Abstract
The objective of this study was to describe the rates and impact of bacterial and viral co-infections of hospitalized children with Mycoplasma pneumoniae. The clinical characteristics, hospital expenses, and differences between single and co-infection MPP were explored. This study included 5,009 children from 2010 to 2014. Infections with various pathogens were identified by the following tests: positive specimens’ culture, direct immunofluorescent antigen test for viruses, mycoplasma or chlamydia detection. The results indicated that 13.6% of them showed positive results, including bacterial pathogens in 2.5% of cases and viral pathogens in 9.8% of cases. The most commonly identified bacteria was Streptococcus pneumonia. Influenza and parainfluenza were the most commonly identified virus. Hospitalization expenses of patients with single infections were less than those who with co-infections. In conclusion, co-infections were more common in recent years. In severe MPP, rates of co-infection were higher than non-severe MPP. The longer the course of infection, the higher the co-infection rate.
Keywords: Mycoplasma pneumonia, co-infection, bacterial, virus, children
Introduction
Mycoplasma pneumonia (MP) has recently become an important pathogen of respiratory infection in children, as well as a common pathogen of community-acquired pneumonia (CAP) of children. MP plays a significant role in CAP in children [1,2]. Recent studies have reported that 7-30% of hospitalized children with CAP have been infected by mixed viral-bacterial infections [3-5].
In recent years, refractory MP pneumonia (MPP) has increased. Two main mechanisms of MPP are proposed: damages to airway directly by MP, and inflammatory reaction caused by MP. Refractory MPP is also associated with many other factors, such as macrolide-resistant MP and combined infection with bacteria or viruses. It has long been suspected that MPP may be associated with preceding or concomitant viral infections, while recent experience with MPP has refocused attention on the role of bacterial co-infections in contributing to disease severity and death in children with viral respiratory tract infections.
There are only fragmentary data on the etiology of childhood MPP, particularly about co-infections of MPP in children. The purpose of this study was to investigate the impact of bacterial and viral co-infection in hospitalized children with MPP.
Materials and methods
Study population
All medical records of patients with MPP who were admitted to Beijing Children’s Hospital from June 2010 to June 2014 were reviewed. The wards of the Pediatric Internal Medicine Department had 6,128 MPP admissions during this time. Cases were eligible for enrollment if data were complete. Diagnosis of pneumonia was performed according to the practical science of Fu-Tang Zhu, clinical practice guidelines in the management of CAP in infants and children and British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011 [6-8]. In addition, antibody for all diagnosed patients was positive. PCR of throat swabs and BALF were positive in some cases.
Patients were excluded if they had congenital immunodeficiency, malignancy or were receiving immunosuppressant agents. Chronic pneumonia was excluded. Cases of tuberculosis, fungi, EBV and CMV were excluded from the study. A total of 5,009 children aged 0-17 years old were included in this analysis.
Microbiological diagnostic methods
The diagnosis of MPP was based on clinical signs and symptoms (cough, fever, dry or productive sputum, dyspnea, chest pain, abnormal breath sounds), and radiological pulmonary abnormalities. For M. pneumoniae, both acute and convalescent serum were obtained and measured for antibody response to M. pneumoniae by enzyme-linked immunosorbent assay methods (SERODIA-MYCOII) [9]. Paired serology (rising titers in antibody complement fixation tests) remains the mainstay for diagnosing M pneumoniae and C pneumoniae infections [8]. Some cases were used PCR to diagnose M pneumonia from throat swabs and BAL. An acute infection could be indicated by a positive IgM result [10]. Patients were evaluated for viral, bacterial, acid-fast bacillus or fungal infections.
For viral etiology, Respiratory Virus Direct Specimen Screening Set (Diagnostic Hybrids, Inc, Athens OH 45701 USA) were performed using sputum or oropharyngeal swabs. Viruses examined included respiratory syncytial virus (RSV), adenovirus, parainfluenza 1, 2 and 3, and influenza A and B.
All patients were screened for pulmonary tuberculosis by the PPD skin test with 5TU purified protein derivative. Some cases were sent for Interferon-gamma Release Assey (TB-SPOT). Blood, pleural effusion and BALF were sent for slide review and culture Bacterial, M. tuberculosis and fungus.
Definitions
Acute MP pneumonia was defined as the course of disease no more than one month with either the test results of PCR or MP-specific antibodies by serology was positive. Chronic pneumonia was defined as the course of disease more than one month. A case with a co-infection was defined as any pathogen except MP detected in any specimen type. A patient was considered to have a single infection if MP was the only pathogen detected. Bacterial infection was defined by a positive result in blood or pleural fluid culture and BALF.
Severe pneumonia was diagnosed according to the practical science of Fu-Tang Zhu, clinical practice guidelines in the management of CAP in infants and children and British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011 [6-8]. Refractory M. pneumoniae pneumonia was defined as follows: 1) prolonged fever for 7 days or more or 2) increasing cough and infiltrates in chest radiograph despite administration of appropriate antibiotics. Patients with severe M. pneumoniae pneumonia who required intensive care unit (ICU) admission were defined by Infectious Diseases Society of America/American Thoracic Society criteria for severe CAP [11]. All patients were treated with macrolide. The symptoms mentioned above was typical of SMPP, and patients without these symptoms could be defined as non-severe MPP. Mortality of MPP in these cases was zero.
Statistical analyses
The demographic data and identified pathogens were entered into database for analysis. Descriptive statistics were performed using SPSS 17.0 (SPSS Inc, Chicago, IL). The differences in age distributions among patients with various pathogens identified were tested by an independent sample t-test. P<0.05 was considered statistically significant. Parametric data were compared with independent sample t-tests. Categorical data were analyzed by using the chi-square test.
Results
Demographic and clinical data
A total of 5,009 children hospitalized with MPP were included in the study, of those who, 2,378 (47.5%) were female and 2,631 (52.5%) were male. Ages ranged from 2 months to 17 years, and 67.6% were over 6 years old. There was no significant difference in gender with different ages. Ages were classified into subgroups (<1, 1-3, 3-6, >6 years, which correspond to different physiological stages). There were 776 (15.5%) patients with severe MPP, 636 (12.7%) with pleural effusions. Characteristics of the 5, 009 children hospitalized with MPP are shown in Table 1.
Table 1.
Clinical characteristics of 5,009 children hospitalized with MPP
| Characteristic | Overall | Single infection | Single infection (percentage) | Co-infection (N) | Co-infection (percentage) |
|---|---|---|---|---|---|
| Total cases | 5,009 | 4,326 | 86.4 | 683 | 13.6 |
| Gender | |||||
| Female | 2,378 | 2051 | 86.2 | 327 | 13.8 |
| Male | 2,631 | 2,275 | 86.5 | 356 | 13.5 |
| Age group | |||||
| <1 y | 50 | 46 | 92 | 4 | 8 |
| 1-3 y | 322 | 265 | 82.3 | 57 | 17.7 |
| 4-6 y | 1,249 | 1,064 | 85.2 | 185 | 14.8 |
| 7-17 y | 3,387 | 2,951 | 87.1 | 436 | 12.9 |
| Severe MPP | 776 | 621 | 80.0 | 155 | 20.0 |
| Pleural effusions | 636 | 536 | 84.3 | 100 | 15.7 |
| Time from onset of symptoms to hospital visit (days) | |||||
| 1-7 | 2,129 | 1868 | 87.7 | 261 | 12.3 |
| 8-15 | 2298 | 1969 | 85.7 | 329 | 14.3 |
| 16-30 | 538 | 445 | 82.7 | 93 | 17.3 |
| Fever | 4956 | ||||
| Cough | 4958 |
Hospitalization expenses of single infections were less than co-infections. Significant differences were observed between single and co-infections. There was no significant difference in duration of hospitalization between patients with single infections and those who with co-infection.
Distribution of pathogens in co-infective MPP
Tests for viral, bacterial, acid-fast bacilli and fungal infections were performed in all patients, and 13.6% (683/5,009) of cases were positive for at least one pathogen in addition to MP (Figure 1A). The 683 cases identified are listed in Table 2. There were 894 times co-infections, including typical bacterial pathogens in 16.9% (151/894) of it. A total of 51.0% of bacterial pathogens were identified SP. Viruses were identified in 75.7% (677/894) of total co-infection, CP were identified in 7.4% (66/894) of total co-infection, while cases mixed EBV,CMV, TB infections and fungal infections were excluded.
Figure 1.
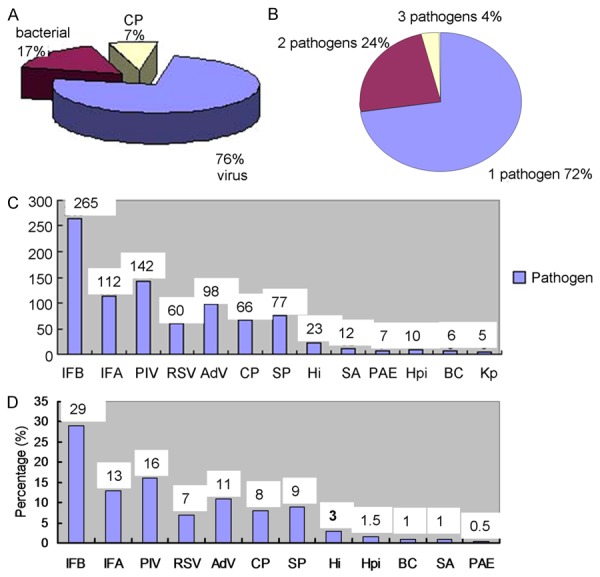
Distribution of pathogens in co-infective MPP. A. Distribution of pathogens in co-infective MPP. B. Percentage of different number pathogens. C. Distribution of pathogens in MPP with co-infection during 4 years. D. Distribution of pathogens in co-infective MPP.
Table 2.
Pathogens identified in 5,009 hospitalized children with mycoplasmal pneumonia
| Pathogen (s) | Cases | Pathogen (s) | Cases | Pathogen (s) | Cases |
|---|---|---|---|---|---|
| IFA + IFB + PIV | 13 | IFB + PAE | 1 | PAE | 6 |
| IFA + IFB + AdV | 2 | IFB + S.P | 2 | E. coli | 1 |
| IFB + PIV + AdV | 1 | IFB + Ab | 1 | Ab | 1 |
| IFB + RSV + AdV | 1 | PIV + RSV | 6 | K p | 4 |
| IFB + BC + Hi | 1 | PIV + S.P | 2 | BCe | 1 |
| IFB + BC + S.P | 1 | PIV + CP | 2 | Sewer coli | 1 |
| IFB + Hpi + S.P | 1 | RSV+ AdV | 7 | ML | 1 |
| PIV + RSV + AdV | 2 | RSV + S.P | 1 | Hpi | 6 |
| AdV + S.P + Hi | 1 | RSV + BC | 1 | SA | 12 |
| AdV + PIV | 11 | AdV + Hpi | 1 | Hi | 15 |
| AdV + Hi | 4 | S.P + Hpi | 2 | IFA | 19 |
| IFA + IFB | 76 | S.P + K p | 1 | IFB | 126 |
| IFA + RSV | 2 | S.P+ Hi | 2 | PIV | 90 |
| IFB + PIV | 15 | S.P + BC | 2 | RSV | 34 |
| IFB + AdV | 11 | Ab + S. coli | 1 | AdV | 57 |
| IFB + RSV | 6 | S.P | 62 | CP | 57 |
| IFB + CP | 7 | Ng | 4 |
Of the 683 cases, one pathogen was identified in 72.2% (496/683), two pathogens were identified in 23.9% (163/683), and three pathogens were identified in 3.5% (24/683) (Figure 1B). Overall, frequency of pathogens in co-infection with MPP was showed in Figure 3. IFB, PIV1, 2, 3 and IFA were the most common source of infection. IFB were detected with 5.3% in all cases.
Figure 3.
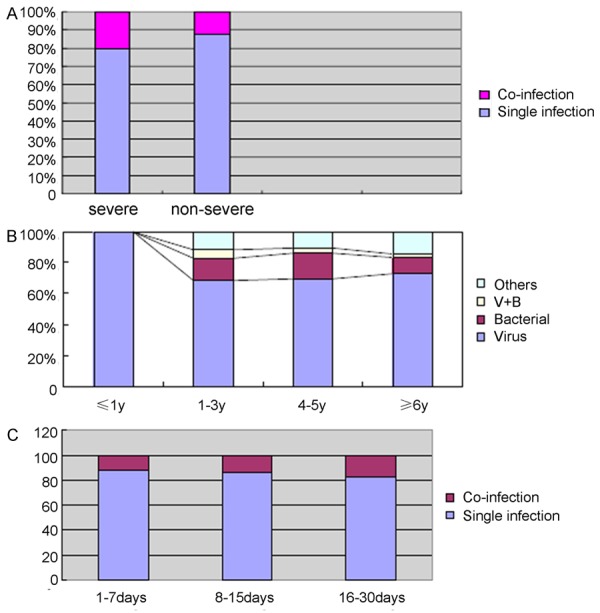
Course distribution of co-infection in MPP. A. Percentage of co-infection in severe cases. B. Mixed bacterial, viral and bacterial/viral occurred in different age children with MPP: Distribution of pathogens in co-infecting MPP, stratified by age. C. Percentage of co-infection cases in different course of disease.
The proportion of IFB infection with MPP increased in 2010, 2013 and 2014 (Figure 1C). The proportion of IFA patients with MPP increased in 2010 and 2014, while PIV increased in 2012 and the proportion of AdV increased in 2013. Pathogens identified are shown in Table 2, and Figure 1D.
Season distribution of co-infection in MPP
The annual rates of co-infection of MPP patients increased from 11.3% in 2011 to 39.6% in 2014 (Figure 2A). Co-infections were more common in autumn and winter (Figure 2B). Viruses were more common in autumn and winter, and bacteria were more common in spring and summer (Figure 2C).
Figure 2.
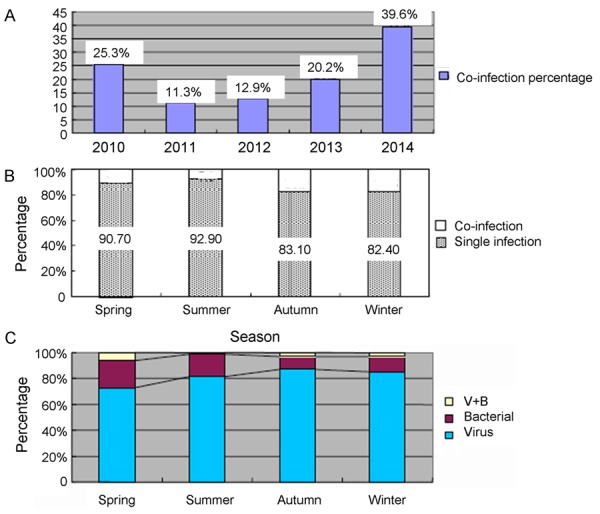
Season distribution of co-infection in MPP. A. Percentage of co-infection in MPP, stratified by year. B. Season distribution of co-infection in MPP. C. Distribution of pathogens in co-infective MPP, stratified by season.
Course distribution of co-infection in MPP
Co-infections were more common in severe MPP than in non-severe MPP, and co-infections were also more serious than single infections (Figure 3A). Viruses accounted for 100% of pathogens were identified in children less than 1 year old (Figure 3B). In children aged from 1 to 3 years old, bacteria were the most common co-infection identified. Similarly, the proportion of identifiable viral infections decreased with increasing ages.
The co-infection rate increased with increasing time from onset of symptoms to hospital visit days (Figure 3C). Hospitalization expenses of single infections were less than co-infections (Table 3). Significant differences were observed between single and co-infections for each pathogen identified (Table 3). Mixed co-infection with Hi, SA, PAE, AB and AdV, or positive bacterial result showed in blood culture often caused the hospitalization expenses increasing.
Table 3.
Hospitalization expenses of co-infections and single infections
| Pathogen | Hospitalization expenses (dollar) | P | |
|---|---|---|---|
|
| |||
| Co-infections | Single infections | ||
| S.P | 17290.0 | 14,607.0 | 0.01 |
| Hi | 25327.0 | 14,607.0 | 0.0001 |
| BC | 16272.6 | 14,607.0 | 0.01 |
| SA | 24443.1 | 14,607.0 | 0.0001 |
| PAE | 19351.3 | 14,607.0 | 0.001 |
| AB | 31304.5 | 14,607.0 | 0.0001 |
| AdV | 24017.8 | 14,607.0 | 0.0001 |
| RSV | 17336.6 | 14,607.0 | 0.01 |
| PIV | 17784.5 | 14,607.0 | <0.001 |
Discussion
In our study, 13.6% of children with MPP were infected with another pathogen. This percentage of MPP with co-infections was less than the report about co-infections of children with MPP in China [12] and the result of Michelow et al. [5]. This difference might be caused by excluding rhinoviruses and enterovirus tests in our data. The incidence of mixed infections with viruses was 9.8%, which was less than the result of Michelow [5] and Hamano-Hasegawa [13].
MPP in children under 4 was rare. In the infants below one year of age, as compared with the other age group, fever was observed frequently less than 30 percent in contrast to 47-60 percent of the other age groups [14]. In our study, the infants below one year of age showed slight fever, but children more than 3 years showed severe fever. Pathy shadow could be easily seen from Chest X-ray performance. It was difficult to distinguish the MPP from different pneumonia. Generally, it was considered that M. pneumoniae infections were mild in infants. Present reports showed that infants below one year of age less frequently had fever or pneumonia, and showed slighter symptoms as compared with elder children. It was easy to be ignored. In our study, there are 372 children under 3 years with MPP. Analysis results suggested that mycoplasma played an important role also in this age group.
A previous study indicated that most viruses exhibited strong seasonal patterns, and some viruses were frequently detected in the cold and rainy season [15]. In our study, virus infections were more common in autumn and winter. This led to more co-infections being identified during these seasons, and could be related to polluted air in these seasons. Such co-infections may be particularly problematic during influenza pandemics. The August and September 2010 H1N1 influenza virus pandemic resulted in sharply increased rates of influenza co-infection of children with MPP. Co-infections of children with MPP were higher in 2010, 2013 and 2014, and were primarily related to viruses being identified more often these years. The prevalence of influenza infections increased during autumn and winter. Respiratory viral infections may be a main reason for the development of MPP, while infection with MP might lead to further deterioration of health. According to previously published reports, enteroviruses were common in spring and summer, but enteroviruses were not evaluated in our study. RSV and influenza viruses are the major viral pathogens during the winter season, and their peak incidences often overlap [16]. In our study, co-infection with MP and RSV was less common than with MP and influenza. In previous study, the delayed and reduced circulation of RSV in 2009-2010 compared with 2008-2009 suggested that the early circulation of the 2009 pandemic IFA (H1N1) viruses had an impact on the RSV epidemic [17].
Age is an important factor that can affect pathogen distribution, and viruses without bacterial co-infection were more frequently identified in young children than in elder children. In other study, Viruses could cause a significant percentage of CAP infections, especially in children who were younger than two years old [18]. In previous studies, respiratory viral infections showed a periodic seasonal distribution and were the main cause of acute respiratory infection in young children [19,20]. Co-infections in 1-3 years old children were increased than other aged children. This result was consistent with that reported by Nakamaya in a previous study [21]. According to previous investigations, viral pneumonia occurred primarily in young children [5], and the major risk factors had been identified as young age and immunologic impairment [14-22]. As a result, viral pneumonia was less common in elder children and adolescents than in young children. In young children, respiratory and immune systems are immature and may be more susceptible to respiratory pathogens [25]. AdV may be associated with complications such as bronchiolitis obliterans or bronchiectasis [26,27]. AdV infections often associated with co-infection of bacterial or viral agents, frequently led to severe clinical consequences in hospital patients [28]. According to our data, the proportion of ADV increased in 2013 and 2014 compared to earlier years. The most common age of AdV infection in our study occurred among children between 2 to 4 years old, while Chen et al. reported the peak values occurred between 4 and 8 years old in Taiwan [29]. Similar to our findings, other studies reported that AdV infections primarily occurred among children under 5 years of age [30,31].
Bacteria were recognized as a common cause of respiratory infections. Co-infection with respiratory bacteria and MP was rare, and in our study this occurred less commonly than co-infection with viruses and MP. Nearly all children in our study received antibiotic treatment. Most pneumococcal pneumonia occurred in children aged 5 years old or younger [25]. SP and Hi were the most commonly identified pathogens for childhood pneumonia in developing countries. 18 SP were the most commonly isolated bacterial pathogens among children with MPP [32]. In our study, SP was the leading cause of bacterial co-infection. In a previous analysis, a sub-analysis stratified by bacteria species, outcomes were worse for co-infected children in the subgroups of children with SA and with no specified bacteria [33]. In our study SA co-infections led to more disease severity in children with MPP compared with single infections. In addition to these bacteria, attention should also be paid to opportunistic bacteria such as BC, which could lead to severe disease in certain conditions.
In our study, co-infections were more severe than single infections, which was consistent with some previous studies [34,35]. In contrast with other previous studies, co-infection did not affect disease severity [19]. In the present study, co-infections were slightly increased in recent years, and could be found in severe cases. Co-infection may lead to severe pneumonia. As for multiple viral infections, previous studies had reported conflicting results when comparing a single infection to multiple pathogens infections and their association with more severe clinical presentation [36].
Co-infections became more common with increasing time from symptom onset to hospital visit. There was no significant difference in duration of hospitalization between patients with single infections and those with co-infection. Hospitalization expenses of patients with single infections were less than those with co-infections. Hospitalization expenses were greatly increased with co-infection with Hi, SA, PAE, AB and AdV, or when they were blood culture positive. In agreement with previous studies, we found that bacterial co-infections with influenza were also associated with more severe illness and worse outcomes in children [37-41]. This was a main reason why expenses were higher for patients with co-infections.
Particular attention should be paid to viral infections in younger children, especially in autumn and winter. In recent years, increased attention had been paid to viruses. Additional studies should focus on the duration of illness that was caused by respiratory viruses, as well as if any of these viruses were part of the normal viral flora. Improvement of etiological insight was needed to lead to better clinical management and prevention. The 2011 pediatric CAP guidelines noted that positive viral test results could modify clinical decision-making in children suspected of having pneumonia by allowing antibacterial therapy to be withheld in the absence of clinical, laboratory, or radiographic findings which suggested bacterial co-infection [41]. Climate change, air pollution in urban areas, and economic or political upheavals that might impact population-level nutritional health, access to health care, and seasonality and other epidemiological aspects of some respiratory pathogens might become important factors regarding CAP and other infections in children in the next few decades.
In conclusion, these co-infections were more common in recent years than in 2010. Co-infections should be considered in refractory MPP. IFB, PIV 1, 2, 3 and IFA were the most common viruses in co-infections. The single most common detected virus was IFB. Co-infection with respiratory bacteria occurred less commonly, and SP and Hi were the most commonly identified bacteria. Co-infections were more common in autumn and winter, and co-infections were more common in severe MPP patients. Mixed infections with viruses were common in young children. Co-infections became more common with increasing time from symptom onset to hospital visit. In our study, co-infections were more severe than single infections, and hospitalization expenses of patients with single infections were less than patients with co-infections, while the duration of hospital stay days were same between patients with single and co-infections. There were several limitations to our study. First, nearly all children in our study received antibiotic treatment. Second, our testing did not include human rhinovirus, human metapneumovirus, bocavirus or enteroviruses; as a result, viral co-infections may be underestimated. Lastly, CP was not detected in many cases.
Acknowledgements
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication: multicenter study on the diagnosis and treatment of children community acquired pneumonia with the number of 2013BAI 09B11.
Disclosure of conflict of interest
None.
References
- 1.Zhou Z, Li F, Chen X, Jin P, Guo Q, Wang H. Mycoplasma pneumonia combined with pulmonary infarction in a child. Int J Clin Exp Med. 2015;8:1482–1486. [PMC free article] [PubMed] [Google Scholar]
- 2.Ferwerda A, Moll HA, de Groot R. Respiratory tract infections by Mycoplasma pneumonia in children: a review of diagnostic and therapeutic measures. Eur J Pediatr. 2001;160:483–491. doi: 10.1007/s004310100775. [DOI] [PubMed] [Google Scholar]
- 3.Juven T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, Eskola J, Saikku P, Ruuskanen O. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wang YJ, Vuori-Holopainen E, Yang Y, Wang Y, Hu Y, Leboulleux D, Hedman K, Leinonen M, Peltola H. Relative frequency of Haemophilus influenzae type b pneumonia in Chinese children as evidenced by serology. Pediatr Infect Dis J. 2002;21:271–277. doi: 10.1097/00006454-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, Kauppila J, Leinonen M, McCracken GH Jr. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 6.Shen KL, Jiang ZF. Mycoplasma pneumonia. In: Hu MYM, Jiang ZF, editors. The practical science of Fu-Tang Zhu. The seventh edition. Beijing: People’s Medical Publishing House; 2014. p. 1205. [Google Scholar]
- 7.Pediatrics of Chinese medical association branch of breathing group. The Chinese journal pediatrics editorial board. The management of community acquired pneumonia in infants and children clinical practice guidelines. Chin J Pediatr. 2013;51:745–752. [Google Scholar]
- 8.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66:ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 9.Hirschberg L, Krook A, Pettersson CA, Vikerfors T. Enzyme-linked immunosorbent assay for detection of Mycoplasma pneumoniae specific immunoglobulin M. Eur J Clin Microbiol Infect Dis. 1998;7:420–423. doi: 10.1007/BF01962354. [DOI] [PubMed] [Google Scholar]
- 10.Sinaniotis CA, Sinaniotis AC. Community-acquired pneumonia in children. Curr Opin Pulm Med. 2005;11:218–225. doi: 10.1097/01.mcp.0000159831.82529.85. [DOI] [PubMed] [Google Scholar]
- 11.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC. American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LL, Cheng YG, Chen ZM, Li SX, LI XJ, Wang YS. Mixed infections in children with Mycoplasma pneumonial pneumonia. Chin J Pediatr. 2012;50:211–215. [PubMed] [Google Scholar]
- 13.Hamano-Hasegawa K, Morozumi M, Nakayama E, Chiba N, Murayama SY, Takayanagi R, Iwata S, Sunakawa K, Ubukata K. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14:424–432. doi: 10.1007/s10156-008-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niitu YM. pneumoniae Respiratory Diseases: Clinical Features-Children. Yale J Biol Med. 1983;56:493–503. [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DL, Smith GE, Edmunds WJ, Joseph C, Gerand E, George RC. The contribution of respiratory pathogens to the seasonality of NHS direct calls. J Infect. 2007;55:240–248. doi: 10.1016/j.jinf.2007.04.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23:S188–S192. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 17.Bicer S, Giray T, Çöl D, Erdag GC, Vitrinel A, Gürol Y, Çelik G, Kaspar Ç, Küçük Ö. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013;39:22. doi: 10.1186/1824-7288-39-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XT, Wang GL, Luo XF, Chen YL, Ou JB, Huang J, Rong JY. Spectrum of pathgens for community-acquired pneumonia in children. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:42–45. [PubMed] [Google Scholar]
- 19.Dawood FS, Chaves SS, Perez A, Reingold Arthur, Meek J, Farley MM, Ryan P, Lynfield R, Morin C, Baumbach J, Bennett NM, Zansky S, Thomas A, Lindegren ML, Schaffner W, Finelli L. Complications and associated bacterial co-infections among children hospitalized with seasonal or pandemic influenza, United States, 2003-2010. J Infect Dis. 2014;209:686–694. doi: 10.1093/infdis/jit473. [DOI] [PubMed] [Google Scholar]
- 20.Williams DJ, Hall M, Brogan TV, Farris RW, Myers AL, Newland JG, Shah SS. Influenza co-infection and outcomes in children with complicated pneumonia. Arch Pediatr Adolesc Med. 2011;165:506–512. doi: 10.1001/archpediatrics.2010.295. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama E, Hasegawa K, Morozumi M, Kobayashi R, Chiba N, Iitsuka T, Tajima T, Sunakawa K, Ubukata K. Rapid optimization of antimicrobial chemotherapy given to pediatric patients with community-acquired pneumonia using PCR techniques with serology and standard culture. J Infect Chemother. 2007;13:305–313. doi: 10.1007/s10156-007-0535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echavarria M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21:704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalpoe JS, van der Heiden PL, Barge RM, Houtzager S, Lankester AC, van Tol MJ, Kroes AC. Assessment of disseminated adenovirus infections using quantitative plasma PCR in adult allogeneic stem cell transplant recipients receiving reduced intensity or myeloablative conditioning. Eur J Haematol. 2007;78:314–321. doi: 10.1111/j.1600-0609.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 24.Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in haematopoietic stem cell transplantation recipients. Blood. 2010;116:5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CJ, Lin PY, Tsai MH, Huang CG, Tsao KC, Wong KS, Chang LY, Chiu CH, Lin TY, Huang YC. Etiology of community acquired pneumonia in hospitalized children in Northern Taiwan. Pediatr Infect Dis J. 2010;31:e196–201. doi: 10.1097/INF.0b013e31826eb5a7. [DOI] [PubMed] [Google Scholar]
- 26.Murtagh P, Guibergia V, Viale D, Bauer G, Pena HG. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr Pulmonol. 2009;44:450–456. doi: 10.1002/ppul.20984. [DOI] [PubMed] [Google Scholar]
- 27.Aguerre V, Castanos C, Pena HG, Grenoville M, Murtagh P. Postinfectious bronchiolitis obliterans in children: clinical and pulmonary function findings. Pediatr Pulmonol. 2010;45:1180–1185. doi: 10.1002/ppul.21304. [DOI] [PubMed] [Google Scholar]
- 28.Berciaud S, Rayne F, Kassab S, Jubert C, Faure-Della Corte M, Salin F, Wodrich H, Lafon ME. Adenovirus infections in Bordeaux University Hospital 2008-2010: Clinical and virological features. J Clin Virol. 2012;54:302–307. doi: 10.1016/j.jcv.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Chen HL, Chiou SS, Hsiao HP, Ke GM, Lin YC, Lin KH, Jong YJ. Respiratory adenoviral infections in children: a study of hospitalized cases in Southern Taiwan in 2001-2002. J Trop Pediatr. 2004;50:279–284. doi: 10.1093/tropej/50.5.279. [DOI] [PubMed] [Google Scholar]
- 30.Cooper RJ, Hallett R, Tullo AB, Klapper PE. The epidemiology of adenovirus infections in Greater Manchester, UK1982-96. Epidemiol Infect. 2000;125:333–345. doi: 10.1017/s0950268899004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong JY, Lee HJ, Piedra PA, Choi EH, Park KH, Koh YY, Kim WS. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32:1423–1429. doi: 10.1086/320146. [DOI] [PubMed] [Google Scholar]
- 32.Techasaensiri B, Techasaensiri C, Mejías A, McCracken GH, Ramilo O. Viral co-infections in children with invasive pneumococcal disease. Pediatr Infect Dis J. 2010;29:519–523. doi: 10.1097/INF.0b013e3181cdafc2. [DOI] [PubMed] [Google Scholar]
- 33.Casalegno JS, Ottmann M, Duchamp MB, Escuret V, Billaud G, Frobert E, Morfin F, Lina B. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect. 2010;16:326–329. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 34.Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aberle JH, Aberle SW, Pracher E, Hutter HP, Knudi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants. Pediatr Infect Dis J. 2005;24:605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 36.Sly PD, Jones CM. Viral co-infection in infants hospitalized with respiratory disease: is it important to detect? J Pediatr (Rio J) 2011;87:277–280. doi: 10.2223/JPED.2117. [DOI] [PubMed] [Google Scholar]
- 37.Schrag SJ, Shay DK, Gershman K, Thomas A, Craig AS, Schaffner W, Harrison LH, Vugia D, Clogher P, Lynfield R, Farley M, Zansky S, Uyeki T. Multistate surveillance for laboratory confirmed influenza-associated hospitalizations in children: 2003-2004. Pediatr Infect Dis J. 2006;25:395–400. doi: 10.1097/01.inf.0000214988.81379.71. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen T, Kyle UG, Jaimon N, Tcharmtchi MH, Coss-Bu JA, Lam F, Teruya J, Loftis L. Co-infection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med. 2012;40:3246–3250. doi: 10.1097/CCM.0b013e318260c7f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palacios G, Hornig M, Cisterna D, Savji N, Bussetti AV, Kapoor V, Hui J, Tokarz R, Briese T, Baumeister E, Lipkin WL. Streptococcus pneumoniae co-infection is correlated with the severity of H1N1 pandemic influenza. PLoS One. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M, Doctor A, Paden M, Flori H, Babbitt C, Graciano AL, Gedeit R, Sanders RC, Giuliano JS, Zimmerman J, Uyeki TM. Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics. 2000;128:e1450–1458. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams DJ, Hall M, Brogan TV, Farris RW, Myers AL, Newland JG, Shah SS. Influenza co-infection and outcomes in children with complicated pneumonia. Arch Pediatr Adolesc Med. 2011;165:506–512. doi: 10.1001/archpediatrics.2010.295. [DOI] [PubMed] [Google Scholar]


