA, USA). The sequences used to amplify the XIAP 3’-UTR were 5’-GCTGATTTAAAGGCTTAG-3’ (forward) and 5’-CAAATGTAGGTAGAGGA-3’ (reverse). A mutant XIAP 3’-UTR bearing a substitution of three nucleotides (TAT to CCC) in the miR-429 target sequence was generated using a Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA). Cells were co-transfected with luciferase reporter plasmids and miR-429 precursor (or control miRNA), along with Renilla Luciferase phRG-TK (Promega, Madison, WI, USA) as an internal control, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Luciferase activity was measured 24 h after transfection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). All experiments were performed in triplicate.
Annexin V-FITC/propidium iodide (PI) staining assay
The annexin V-FITC/PI assay was performed using a commercial kit (BD Bioscience, San Jose, CA, USA). Briefly, cells were washed twice with PBS and incubated in 500 μL binding buffer containing annexin V-FITC and PI in the dark for 10 min at room temperature. The stained samples were then analyzed on a FACSort flow cytometer, as instructed by the manufacturer.
Caspase-3 activity assay
Caspase-3 activity was determined by using a fluorometric assay kit as described by the manufacturer (Roche, Indianapolis, IN, USA). Cleavage of the fluorogenic substrate, Ac-DEVD-AFC (7-amino-4-trifluoromethylcoumarin, N-acetyl-L-aspartyl-Lglutamyl-L-valyl-L-aspartic acid amide) by caspase-3 was measured at an excitation wavelength of 380 nm and an emission wavelength of 490 nm. The increase in caspase-3 activity was determined by comparing results with control. Experiments were performed in triplicate.
Cell death ELISA assay
Cell death as a result of apoptosis was quantified by measuring mono- and oligonucleosome release using the Cell Death Detection ELISA Kit (Roche, Indianapolis, IN, USA), following the manufacturer’s instructions. Briefly, cells were seeded in a six-well plate, treated with control-miRNA, miR-429 precursor, and miR-429 inhibitor and grown either in the presence or absence of δ-tocotrienol for 24 h at 37°C. Cells were lysed in buffer for 30 min at 25°C, and the supernatant was diluted 1:10 with incubation buffer and transferred into the MP-modules. The conjugate solution and substrate solution were added following the protocol. The absorbance was read in a micro-plate reader at 405 nm. Experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using one-way ANOVA or Student’s t-test. Values of P<0.05 were considered significant. Data were represented as the mean ± S.D. GraphPad Prism 5.0 software was used for all data analysis.
Results
δ-tocotrienol inhibits cell proliferation and induces apoptosis in TNBC cells
First, we evaluated the effects of δ-tocotrienol on exponentially growing TNBC cells. MDA-MB-231 and MDA-MB-468 cells, also known as human triple negative breast cancer cells, were treated in the presence of various concentrations (0-100 μM) of δ-tocotrienol for 24 hours, and the cell viability rate was measured using an MTT assay. Treatment with δ-tocotrienol inhibited the proliferation of MDA-MB-231 and MDA-MB-468 cells in a dose-dependent manner (Figure 1B). Then, an annexin V/propidium iodide flow cytometry assay was performed to determine if the growth inhibition by δ-tocotrienol was associated with the induction of apoptosis. As expected, we determined the induction of apoptosis of MDA-MB-231 and MDA-MB-468 cells by δ-tocotrienol at 24 hours (Figure 1C).
miR-429 mediated induction of apoptosis in TNBC cells treated with δ-tocotrienol
To evaluate if miR-429 was involved in the δ-tocotrienol-induced apoptosis in TNBC cells, a real-time PCR assay was performed. Interesting, miR-429 was up-regulated in MDA-MB-231 cells by δ-tocotrienol, but the expression was suppressed when treated with both δ-tocotrienol and miR-429 inhibitor (Figure 2A). Then, we tried to explore if the expression of miR-429 was required for the δ-tocotrienol-induced apoptosis in MDA-MB-231 cells. We perform caspase-3 activity assay as well as cell death ELISA assay to detect apoptosis in MDA-MB-231 cells which were transfected with either control miRNA or miR-429 inhibitor and grown either in the presence or absence of δ-tocotrienol (30 μM) for 24 hours. The δ-tocotrienol treatment resulted in a marked induction of caspase-3 activity and cell apoptosis in the control-transfected cells. On the other hand, the effect of δ-tocotrienol, although not completely extinguished, was significantly reduced in MDA-MB-231 cells transfected with the miR-429 inhibitor (Figure 2B and 2C).
Figure 2.
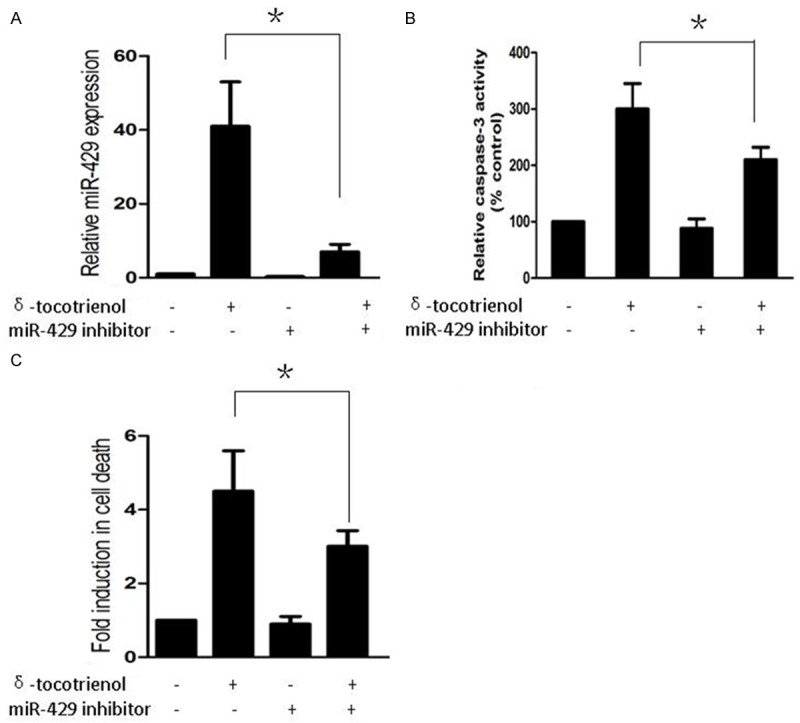
miR-429 mediated induction of apoptosis in TNBC cells treated with δ-tocotrienol. A. Real-time PCR was used to measure expression of miR-429 in MDA-MB-231 cells treated with vehicle, δ-tocotrienol, miR-429 inhibitor or both. *P<0.05. B. Caspase-3 activity assay was used to measure cell caspase-3 activity in MDA-MB-231 cells treated with vehicle, δ-tocotrienol, miR-429 inhibitor or both. *P<0.05. C. Cell death ELISA assay was used to measure cell apoptosis in MDA-MB-231 cells treated with vehicle, δ-tocotrienol, miR-429 inhibitor or both. *P<0.05.
Forced expression of miR-429 induces apoptosis in TNBC cells via suppressing XIAP
To evaluate if over-expression of miR-429 was enough to induce apoptosis in TNBC cells, we used a cell death ELISA assay to measure cell apoptosis in MDA-MB-231 cells treated with control miRNA or miR-429 precursor. We also determined the induction of apoptosis of MDA-MB-231 cells by the forced expression of miR-429 at 24 hours (Figure 3A). Our results indicate that miR-429 plays a major role in the induction of apoptosis in TNBC cells.
Figure 3.
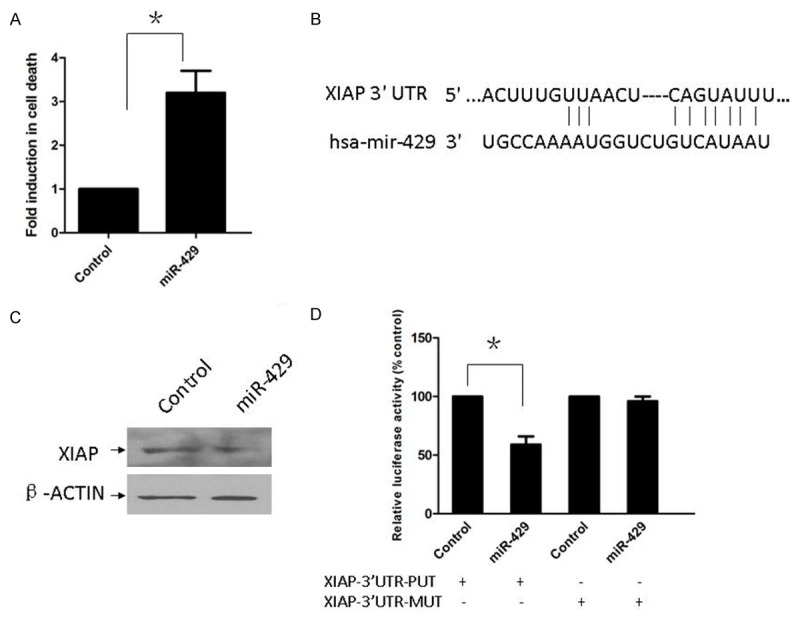
Forced expression of miR-429 induces apoptosis in TNBC cells via suppressing XIAP. A. Cell death ELISA assay was used to measure cell apoptosis in MDA-MB-231 cells treated with control microRNA or miR-429 precursor. *P<0.05. B. Sequence alignment of miR-429 and its conserved target site in XIAP 3’-UTR (downloaded from Target-Scan 6.2). C. XIAP protein expression was detected by western-blot and normalized to β-actin protein levels. D. Relative luciferase reporter activity of XIAP 3’-UTR (wild type and mutant type) in MDA-MB-231 cells after transfected with control microRNA or miR-429 precursor. *P<0.05.
To explore the mechanism of miR-429 activity in MDA-MB-231 cells, we used TargetScan 6.2 (http://www.targetscan.org) to search for the target gene of miR-429, especially for genes with potential roles in promoting tumor cell proliferation. TargetScan6.2 predicted that XIAP was the common target gene of the miR-429 (Figure 3B). The influence of miR-429 on the endogenous expression of XIAP proteins was examined. The expression of XIAP was down-regulated in MDA-MB-231 cells transfected with miR-429 precursor (Figure 3C). To explore whether XIAP was the common target gene of miR-429, we constructed two luciferase reporter plasmids with the putative XIAP 3’UTR target site for miR-429 (pMIR-XIAP-3’UTR-PUT) and a mutant XIAP 3’UTR target site for miR-429 (pMIR-XIAP-3’UTR-MUT). Co-transfection with the miR-429 precursor was found to decrease wild type XIAP 3’-UTR reporter activity (P<0.05) compared with co-transfection with control miRNA in MDA-MB-231 cells. However, cotransfection with the miR-429 precursor did not significantly alter mutant XIAP 3’-UTR reporter activity (Figure 3D). These results demonstrate that miR-429 targets the predicted site within the 3’-UTRs of XIAP mRNA in the TNBC cell line.
Discussion
The recent discovery of a class of small non-coding RNAs called microRNAs has received significant attention in cancer research [17,18]. The over-expression of tumor suppressor miRNAs may repress cancer cell proliferation and result in apoptosis, but the mechanisms by which miRNAs affect oncogenesis remain to be elucidated.
It was reported that the reduced expression of the miR-200 family was involved in the epithelial-to-mesenchymal (EMT) transition of tumor cells [19,20], and overexpression of miR-429 may induce mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells [21]. It was also reported that over-expression of miR-200b/c/429 was sufficient to induce apoptosis in breast cancer cells [22], but little is known about the role of miR-429 in the δ-tocotrienol-induced apoptosis of TNBC cells.
Vitamin E δ-tocotrienol, a major bioactive compound found in cereal grains, oats, barley, annatto beans and palm, is one of eight natural lipid-soluble vitamin E compounds. Extensive in vitro and in vivo studies have indicated that δ-tocotrienol is a cancer-suppressing bioactive micronutrient that inhibits cell proliferation and induces tumor cell apoptosis, but the precise molecular mechanism by which it inhibits the proliferation of cancer cells remains unclear.
Multiple signaling pathways were reported to be involved in δ-tocotrienol’s anti-tumor activity, such as NF-κB, STAT3, and Ras [23-25]. We have shown in vitro as well as in vivo studies that δ-tocotrienol may activate EGR-1/Bax to exert its function in Miapaca-2 cells, a pancreatic cancer cell line [7], but little is known about its mechanism in TNBC cells.
In this study, we demonstrated that δ-tocotrienol exerted a significant cell growth inhibition in TNBC cells and resulted in apoptosis. miR-429 was up-regulated in MDA-MB-231 cells, and inhibition of miR-429 may partially rescue the effect of δ-tocotrienol. We also determined that ectopic over-expression of miR-429 was enough to induce apoptosis in MDA-MB-231 cells. Moreover, we showed using a luciferase assay that the 3’-UTR of XIAP has a functional target of miR-429.
XIAP is a member of the IAP family of proteins that contain baculovirus IAP repeat (BIR) domains [26], and it has been demonstrated that it is able to bind to caspase-3, 7 and 9 in vitro [27]. Up-regulation of XIAP is a frequent event in breast cancer, and XIAP positive nuclear labeling is a sign of an unfavorable prognosis in breast invasive ductal carcinoma [28]. It was reported that XIAP knockdown by siRNA might inhibit proliferation and induce apoptosis in breast cancer cells [29]. Therefore, the identification of XIAP as a miR-429 target gene may explain, at least in part, the molecular mechanism of tumor suppression by miR-429 in TNBC cells.
In summary, this is the first study to focus on the function of miR-429 and the effect of δ-tocotrienol in triple-negative breast cancer cells. Our data support that the up-regulation of miR-429 is required for δ-tocotrienol’s anti-tumor activity in TNBC cells. Furthermore, we identified XIAP as one of miR-429’s target genes, which may partially explain the role of miR-429 in TNBC cells. On the basis of our results, δ-tocotrienol may be an effective anti-cancer compound in triple-negative breast cancer, and the activation of miR-429 may be a potentially useful novel strategy for inhibiting TNBC growth.
Acknowledgements
This work was supported by grants from the National Science & Technology Pillar Program (2015BAI12B15) and Affiliated Hospital of Chinese People’s Armed Police Forces Logistics Institute (Grant No: FYM201527).
Disclosure of conflict of interest
None.
References
- 1.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 2.Tomao F, Papa A, Zaccarelli E, Rossi L, Caruso D, Minozzi M, Vici P, Frati L, Tomao S. Triple-negative breast cancer: new perspectives for targeted therapies. Onco Targets Ther. 2015;8:177–193. doi: 10.2147/OTT.S67673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramdas P, Rajihuzzaman M, Veerasenan SD, Selvaduray KR, Nesaretnam K, Radhakrishnan AK. Tocotrienol-treated MCF-7 human breast cancer cells show down-regulation of API5 and up-regulation of MIG6 genes. Cancer Genomics Proteomics. 2011;8:19–31. [PubMed] [Google Scholar]
- 4.Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem. 2011;112:2773–2283. doi: 10.1002/jcb.23184. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Xiao H, Jin H, Koo PT, Tsang DJ, Yang CS. Synergistic actions of atorvastatin with gamma-tocotrienol and celecoxib against human colon cancer HT29 and HCT116 cells. Int J Cancer. 2010;126:852–863. doi: 10.1002/ijc.24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendran P, Li F, Manu KA, Shanmugam MK, Loo SY, Kumar AP, Sethi G. γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br J Pharmacol. 2011;163:283–298. doi: 10.1111/j.1476-5381.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Husain K, Zhang A, Centeno BA, Chen DT, Tong Z, Sebti SM, Malafa MP. EGR-1/Bax pathway plays a role in vitamin E δ-tocotrienol-induced apoptosis in pancreatic cancer cells. J Nutr Biochem. 2015;26:797–807. doi: 10.1016/j.jnutbio.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 9.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Zheng X, Shen C, Shi Y. MicroRNA-203 suppresses cell proliferation and migration by targeting BIRC5 and LASP1 in human triple-negative breast cancer cells. J Exp Clin Cancer Res. 2012;31:58. doi: 10.1186/1756-9966-31-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 14.Hidaka H, Seki N, Yoshino H, Yamasaki T, Yamada Y, Nohata N, Fuse M, Nakagawa M, Enokida H. Tumor suppressive microRNA-1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma. Oncotarget. 2012;3:44–57. doi: 10.18632/oncotarget.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun T, Wang C, Xing J, Wu D. miR-429 modulates the expression of c-myc in human gastric carcinoma cells. Eur J Cancer. 2011;47:2552–2559. doi: 10.1016/j.ejca.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Liu Y, Wu S, Shi X, Li L, Zhao J, Xu H. Tumor-suppressing effects of miR-429 on human osteosarcoma. Cell Biochem Biophys. 2014;70:215–224. doi: 10.1007/s12013-014-9885-8. [DOI] [PubMed] [Google Scholar]
- 17.Hummel R, Hussey DJ, Haier J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer. 2010;46:298–311. doi: 10.1016/j.ejca.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–2074. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelialmesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Wang L, Matyunina LV, Hill CG, McDonald JF. Overexpression of miR-429 induces mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells. Gynecol Oncol. 2011;121:200–205. doi: 10.1016/j.ygyno.2010.12.339. [DOI] [PubMed] [Google Scholar]
- 22.Uhlmann S, Zhang JD, Schwager A, Mannsperger H, Riazalhosseini Y, Burmester S, Ward A, Korf U, Wiemann S, Sahin O. miR-200bc/429 cluster targets PLCgamma1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene. 2010;29:4297–4306. doi: 10.1038/onc.2010.201. [DOI] [PubMed] [Google Scholar]
- 23.Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E δ-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-κB activation in pancreatic cancer. Mol Cancer Ther. 2011;10:2363–2372. doi: 10.1158/1535-7163.MCT-11-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye C, Zhao W, Li M, Zhuang J, Yan X, Lu Q, Chang C, Huang X, Zhou J, Xie B, Zhang Z, Yao X, Yan J, Guo H. δ-Tocotrienol Induces Human Bladder Cancer Cell Growth Arrest, Apoptosis and Chemosensitization through Inhibition of STAT3 Pathway. PLoS One. 2015;10:e0122712. doi: 10.1371/journal.pone.0122712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husain K, Centeno BA, Chen DT, Fulp WJ, Perez M, Zhang Lee G, Luetteke N, Hingorani SR, Sebti SM, Malafa MP. Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+; Pdx-1-Cre mice by vitamin E δ-tocotrienol. Carcinogenesis. 2013;34:858–863. doi: 10.1093/carcin/bgt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 27.Dean EJ, Ranson M, Blackhall F, Dive C. X-linked inhibitor of apoptosis protein as a therapeutic target. Expert Opin Ther Targets. 2007;11:1459–1471. doi: 10.1517/14728222.11.11.1459. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhu J, Tang Y, Li F, Zhou H, Peng B, Zhou C, Fu R. X-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinoma. Diagn Pathol. 2011;6:49. doi: 10.1186/1746-1596-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang Y, Gao W, Zhang R, Han X, Jia M, Guan W. Transfer of siRNA against XIAP induces apoptosis and reduces tumor cells growth potential in human breast cancer in vitro and in vivo. Breast Cancer Res Treat. 2006;96:267–277. doi: 10.1007/s10549-005-9080-0. [DOI] [PubMed] [Google Scholar]