Abstract
Background: Epidermal growth factor receptor (EGFR) is a new target for nasopharyngeal carcinoma (NPC) therapy. This prospective phase I study sought to determine the safety and recommended phase II dose of icotinib, a novel highly selective oral EGFR tyrosine kinase inhibitor, in combination with intensity-modulated radiotherapy (IMRT) in patients with NPC. Methods: Eligible patients with NPC received escalating doses of icotinib during IMRT. We treated six patients at a particular dose level until the maximum tolerated dose (MTD) was determined. The starting dose was 125 mg, once-daily and the dose was escalated to another level 125 mg, twice- and thrice- daily, until dose-limiting toxicity (DLT) occurred in two or more patients at a dose level. Expression and mutation analysis of EGFR were performed in all cases. Results: A total of twelve patients were enrolled. Three patients experienced DLT (250 mg/day cohort) and MTD was 125 mg/day. Mucositis toxicity appears to be the major DLT. While EGFR expression in tumor tissue was detected in 75% (9/12) patients, EGFR mutation was detected in 16.67% (1/6) patients in 125 mg/day cohort, and 50% (3/6) in 250 mg/day cohort. Conclusion: The combination of icotinib (125 mg/day) and IMRT in patients with locally NPC had an acceptable safety profile and was well tolerated.
Keywords: Nasopharyngeal carcinoma, icotinib hydrochloride, intensity-modulated radiotherapy, dose-limiting toxicity
Introduction
Nasopharyngeal carcinoma (NPC) is a common disease in the Asian population [1]. Radiation therapy with or without chemotherapy is a standard therapy for patients with NPC [2]. Intensity-modulated radiotherapy (IMRT) for NPC is promising in terms of modulating the radiation beams so that a high dose can be delivered to the tumor while the dose to the normal tissues is reduced. Although NPC is a relatively radiosensitive disease, distant metastases have been the main reason for failure of IMRT whether in the early or advanced local nasopharyngeal carcinoma due to radioresistance [3,4]. Cytotoxic chemotherapy plays an important role in the curative treatment of advanced NPC [5,6]. However, concurrent chemoradiotherapy significantly increases local and systemic toxic effects, which may preclude many NPC patients from proceeding with combined therapy. Hence, with the advance of targeted therapies in cancer, development of novel molecular targeted therapies for NPC is urgent for improving patient survival and prognosis.
It has been reported that epidermal growth factor receptor (EGFR) gene is amplified in 40% of NPC patients and EGFR protein is overexpressed in over 80% [7-9]. EGFR overexpression is also associated with shorter survival following chemoradiotherapy in locoregionally advanced NPC [10]. This indicates that EGFR may be a new target for NPC therapy. Radiotherapy promotes epithelial-mesenchymal transition (EMT) and boosts tumor stem cells in the blood circulation, which may increase the occurrence of distant metastasis [11]. Additionally, it has been proved that EGFR tyrosine kinase inhibitor (TKI) can increase the radiosensitivity and reduce EMT in squamous cell carcinomas [12,13]. An antiproliferative effect of EGFR TKI on human NPC cells has also been shown in vitro studies. However, the effects of EGFR TKI in NPC patients remain unclear.
Icotinib has a low mammalian toxicity and short half-life EGFR TKI and has a proven survival benefit, a favorable safety and tolerability profile in Chinese patients with lung cancer [14]. However, whether the skin and mucosa toxic effects of EGFR TKI may be increased in combination therapy with icotinib and IMRT remains unclear. This prospective phase I study aimed to assess the tolerability and safety of icotinib combined with IMRT in patients with NPC, and to define the recommended dose for phase II trial.
Materials and methods
Study design
The primary objective of this study was to determine the recommended phase II dose of icotinib combined with IMRT in patients with NPC. We also sought to determine safety and tolerability and their correlation with EGFR mutation status. This trial has been approved by the local research ethics committees and registered at ClinicalTrials.gov (NCT01534585). The study was performed in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Patient evaluation
Patients were enrolled if the biopsy was a histological proven NPC without distant metastasis. Additional eligibility criteria included: age 18-70 years old at enrollment; with a Karnofsky performance score (KPS) of 70 or higher; adequate renal, hepatic, and bone marrow function. All patients gave written informed consent.
Treatment plan
Patients began oral icotinib on day one and continued the therapy until completion of radiotherapy. Radiation therapy was implemented 3-6 hours after oral icotinib treatment. The initial plan was to accrue six patients to each dose level (125 mg, once-, twice- and thrice- daily) in each cohort. If one or none of six patients had dose-limiting toxicity (DLT), then escalation proceeded. If DLT occurred in two or more patients at a dose level, then escalation was stopped. The dose level below that at which two of six patients experienced a DLT was defined as the maximum-tolerated dose (MTD). A minimum of four weeks of observation was required after completion of radiation within each icotinib dose level before accrual to the next level. Adjuvant chemotherapy (AC) that consisted of two cycles of paclitaxel 135 mg/m2 plus carboplatin AUC (area under the curve) 5 on day one was started four weeks after the end of RT in advanced NPC.
The planning target volume (PTV) was defined by adding a 3-mm margin to the clinical target volume (CTV) to compensate for the variability of treatment setup and internal organ motion. A total of 70-76Gy (2.12-2.3Gy/fraction), 66-70Gy (2.0-2.12Gy/fraction), 60-66Gy (1.8-2.0Gy/fraction), and 56-60Gy (1.7-1.8Gy/fraction) were delivered to primary tumor volume (PTV) of the primary nasopharyngeal tumor (GTVnx), the involved lymph nodes (GTVnd), the clinical target volume included the high-risk regions (CTV1) and the low-risk regions CTV2, respectively, in 33 fractions with simultaneous integrated boost. PTV Critical structures limit dose were planned according to the RTOG0225 trial [4]. IMRT plans were approved and generated for each patient as our previous paper described [15].
Dose-limiting toxicity (DLT)
The definition of DLT followed the generally accepted parameters in published phase I trials and aimed to identify any amplification of the expected toxicities of standard radiotherapy by addition of icotinib. Therefore, DLT was defined as grade 4 mucositis and skin, or grade 3 mucositis with more than one weeks of toxicity-related treatment delay; or any other grade 3 nonhematologic toxicity (except nausea and vomiting) that in the opinion of the investigator was considered dose limiting.
Histological immunohistochemistry
Streptavidin-perosidase (SP) method was performed to Immunohistochemistry. Rabbit-anti-EGFR antibody (1:100) was applied. EGFR staining in NPC tissues was determined by two pathologists. Staining for EGFR was defined as positive (+) when there was a reaction in up to 10% of tumor cells, moderate (++) with a reaction in more than 10% to 50%, and strong (+++) with a reaction in more than 50% of tumor cells. Cases with no positive cells were regarded as negative (-) [16].
Gene mutation
EGFR mutational analysis was performed in the tumor paraffin-embedded tissue and blood samples by fluorescent quantitative real-time PCR-HRM method as previously described [17]. DNA was extracted from specimens in the tumor paraffin-embedded tissue and blood samples. Exons 18 and exons 19 and exons 20 and exons 21 were amplified, and uncloned polymerase-chain-reaction (PCR) fragments were sequenced and analyzed in both sense and antisense directions for the presence of heterozygous mutations using the LightCycler 480 Real-Time PCR System.
Results
Patient demographics
Between December 2011 and June 2012, a total of twelve patients (four females, eight males) were enrolled, with median age 55 years (range, 18-70 years). Among them, six patients were accrued to dose level of 125 mg/d qd (dose level 1) and six patients were accrued to dose level of 125 mg bid (dose level 2). Baseline characteristics of the patients are listed in Table 1.
Table 1.
Clinical characteristic of the 12 patients
| Case No. | Dose (125 mg) | Gender | Age | KPS | Smoke | Pathology (Non-keratinizing carcinomas) | T | N | Stage* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | qd | Female | 18 | 70 | 0 | Differentiated | 3 | 1 | III |
| 2 | qd | Male | 70 | 70 | 1 | Undifferentiated | 4 | 1 | IVa |
| 3 | qd | Male | 55 | 80 | 1 | Differentiated | 1 | 1 | II |
| 4 | qd | Male | 63 | 80 | 0 | Differentiated | 3 | 0 | III |
| 5 | qd | Female | 56 | 100 | 0 | Undifferentiated | 1 | 0 | I |
| 6 | qd | Male | 40 | 90 | 0 | Differentiated | 2 | 1 | II |
| 7 | bid | Male | 70 | 80 | 1 | Differentiated | 1 | 1 | II |
| 8 | bid | Male | 43 | 80 | 0 | Undifferentiated | 1 | 1 | II |
| 9 | bid | Female | 66 | 70 | 0 | Undifferentiated | 4 | 2 | IVa |
| 10 | bid | Male | 52 | 90 | 1 | Differentiated | 2 | 0 | II |
| 11 | bid | male | 55 | 80 | 0 | Differentiated | 1 | 1 | II |
| 12 | bid | male | 61 | 80 | 1 | Differentiated | 3 | 2 | III |
Abbreviations: KPS = Karnofsky Performance Status.
Patients were staged according to the 7th edition AJCC staging system.
EGFR expression
As shown in Table 2 and Figure 1, EGFR expression was detected in 83.3% (5/6) patients who received the dose levels 1 and in 66.67% (4/6) patients who received the dose level 2.
Table 2.
Epidermal growth factor receptor (EGFR) expression and mutation in the 12 patients
| Case No. | Dose (125 mg) | Tissue EGFR Expression | Tissue EGFR mutation | Blood EGFR mutation |
|---|---|---|---|---|
| 1 | qd | + | - | - |
| 2 | qd | - | - | - |
| 3 | qd | ++ | - | - |
| 4 | qd | + | E18/E20+ | - |
| 5 | qd | ++ | - | - |
| 6 | qd | + | - | - |
| 7 | bid | + | - | - |
| 8 | bid | + | E20+ | - |
| 9 | bid | + | E20+ | - |
| 10 | bid | + | - | - |
| 11 | bid | - | - | - |
| 12 | bid | - | E20+ | - |
Abbreviations: E = Exon; qd = once a day; bid = twice a day.
Figure 1.

Representative images of EGFR expression in nasopharyngeal carcinoma tissues. Immunostainging of EGFR performed in nasopharyngeal carcinoma tissues from Case 5 (++) and Case 11 (-).
EGFR mutation
No EGFR mutation was detected in all blood samples. EGFR mutation in tumor tissue samples was detected in 16.67% (1/6) patients at dose levels 1 of 125 mg/d, and 50.0% (3/6) patients at dose level 2 of 250 mg/d (Table 2 and Figure 2).
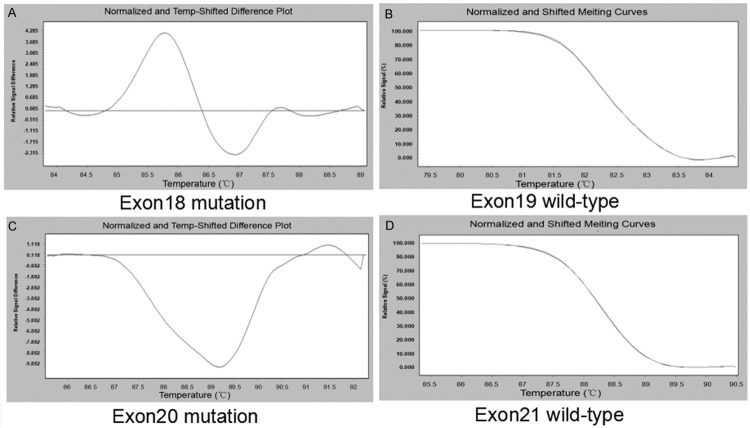
Figure 2.
The EGFR Mutation in nasopharyngeal carcinoma tissues. Case 4 EGFR were mutation in exon 18 (A) and 20 (C), but wild-type in exon 19 (B) and 21 (D).
MTD
No DLT was encountered at dose level 1 (125 mg/d). Three patients experienced a DLT at dose level 2 (250 mg/d) and two of them have EGFR mutation. Mucositis toxicity appears to be the major DLT (Table 3).
Table 3.
Clinical efficacy and side effects of icotinib hydrochloride
| Case No. | Dose (125 mg) | Efficacy* | Dry mouth | Dermatitis | Mucositis | Nausea | Marrow | Rash | Diarrhea |
|---|---|---|---|---|---|---|---|---|---|
| 1 | qd | PR | 2 | 1 | 1 | 1 | 0 | 1 | 0 |
| 2 | qd | PR | 1 | 1 | 2 | 1 | 0 | 0 | 0 |
| 3 | qd | CR | 1 | 1 | 2 | 1 | 0 | 1 | 0 |
| 4 | qd | PR | 1 | 1 | 2 | 1 | 0 | 1 | 0 |
| 5 | qd | CR | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 6 | qd | PR | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 7 | bid | CR | 2 | 1 | 3& | 2 | 1 | 2 | 0 |
| 8 | bid | CR | 2 | 1 | 2 | 2 | 1 | 1 | 0 |
| 9 | bid | - | 0 | 0 | 4 | 1 | 4 | 2 | 0 |
| 10 | bid | PR | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| 11 | bid | PR | 1 | 2 | 2 | 2 | 0 | 1 | 0 |
| 12 | bid | CR | 1 | 2 | 4 | 2 | 1 | 2 | 0 |
Evaluation of the curative effect of icotinib hydrochloride 1 month after radiotherapy.
-Not evaluated due to death.
Therapy was stopped for 2 weeks during the treatment due to severe side effects.
Abbreviations: qd = once a day; bid = twice a day; PR = Partial response; CR = Complete response.
Toxicity
The side effects reported during radiotherapy are listed in Table 3. The most commonly reported adverse events were radiation mucositis (≥ grade 2, 8 patients, 66.7%) and radiation dermatitis (≥ grade 2, 2 patients, 16.7%). No patient showed ≥ grade 3 radiation mucositis in dose level 1. Three patients with ≥ grade 3 radiation mucositis were reported in dose level 2. Two patients with grade 4 radiation mucositis (EGFR mutation) were reported in dose level 2. Among them, one patient (case 7) had grade 3 radiation mucositis at 16 fraction during radiotherapy and two weeks delay. The second patient had (case 9) had grade 4 radiation mucositis at 22 fraction during radiotherapy, followed by severe bleeding in mucosa irradiated and grade 4 thrombopenia. The patient gave up the treatment and died one week later. The third patient (case 12) experienced severe local ulcer due to the pharynx posterior wall tumor retreating after treatment. The ulcer gradually healed six months after treatment.
Recommended phase II dose
Targeted therapy by icotinib combined IMRT has adverse effects in patents with NPC. Mucositis toxicity appears to be the major DLT. Dose level 1 of 125 mg qd is the recommended phase II dose.
Response rates
As listed in Table 3 and Figure 3, the objective response rate (ORR) was 11 out of 12 (91.2%) one month after radiotherapy, with 5 complete responses (CRs) and 6 (partial responses (PRs). One case died during radiotherapy.
Figure 3.
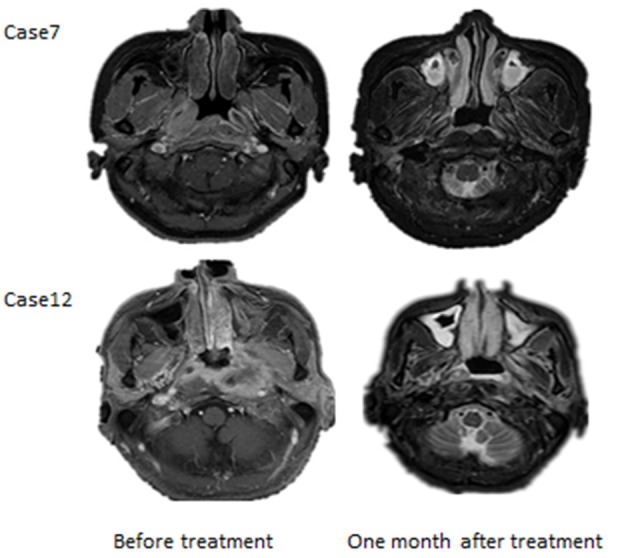
Representative images of curative Effect. MRI of Case 7 and Case 12 before and one month after IMRT.
Discussion
IMRT is becoming a standard radiotherapy technique ensuring high local and regional control at reduced toxicity rates [18]. Distant metastases were reported to be the first site of failure [5,6]. Unexpectedly, it has been recently reported that the main failure reason for concurrent chemoradiotherapy and/or adjuvant chemotherapy is still distant metastasis [19].
Overexpression of EGFR occurs in up to 60% of NPC and affects tumor development, growth, angiogenesis, and invasion [20,21]. EGFR has been implicated in resistance to chemotherapy and radiation. Therefore, inhibition of EGFR may increase the efficacy of chemoradiotherapy through enhanced radiosensitivity of tumor cells [22]. Inhibition of EGFR also decreases the occurrence of distant metastasis through reducing cellular proliferation, EMT and release of tumor stem cells into blood circulation during radiotherapy [23]. There are limited reports available on anti-EGFR therapy in patients with NPC. In recurrent/metastatic setting, gefitinib (in monotherapy), cetuximab (in combination with carboplatin), erlotinib (as a maintenance treatment after chemotherapy), and sorafenib (in monotherapy) have been studied in several small phase II trials [9,24,25]. However, the effects were not ideal. Recently, complete and partial response was observed in patients with stage III-IVb NPC, when concomitant administration of cisplatin and cetuximab combined with radiotherapy [26]. In a phase II study of concurrent cetuximab-cisplatin and IMRT in locoregionally advanced nasopharyngeal carcinoma, thirty patients with nonkeratinizing NPC were enrolled [27]. Grade 3-4 oropharyngeal mucositis occurred in 26 (87%) patients and 10 (33%) of those patients required short-term nasogastric feeding. Grade 3 radiotherapy-related dermatitis occurred in six patients (20%) and three patients (10%) had grade 3 cetuximab-related acneiform rash. Also it was deemed that concurrent administration of cetuximab, weekly cisplatin and IMRT is a feasible strategy against locoregionally advanced NPC. However, no studies have been reported on the relationship between efficacy and side effects and EGFR expression and mutation status. Given that the targeted drugs have side effects on skin and mucous membrane, this kind of drug may increase the main side effects of radiation therapy. Therefore, we chose the icotinib hydrochloride (short half-life) to conduct Phase I study.
Icotinib hydrochloride {4-[(3-ethynylphenyl) amino]-6, 7-benzo-12-crown-4-quinazoline hydrochloride} is a novel EGFR-TKI [28]. It was chemically synthesized by Zhejiang Bata Pharma Inc. (Zhejiang, China). Pharmacodynamic studies indicated that icotinib significantly inhibits numerous human tumor cell lines in nude mice [14]. Moreover, icotinib exhibited excellent tolerance among healthy Chinese population in a recent tolerance clinical trial, which demonstrated the highest safe dose of 1025 mg [28]. Oral icotinib in clinical trials stage I, II and III was generally well tolerated with positive clinical anti-tumor activities in patients with advanced NSCLC [14,29,30]. Additionally, icotinib can be absorbed rapidly, and the median T max was within the range of 0.5-3 h. It is a short-acting EGFR TKI inhibitor, and the half-life in body is six hours [31]. This feature can accord with the characteristics of our human digestive tract mucosa growth at night, which may reduce mucosa side effects. In the present study, radiation was performed 3-6 hours after oral icotinib, with high blood drug concentration of icotinib. It can reduce the EMT and the stem cells entering the blood circulation, therefore may reduce the chance of distant metastasis.
In our study, no DLT was encountered at dose level 1. At dose level 2, three patients experienced DLT. We did not find association between EGFR expression and occurrence of side effects. Among the patients who experienced DLT, 2 patients with EGFR mutation appeared to have more serious side effects than the patient without EGFR mutation. Whether EGFR mutation status, especially more mutations in exon 18 and 20, has a certain relationship with the adverse event needs to be further clarified.
In summary, the current study indicated that the combination of icotinib (125 mg) and IMRT in patients with locally NPC had an acceptable safety profile and was well tolerated, while mucositis toxicity appears to be the major DLT. Icotinib 125 mg per day with IMRT is the recommended dose for phase II trial in patients with NPC.
Acknowledgements
This study was supported by Zhejiang Provincial Medicine and Health Foundation (2013KYB290) of China and Research Foundation of Science and Technology Department of Zhejiang Province (2015C33257).
Disclosure of conflict of interest
None.
References
- 1.Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978;4:182–198. [PubMed] [Google Scholar]
- 2.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE, Ensley JF. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 3.Saleh-Ebrahimi L, Zwicker F, Muenter MW, Bischof M, Lindel K, Debus J, Huber PE, Roeder F. Intensity modulated radiotherapy (IMRT) combined with concurrent but not adjuvant chemotherapy in primary nasopharyngeal cancer-a retrospective single center analysis. Radiat Oncol. 2013;8:20. doi: 10.1186/1748-717X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, Jones C, Ang KK. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J. Clin. Oncol. 2009;27:3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guigay J, Temam S, Bourhis J, Pignon JP, Armand JP. Nasopharyngeal carcinoma and therapeutic management: the place of chemotherapy. Ann Oncol. 2006;17(Suppl 10):x304–307. doi: 10.1093/annonc/mdl278. [DOI] [PubMed] [Google Scholar]
- 6.Ji X, Xie C, Hu D, Fan X, Zhou Y, Zheng Y. Survival benefit of adding chemotherapy to intensity modulated radiation in patients with locoregionally advanced nasopharyngeal carcinoma. PLoS One. 2013;8:e56208. doi: 10.1371/journal.pone.0056208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan L, Li XH, Wan XX, Yi H, Li C, Li MY, Zhang PF, Zeng GQ, Qu JQ, He QY, Li JH, Chen Y, Chen ZC, Xiao ZQ. Analysis of EGFR signaling pathway in nasopharyngeal carcinoma cells by quantitative phosphoproteomics. Proteome Sci. 2011;9:35. doi: 10.1186/1477-5956-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma BB, Lui VW, Poon FF, Wong SC, To KF, Wong E, Chen H, Lo KW, Tao Q, Chan AT, Ng MH, Cheng SH. Preclinical activity of gefitinib in non-keratinizing nasopharyngeal carcinoma cell lines and biomarkers of response. Invest New Drugs. 2010;28:326–333. doi: 10.1007/s10637-009-9316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma BB, Poon TC, To KF, Zee B, Mo FK, Chan CM, Ho S, Teo PM, Johnson PJ, Chan AT. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma--a prospective study. Head Neck. 2003;25:864–872. doi: 10.1002/hed.10307. [DOI] [PubMed] [Google Scholar]
- 10.Park JK, Jang SJ, Kang SW, Park S, Hwang SG, Kim WJ, Kang JH, Um HD. Establishment of animal model for the analysis of cancer cell metastasis during radiotherapy. Radiat Oncol. 2012;7:153. doi: 10.1186/1748-717X-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Kong L, Lin S, Chen G, Chen Q, Lu JJ. The clinical significance of coexpression of cyclooxygenases-2, vascular endothelial growth factors, and epidermal growth factor receptor in nasopharyngeal carcinoma. Laryngoscope. 2008;118:1970–1975. doi: 10.1097/MLG.0b013e3181805134. [DOI] [PubMed] [Google Scholar]
- 12.Shintani S, Kiyota A, Mihara M, Sumida T, Kayahara H, Nakashiro K, Hamakawa H. Enhancement of radiosensitivity in head and neck cancer cells by ZD1839 (‘IRESSA’), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Am J Clin Oncol. 2003;26:e150–156. doi: 10.1097/01.coc.0000091356.25759.69. [DOI] [PubMed] [Google Scholar]
- 13.Basu D, Bewley AF, Sperry SM, Montone KT, Gimotty PA, Rasanen K, Facompre ND, Weinstein GS, Nakagawa H, Diehl JA, Rustgi AK, Herlyn M. EGFR inhibition promotes an aggressive invasion pattern mediated by mesenchymal-like tumor cells within squamous cell carcinomas. Mol Cancer Ther. 2013;12:2176–2186. doi: 10.1158/1535-7163.MCT-12-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q, Shentu J, Xu N, Zhou J, Yang G, Yao Y, Tan F, Liu D, Wang Y, Zhou J. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer. 2011;73:195–202. doi: 10.1016/j.lungcan.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Yang H, Hu W, Shan G, Ding W, Yu C, Wang B, Wang X, Xu Q. Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:617–621. doi: 10.1016/j.ijrobp.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Matkovic B, Juretic A, Separovic V, Novosel I, Separovic R, Gamulin M, Kruslin B. Immunohistochemical analysis of ER, PR, HER-2, CK 5/6, p63 and EGFR antigen expression in medullary breast cancer. Tumori. 2008;94:838–844. doi: 10.1177/030089160809400611. [DOI] [PubMed] [Google Scholar]
- 17.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 18.Rottey S, Madani I, Deron P, Van Belle S. Modern treatment for nasopharyngeal carcinoma: current status and prospects. Curr Opin Oncol. 2011;23:254–258. doi: 10.1097/CCO.0b013e328344f527. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Sun Y, Mao YP, Tang LL, Chen YM, Liu MZ, Ma J. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 20.Leong JL, Loh KS, Putti TC, Goh BC, Tan LK. Epidermal growth factor receptor in undifferentiated carcinoma of the nasopharynx. Laryngoscope. 2004;114:153–157. doi: 10.1097/00005537-200401000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Hu L, Chen F, Christensson B. Expression of Ki67 antigen, epidermal growth factor receptor and Epstein-Barr virus-encoded latent membrane protein (LMP1) in nasopharyngeal carcinoma. Eur J Cancer B Oral Oncol. 1994;30B:290–295. doi: 10.1016/0964-1955(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 22.Milas L, Raju U, Liao Z, Ajani J. Targeting molecular determinants of tumor chemo-radioresistance. Semin Oncol. 2005;32:S78–81. doi: 10.1053/j.seminoncol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 23.De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–494. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razak AR, Siu LL, Le Tourneau C. Molecular targeted therapies in all histologies of head and neck cancers: an update. Curr Opin Oncol. 2010;22:212–220. doi: 10.1097/CCO.0b013e328338001f. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Wang Y, Li Y, Guo K, He Y. Role of sorafenib and sunitinib in the induction of expressions of NKG2D ligands in nasopharyngeal carcinoma with high expression of ABCG2. J Cancer Res Clin Oncol. 2011;137:829–837. doi: 10.1007/s00432-010-0944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu T, Zhao C, Chen C, Gao L, Lang J, Pan J, Hu C, Jin F, Wang R, Xie C. An open, multicenter clinical study on cetuximab combined with intensity modulated radiotherapy (IMRT) plus concurrent chemotherapy in nasopharyngeal carcinoma (NPC): Preliminary report. J Clin Oncol (Meeting Abstracts) 2010;28:5577. [Google Scholar]
- 27.Ma BB, Kam MK, Leung SF, Hui EP, King AD, Chan SL, Mo F, Loong H, Yu BK, Ahuja A, Chan AT. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23:1287–1292. doi: 10.1093/annonc/mdr401. [DOI] [PubMed] [Google Scholar]
- 28.Ruan CJ, Liu DY, Jiang J, Hu P. Effect of the CYP2C19 genotype on the pharmacokinetics of icotinib in healthy male volunteers. Eur J Clin Pharmacol. 2012;68:1677–1680. doi: 10.1007/s00228-012-1288-4. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, Wang D, Li Q, Qin S, Hu C, Zhang Y, Chen J, Cheng Y, Feng J, Zhang H, Song Y, Wu YL, Xu N, Zhou J, Luo R, Bai C, Jin Y, Liu W, Wei Z, Tan F, Wang Y, Ding L, Dai H, Jiao S, Wang J, Liang L, Zhang W, Sun Y. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–961. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhao JZ, Shentu N, Xu , Tan F. A phase I/IIa study of icotinib hydrochloride, a novel oral EGFR-TKI, to evaluate its safety, tolerance, and preliminary efficacy in advanced NSCLC patients in China. J Clin Oncol (Meeting Abstracts) 2010;28:7574. [Google Scholar]
- 31.Liu D, Jiang J, Hu P, Tan F, Wang Y. Quantitative determination of icotinib in human plasma and urine using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3781–3786. doi: 10.1016/j.jchromb.2009.08.055. [DOI] [PubMed] [Google Scholar]